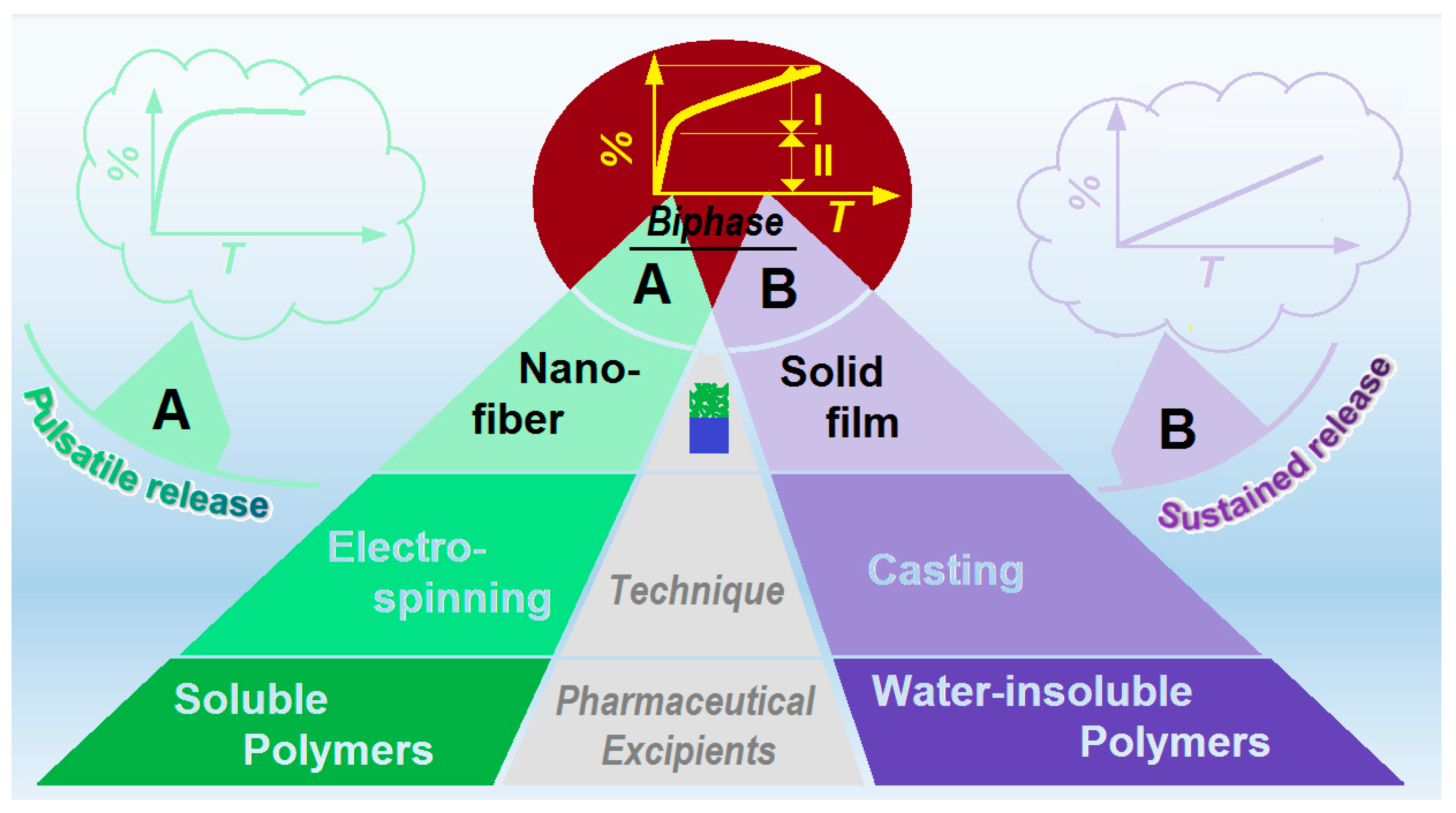
Hybrid Films Prepared from a Combination of Electrospinning and Casting for Offering a Dual-Phase Drug Release
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
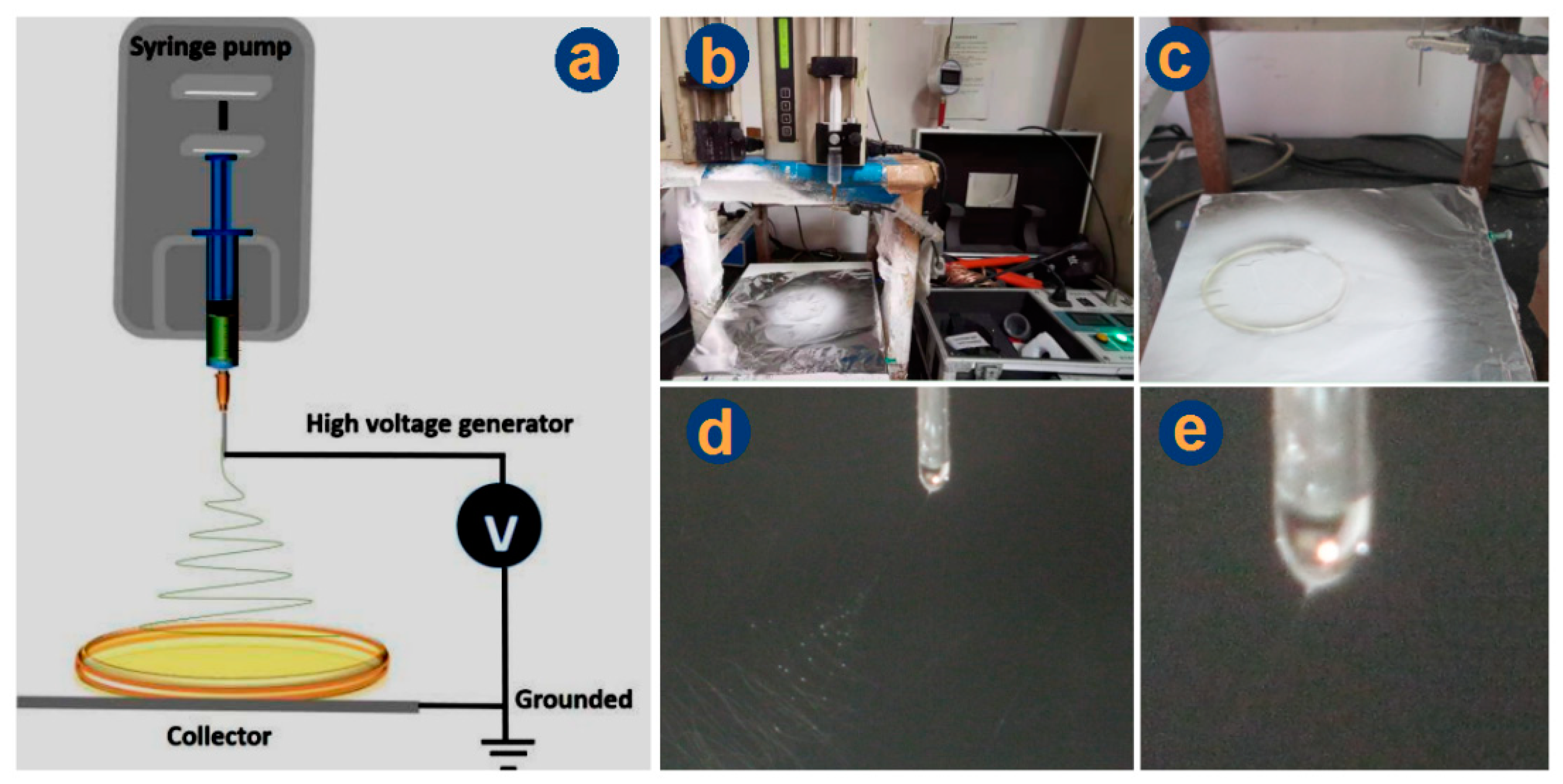
2.2. Preparations and Optimizations
2.3. Morphology
2.4. Physical Forms and Compatibility
2.5. In Vitro Dissolution Tests
3. Results and Discussion
3.1. Optimization of the Electrospinning Conditions
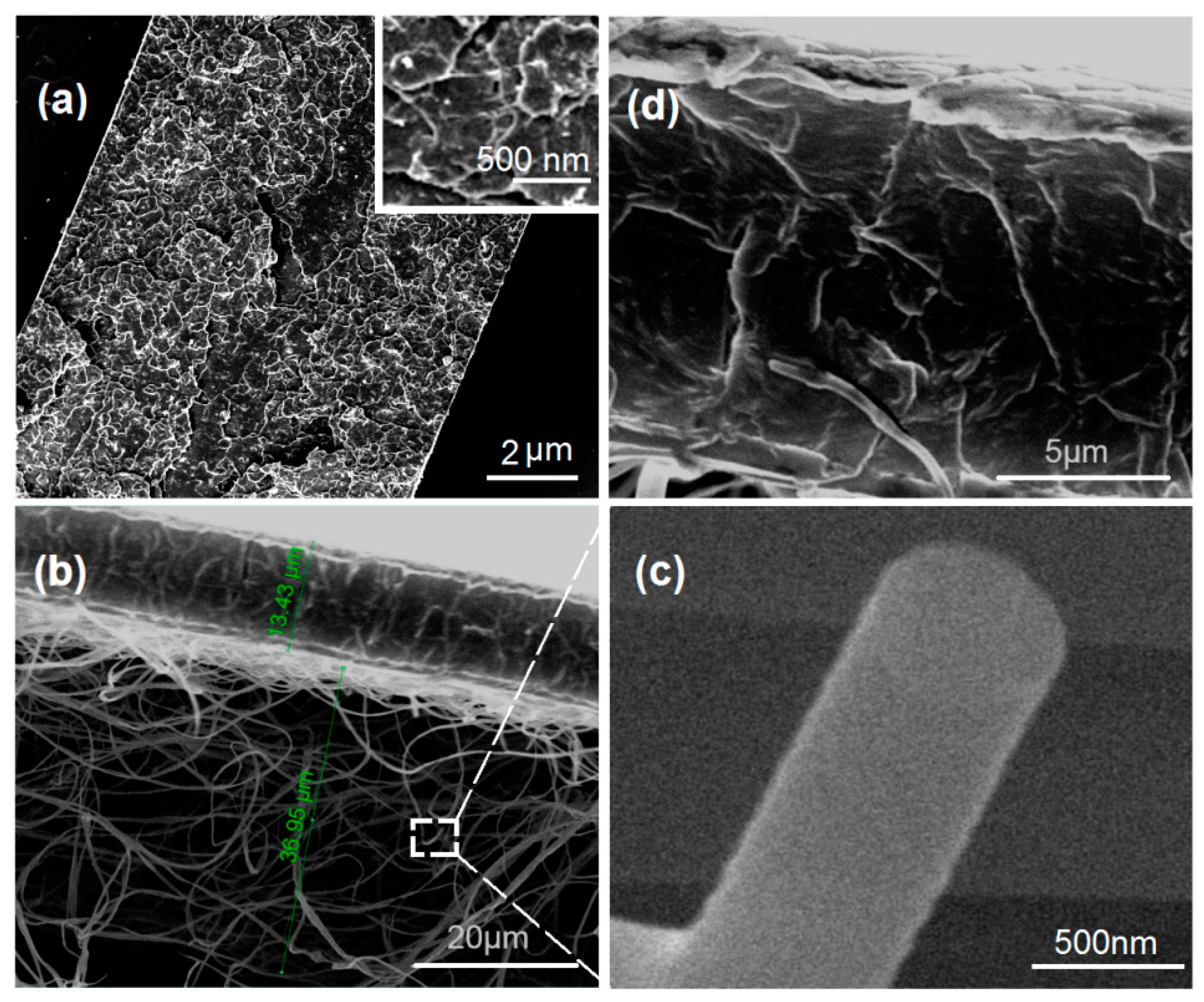
3.2. Morphology and Janus Structure
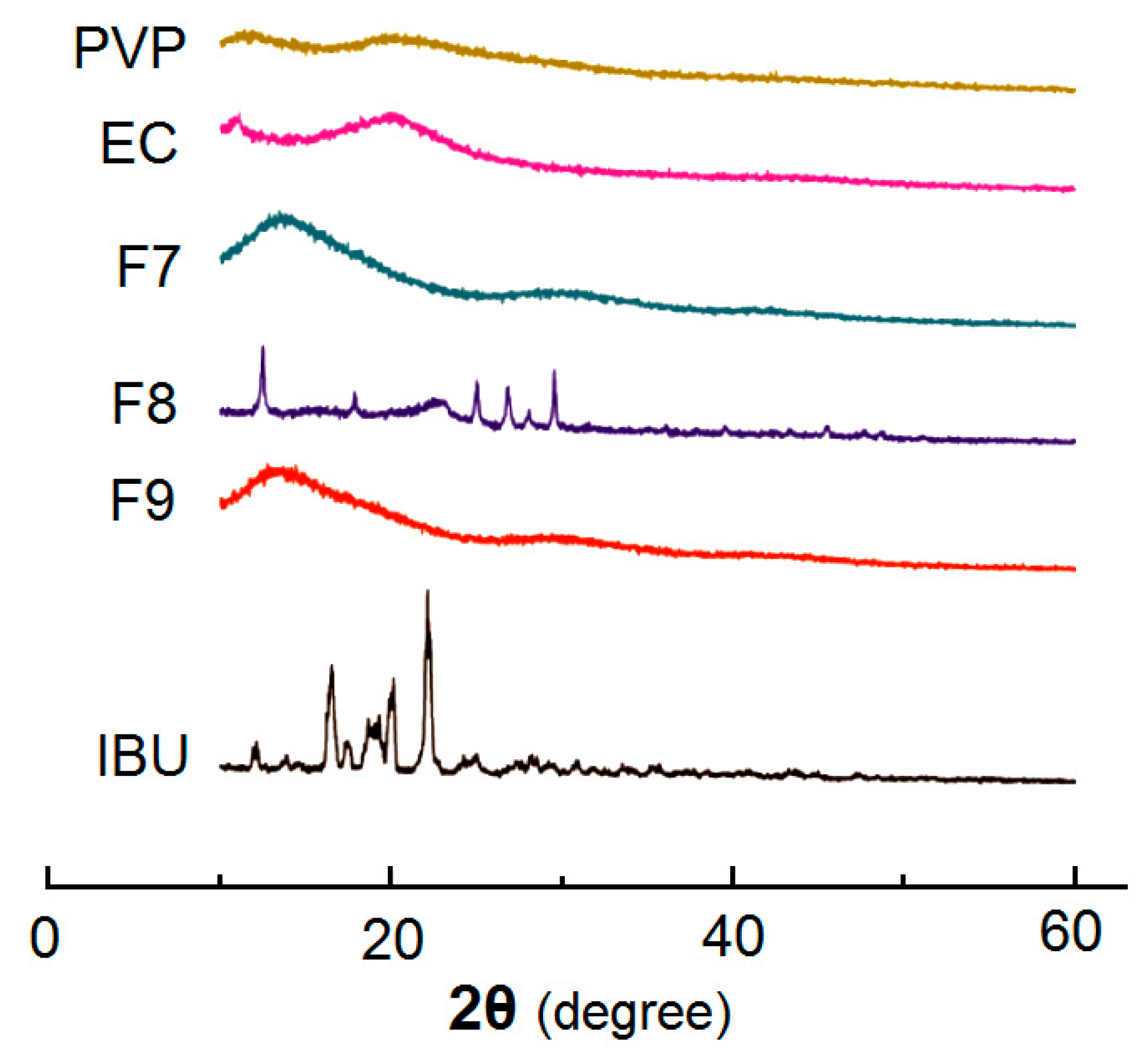
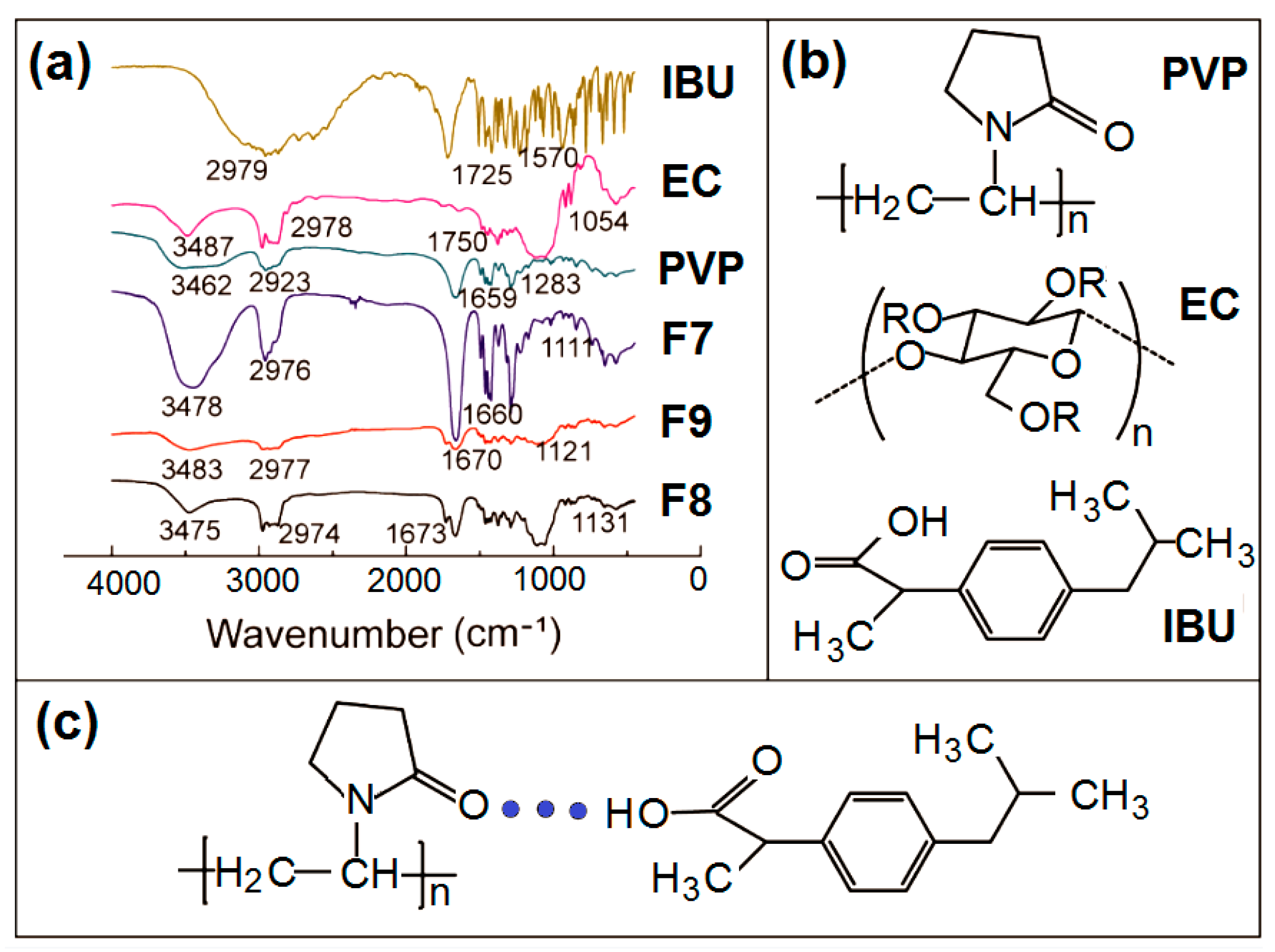
3.3. Physical State and Compatibility
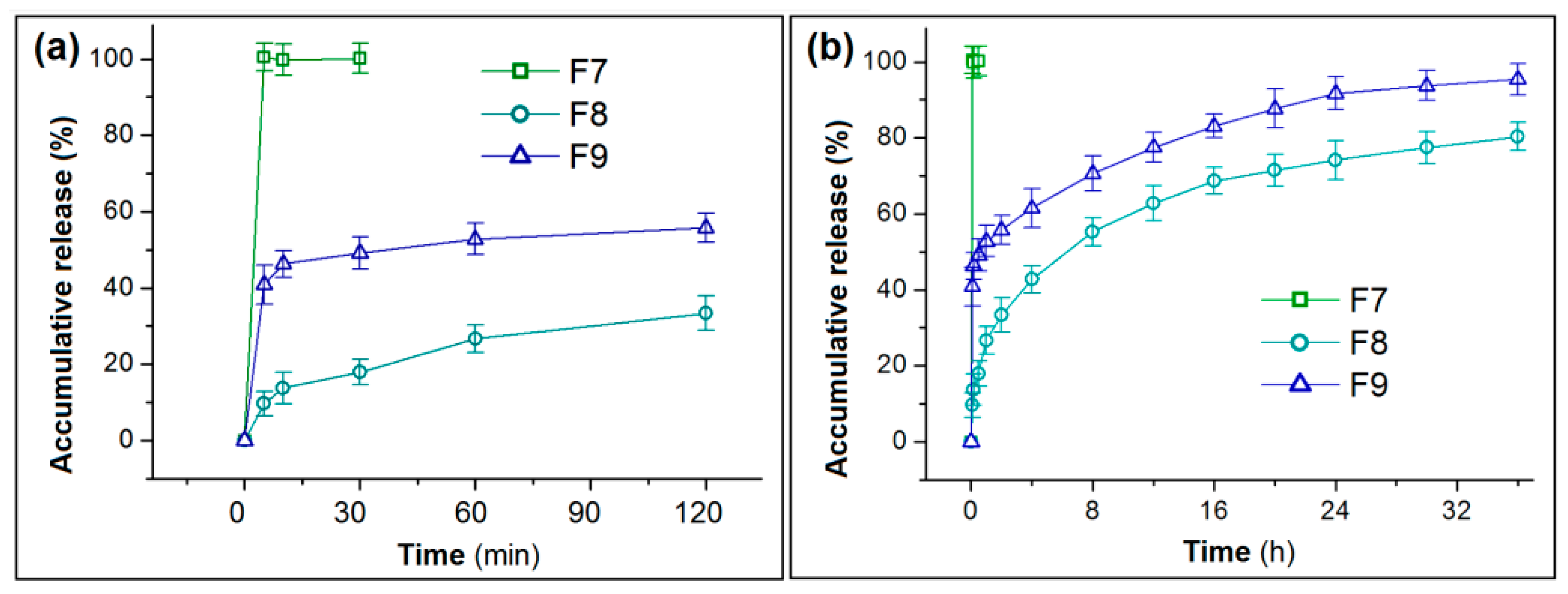
3.4. In Vitro Drug Release Profiles
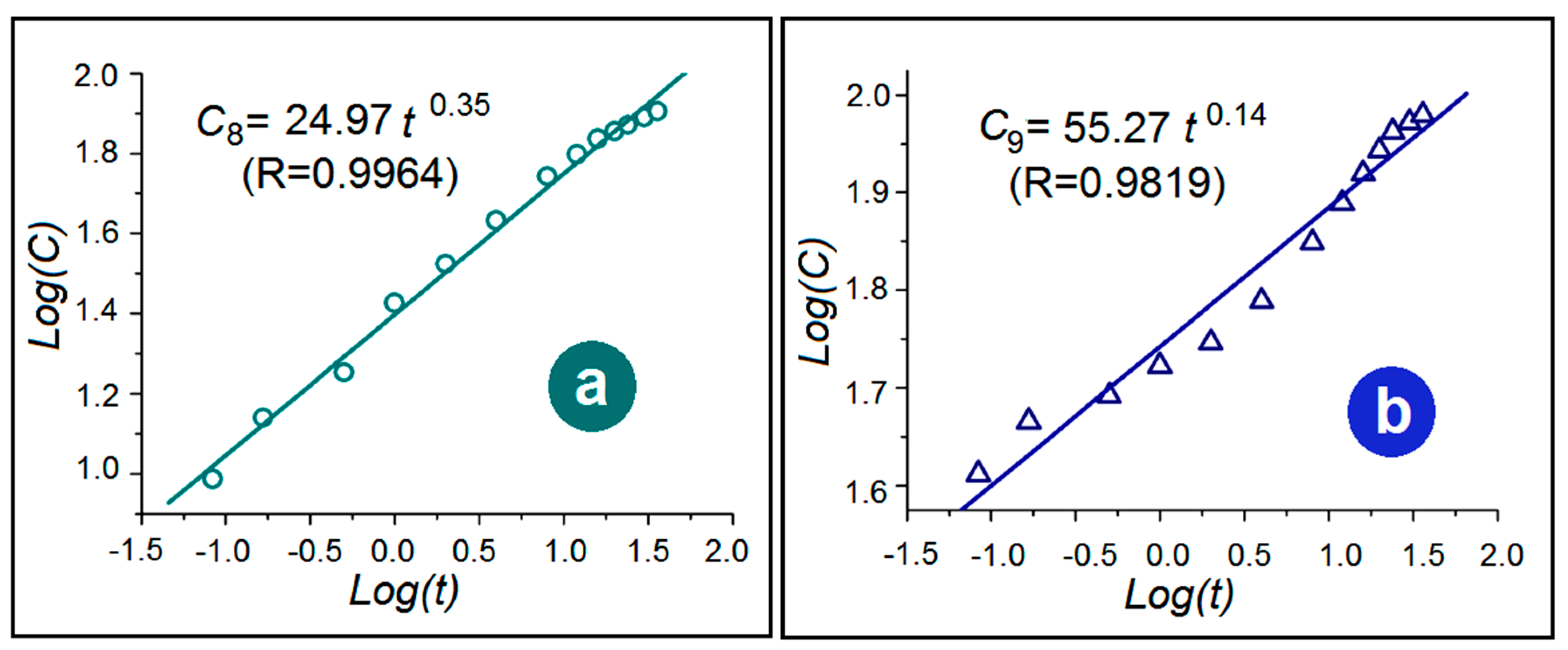
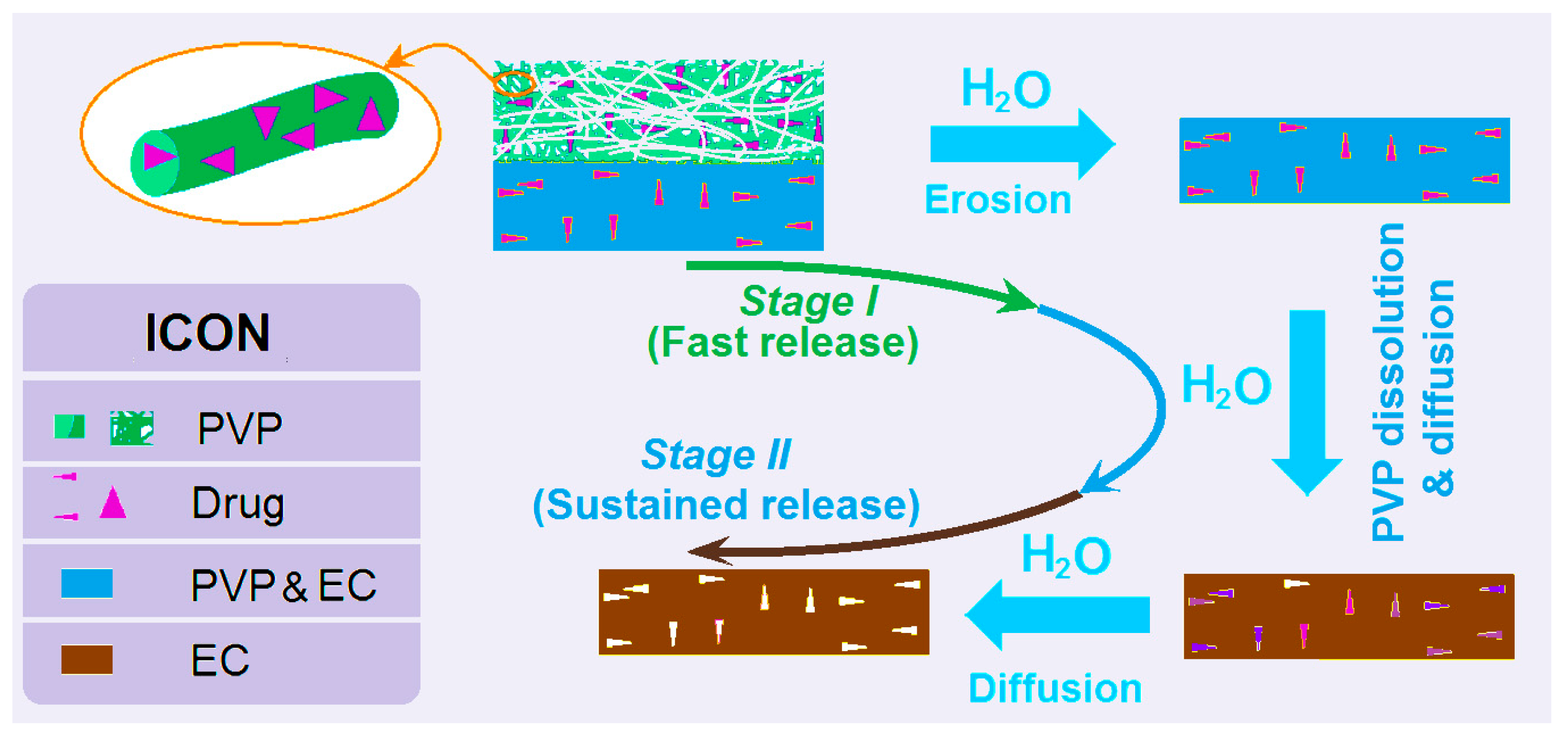
3.5. The Dual-Phase Release Mechanism from the DHFs
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saraogi, G.K.; Tholiya, S.; Mishra, Y.; Mishra, V.; Albutti, A.; Nayak, P.; Tambuwala, M.M. Formulation development and evaluation of pravastatin-loaded nanogel for hyperlipidemia management. Gels 2022, 8, 81. [Google Scholar] [CrossRef]
- Wlodarczyk, J.; Stojko, M.; Musial-Kulik, M.; Karpeta-Jarzabek, P.; Pastusiak, M.; Janeczek, H.; Dobrzynski, P.; Sobota, M.; Kasperczyk, J. Dual-jet electrospun PDLGA/PCU nonwovens and their mechanical and hydrolytic degradation properties. J. Mech. Behav. Biomed. 2022, 126, 105050. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Zhao, Y.; Zhou, M.; Zhong, A.; Sun, J. Promotion of skin regeneration through co-axial electrospun fibers loaded with basic fibroblast growth factor. Adv. Compos. Hybrid Mater. 2022, 5, 1–15. [Google Scholar] [CrossRef]
- Kazsoki, A.; Palcsó, B.; Alpár, A.; Snoeck, R.; Andrei, G.; Zelkó, R. Formulation of acyclovir (core)-dexpanthenol (sheath) nanofibrous patches for the treatment of herpes labialis. Int. J. Pharm. 2022, 611, 121354. [Google Scholar] [CrossRef]
- Mary, A.S.; Raghavan, V.S.; Kagula, S.; Krishnakumar, V.; Kannan, M.; Gorthi, S.S.; Rajaram, K. Enhanced in vitro wound healing using PVA/B-PEI nanofiber mats: A promising wound therapeutic agent against ESKAPE and opportunistic pathogens. ACS Appl. Bio Mater. 2021, 4, 8466–8476. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Y.; Li, D.; Xu, G.; Yin, D.; Xiao, Y.; Xu, T.; Chen, X.; Zhu, X.; Shi, X. Negative isolation of circulating tumor cells using a microfluidic platform integrated with streptavidin-functionalized PLGA nanofibers. Adv. Fiber Mater. 2021, 3, 192–202. [Google Scholar] [CrossRef]
- Yu, D.G. Preface—Bettering drug delivery knowledge from pharmaceutical techniques and excipients. Curr. Drug Deliv. 2021, 18, 2–3. [Google Scholar] [CrossRef]
- Song, X.; Jiang, Y.; Zhang, W.; Elfawal, G.; Wang, K.; Jiang, D.; Hong, H.; Wu, J.; He, C.; Mo, X.; et al. Transcutaneous tumor vaccination combined with anti-programmed death-1 monoclonal antibody treatment produces a synergistic antitumor effect. Acta Biomater. 2022, 140, 247–260. [Google Scholar] [CrossRef]
- El-Shanshory, A.A.; Agwa, M.M.; Abd-Elhamid, A.I.; Soliman, H.M.A.; Mo, X.; Kenawy, E.-R. Metronidazole topically immobilized electrospun nanofibrous scaffold: Novel secondary intention wound healing accelerator. Polymers 2022, 14, 454. [Google Scholar] [CrossRef]
- Feng, X.C.; Hao, J.S. Identifying new pathways and targets for wound healing and therapeutics from natural sources. Curr. Drug Deliv. 2021, 18, 1064–1084. [Google Scholar] [CrossRef]
- Li, J.K.; Guan, S.M.; Su, J.J.; Liang, J.H.; Cui, L.L.; Zhang, K. The Development of hyaluronic acids used for skin tissue regeneration. Curr. Drug Deliv. 2021, 18, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Liguori, A.; Uranga, J.; Panzavolta, S.; Guerrero, P.; Caba, K.; Focarete, M.L. Electrospinning of fish gelatin solution containing citric acid: An environmentally friendly approach to prepare crosslinked gelatin fibers. Materials 2019, 12, 2808. [Google Scholar] [CrossRef] [Green Version]
- Sa’adon, S.; Ansari, M.N.M.; Razak, S.I.A.; Anand, J.S.; Nayan, N.H.M.; Ismail, A.E.; Khan, M.U.A.; Haider, A. Preparation and physicochemical characterization of a diclofenac sodium-dual layer polyvinyl alcohol patch. Polymers 2021, 13, 2459. [Google Scholar] [CrossRef]
- Anand, S.; Pandey, P.; Begum, M.Y.; Chidambaram, K.; Arya, D.K.; Gupta, R.K.; Sankhwar, R.; Jaiswal, S.; Thakur, S.; Rajinikanth, P.S. Electrospun biomimetic multifunctional nanofibers loaded with ferulic acid for enhanced antimicrobial and wound-healing activities in STZ-Induced diabetic rats. Pharmaceuticals 2022, 15, 302. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Jiang, D.; Pei, Y.; Wang, Z.; Zhou, X.; Wu, J.; Mo, X.; Wang, H. Delivery of mRNA vaccines and anti-PDL1 siRNA through non-invasive transcutaneous route effectively inhibits tumor growth. Compos. Part B Eng. 2022, 233, 109648. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R.; et al. Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial Activities. Nano Res. 2022, 15. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, M.; Zhou, Y.; Sun, M.; Xie, Y.; Yu, D.-G. Comparisons of antibacterial performances between electrospun polymer@drug nanohybrids with drug-polymer nanocomposites. Adv. Compos. Hybrid Mater. 2022, 5, 1–13. [Google Scholar] [CrossRef]
- Lukiev, I.V.; Antipina, L.S.; Goreninskii, S.I.; Tverdokhlebova, T.S.; Vasilchenko, D.V.; Nemoykina, A.L.; Goncharova, D.A.; Svetlichnyi, V.A.; Dambaev, G.T.; Bouznik, V.M.; et al. Antibacterial ferroelectric hybrid membranes fabricated via electrospinning for wound healing. Membranes 2021, 11, 986. [Google Scholar] [CrossRef]
- Mohammad, M.H.; Mohammad-Taghi, G.; Niloufar, S.; Mehrdad, N. In-vitro and in-silico characterization of zein fiber incorporating cuminaldehyde. Food Bioprod. Process 2021, 128, 166–176. [Google Scholar] [CrossRef]
- Vu, Q.M.; Nguyen, T.C.; Dam, D.M.N.; Vu, Q.T.; Le, T.L.; Hoang, T.D.; Tran, T.K.N.; Nguyen, T.A.; Nguyen, P.H.; Thai, H.A. Novel method for preparation of carrageenan/fish scale collagen/allopurinol biocomposite film. Int. J. Polym. Sci. 2021, 2021, 9960233. [Google Scholar] [CrossRef]
- Huang, C.; Dong, J.; Zhang, Y.; Chai, S.; Wang, X.; Kang, S.; Yu, D.; Wang, P.; Jiang, Q. Gold nanoparticles-loaded polyvinylpyrrolidone/ethylcellulose coaxial electrospun nanofibers with enhanced osteogenic capability for bone tissue regeneration. Mater. Des. 2021, 212, 110240. [Google Scholar] [CrossRef]
- Yuan, Z.; Sheng, D.; Jiang, L.; Shafifiq, M.; Khan, A.U.R.; Hashim, R.; Chen, Y.; Li, B.; Xie, X.; Chen, J.; et al. Vascular endothelial growth factor-Capturing aligned electrospun polycaprolactone/gelatin nanofifibers promote patellar ligament regeneration. Acta Biomater. 2022, 140, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Azimi, B.; Ismaeilimoghadam, S.; Danti, S. Poly(lactic acid)-based electrospun fibrous structures for biomedical applications. Appl. Sci. 2022, 12, 3192. [Google Scholar] [CrossRef]
- Solovieva, A.O.; Permyakova, E.S.; Ershov, K.I.; Bakhareva, K.I.; Miroshnichenko, S.M.; Kiryukhantsev-Korneev, P.V.; Konopatsky, A.S.; Polčak, J.; Shtansky, D.V.; Manakhov, A.M. Plasma-coated PCL scaffolds with immobilized platelet-rich plasma enhance the wound healing in diabetics mice. Plasma Process Polym. 2022, 19, e2200032. [Google Scholar] [CrossRef]
- Yu, D.-G.; Lv, H. Preface-striding into nano drug delivery. Curr. Drug Deliv. 2022, 19, 1–3. [Google Scholar] [CrossRef]
- Ejeta, F.; Gabriel, T.; Joseph, N.M.; Belete, A. Formulation, optimization and in vitro evaluation of fast disintegrating tablets of salbutamol sulphate using a combination of superdisintegrant and subliming agent. Curr. Drug Deliv. 2021, 19, 129–141. [Google Scholar] [CrossRef]
- Ignatova, M.; Nachev, N.; Spasova, M.; Manolova, N.; Rashkov, I.; Naydenov, M. Electrospun 5-Chloro-7-iodo-8-hydroxyquinoline (clioquinol)-containing poly(3-hydroxybutyrate)/ polyvinylpyrrolidone antifungal materials prospective as active dressings against esca. Polymers 2022, 14, 367. [Google Scholar] [CrossRef]
- Bigogno, E.R.; Soares, L.; Mews, M.H.R.; Zétola, M.; Bazzo, G.C.; Stulzer, H.K.; Pezzini, B.R. It is possible to achieve tablets with good tabletability from solid dispersions—The case of the high dose drug gemfibrozil. Curr. Drug Deliv. 2021, 18, 460–470. [Google Scholar] [CrossRef]
- Guo, S.; Jiang, W.; Shen, L.; Zhang, G.; Gao, Y.; Yang, Y.Y.; Yu, D.G. Electrospun hybrid films for fast and convenient delivery of active herbs extracts. Membranes 2022, 12, 398. [Google Scholar] [CrossRef]
- Song, Y.; Huang, H.; He, D.; Yang, M.; Wang, H.; Zhang, H.; Li, J.; Li, Y.; Wang, C. Gallic acid/2-hydroxypropyl-β-cyclodextrin inclusion complexes electrospun nanofibrous webs: Fast dissolution, improved aqueous solubility and antioxidant property of gallic acid. Chem. Res. Chin. Univ. 2021, 37, 450–455. [Google Scholar] [CrossRef]
- Ning, T.; Zhou, Y.; Xu, H.; Guo, S.; Wang, K.; Yu, D.-G. Orodispersible membranes from a modified coaxial electrospinning for fast dissolution of diclofenac sodium. Membranes 2021, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Manakhov, A.M.; Sitnikova, N.A.; Tsygankova, A.R.; Alekseev, A.Y.; Adamenko, L.S.; Permyakova, E.; Baidyshev, V.S.; Popov, Z.I.; Blahová, L.; Eliáš, M.; et al. Electrospun biodegradable nanofibers coated homogenously by Cu magnetron sputtering exhibit fast ion release. Computational and experimental study. Membranes 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; De Marco, I. The use of Poly (N-vinyl pyrrolidone) in the delivery of drugs: A review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, G.R. Energy-saving electrospinning with a concentric teflon-core rod spinneret to create medicated nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef] [PubMed]
- Khalid, G.M.; Selmin, F.; Musazzi, U.M.; Gennari, C.G.M.; Minghetti, P.; Cilurzo, F. Trends in the characterization methods of orodispersible films. Curr. Drug Deliv. 2021, 18, 941–952. [Google Scholar] [CrossRef]
- Ortega, C.A.; Favier, L.S.; Cianchino, V.A.; Cifuente, D.A. New orodispersible mini tablets of enalapril maleate by direct compression for pediatric patients. Curr. Drug Deliv. 2020, 17, 505–510. [Google Scholar] [CrossRef]
- Garcia Cervantes, M.Y.; Han, L.; Kim, J.; Chitara, B.; Wymer, N.; Yan, F. N-halamine-decorated electrospun polyacrylonitrile nanofibrous membranes: Characterization and antimicrobial properties. React. Funct. Polym. 2021, 168, 105058. [Google Scholar] [CrossRef]
- Yang, J.; Yao, J.; Ma, Y. A highly flexible, renewable and green alginate polymer for electroactive biological gel paper actuators reinforced with a double-side casting approach. Cellulose 2021, 28, 3647–3662. [Google Scholar] [CrossRef]
- Jain, D.D.; Tambe, S.M.; Amin, P.D. Formulation performance window for manufacturing cellulose-based sustained-release mini-matrices of highly water-soluble drug via hot-melt extrusion technology. Cellulose 2022, 29, 3323–3350. [Google Scholar] [CrossRef]
- Peng, Y.; Via, B. The effect of cellulose nanocrystal suspension treatment on suspension viscosity and casted film property. Polymers 2021, 13, 2168. [Google Scholar] [CrossRef]
- Kose, M.D.; Ungun, N.; Bayraktar, O. Eggshell MembraneBased turmeric extract loaded orally disintegrating films. Curr. Drug Deliv. 2021, 19, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.-G. Electrospun structural hybrids of acyclovir-polyacrylonitrile at acyclovir for modifying drug release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Liu, Y.; Gao, Y.; Liu, P. Electrospun coaxial fibers to optimize the release of poorly water-soluble drug. Polymers 2022, 14, 469. [Google Scholar] [CrossRef]
- Leonarta, F.; Lee, C.-K. Nanofibrous membrane with encapsulated glucose oxidase for self-sustained antimicrobial applications. Membranes 2021, 11, 997. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Zhang, C.; Que, F.; Weiss, J.; Zhang, H. Characterization and antioxidant activity of trilayer gelatin/dextran-propyl gallate/gelatin films: Electrospinning versus solvent casting. LWT-Food Sci. Technol. 2020, 128, 109536. [Google Scholar] [CrossRef]
- Sultana, M.; Sultana, S.; Hussain, K.; Saeed, T.; Butt, M.A.; Raza, S.A.; Mahmood, R.; Hassan, S.; Anwer, U.U.; Bukhari, N.I. Enhanced mefenamic acid release from poloxamer-silicon dioxide gel filled in hard gelatin capsules—An application of liquid semisolid matrix technology for insoluble. Curr. Drug Deliv. 2021, 19, 801–811. [Google Scholar] [CrossRef]
- Murugesan, R.; Raman, S. Recent trends in carbon nanotubes based prostate cancer therapy: A biomedical hybrid for diagnosis and treatment. Curr. Drug Deliv. 2022, 19, 229–237. [Google Scholar] [CrossRef]
- Mehra, N.K.; Palakurthi, S. Interactions between carbon nanotubes and bioactives: A drug delivery perspective. Drug Discov. Today 2016, 21, 585–597. [Google Scholar] [CrossRef]
- Esim, O.; Hascicek, C. Lipid-coated nanosized drug delivery systems for an effective cancer therapy. Curr. Drug Deliv. 2021, 18, 147–161. [Google Scholar] [CrossRef]
- Kamal, M.M.; Akter, S.; Al Hagbani, T.; Salawi, A.; Nazzal, S. Sustained release of curcumin self-emulsifying drug delivery system (SEDDS) from solvent-cast Soluplus(R) films. Pharm. Dev. Technol. 2021, 26, 1102–1109. [Google Scholar] [CrossRef]
- Jackson, J.; Plackett, D.; Hsu, E.; Lange, D.; Evans, R.; Burt, H. The development of solvent cast films or electrospun nanofiber membranes made from blended poly vinyl alcohol materials with different degrees of hydrolyzation for optimal hydrogel dissolution and sustained release of Anti-infective silver salts. Nanomaterials 2021, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Hou, J.S.; Yu, D.G.; Li, S.Y.; Zhu, J.W.; Chen, Z.Z. Electrospun tri-layer nanodepots for sustained release of acyclovir. J. Alloy Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Bukhary, H.; Williams, G.R.; Orlu, M. Fabrication of electrospun levodopa-carbidopa fixed-dose combinations. Adv. Fiber Mater. 2020, 2, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Obeidat, W.M.; Gharaibeh, S.F.; Jaradat, A.A.; Abualsuod, O. Preparation and evaluation of ternary polymeric blends for controlled release matrices containing weakly basic model drug. Curr. Drug Deliv. 2021, 18, 54–64. [Google Scholar] [CrossRef]
- He, H.; Wu, M.; Zhu, J.; Yang, Y.; Ge, R.; Yu, D.-G. Engineered spindles of little molecules around electrospun nanofibers for biphasic drug release. Adv. Fiber Mater. 2021, 3, 305–317. [Google Scholar] [CrossRef]
- Yu, D.-G.; Wang, X.; Li, X.Y.; Chian, W.; Li, Y.; Liao, Y.Z. Electrospun biphasic drug release polyvinylpyrrolidone/ ethyl cellulose core/sheath nanofibers. Acta Biomater. 2013, 9, 5665–5672. [Google Scholar] [CrossRef]
- Liu, R.R.; Hou, L.L.; Yue, G.C.; Li, H.K.; Zhang, J.S.; Liu, J.; Miao, B.B.; Wang, N.; Bai, J.; Cui, Z.M.; et al. Progress of fabrication and applications of electrospun hierarchically porous nanofibers. Adv. Fiber Mater. 2022, 4, 1–27. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Yang, D.; Nie, J.; Ma, G. Electrospun core–shell fibrous 2D scaffold with biocompatible poly (glycerol sebacate) and poly-l-lactic acid for wound healing. Adv. Fiber Mater. 2020, 2, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhang, F.; Wang, M.; Lv, H.; Yu, D.G.; Liu, X.; Shen, H. Electrospun hierarchical structural films for effective wound healing. Biomater. Adv. 2022, 1, 212795. [Google Scholar] [CrossRef]
- Zhao, K.; Lu, Z.-H.; Zhao, P.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Modified tri–axial electrospun functional core–shell nanofibrous membranes for natural photodegradation of antibiotics. Chem. Eng. J. 2021, 425, 131455. [Google Scholar] [CrossRef]
- Komijani, M.; Mohebbi, M.; Ghorani, B. Assembly of electrospun tri-layered nanofibrous structure of zein/basil seed gum/zein for increasing the bioaccessibility of lycopene. Lwt Food Sci. Technol. 2022, 161, 113328. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, K.; Yu, D.G.; Zheng, X.; Huang, C. Advances in biosensing and environmental monitoring based on electrospun nanofibers. Adv. Fiber Mater. 2022, 4, 404–435. [Google Scholar] [CrossRef]
- Zheng, G.; Peng, H.; Jiang, J.; Kang, G.; Liu, J.; Zheng, J.; Liu, Y. Surface functionalization of PEO nanofibers using a TiO2 suspension as sheath fluid in a modified coaxial electrospinning process. Chem. Res. Chin. Univ. 2021, 37, 571–577. [Google Scholar] [CrossRef]
- Zhan, L.; Deng, J.; Ke, Q.; Li, X.; Ouyang, Y.; Huang, C.; Liu, X.; Qian, Y. Grooved fibers: Preparation principles through electrospinning and potential applications. Adv. Fiber Mater. 2021, 3, 203–213. [Google Scholar] [CrossRef]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Polymer-based nanofiber-nanoparticle hybrids and their medical applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Qin, Y.; Li, C. Functional electrospun nanocomposites for efficient oxygen reduction reaction. Chem. Res. Chin. Univ. 2021, 37, 379–393. [Google Scholar] [CrossRef]
- Jiang, S.; Schmalz, H.G.; Agarwal, S.; Greiner, A. Electrospinning of ABS nanofibers and their high filtration performance. Adv. Fiber Mater. 2020, 2, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Surendranath, M.; Rajalekshmi, R.; Ramesan, R.M.; Nair, P.; Parameswaran, R. UV-Crosslinked electrospun Zein/PEO fibroporous membranes for wound dressing. ACS Appl. Bio Mater. 2021, 5, 1538–1551. [Google Scholar] [CrossRef]
- Klebeko, J.; Ossowicz-Rupniewska, P.; Nowak, A.; Janus, E.; Duchnik, W.; Adamiak-Giera, U.; Kucharski, Ł.; Prowans, P.; Petriczko, J.; Czapla, N.; et al. Permeability of ibuprofen in the form of free acid and salts of l-valine alkyl esters from a hydrogel formulation through strat-m™ membrane and human skin. Materials 2021, 14, 6678. [Google Scholar] [CrossRef]
- Obeidat, W.M.; Al-Natour, M.A. Assessment of once daily controlled-release ibuprofen matrix tablets prepared using Eudragit (R) E100/carbopol (R) 971P NF polymers and their salt combinations. Curr. Drug Deliv. 2022, 19, 74–85. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.G.; Wang, K. Electrospun nanofiber membranes for air filtration: A review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating current electrospinning: The impacts of various high-voltage signal shapes and frequencies on the spinnability and productivity of polycaprolactone nanofifibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Lv, H.; Zhou, Y.; Yang, Y.; Yu, D.-G. Electrospun polyacrylonitrile-based lace nanostructures and their Cu(II) adsorption. Sep. Purif. Technol. 2022, 288, 120643. [Google Scholar] [CrossRef]
- Ghazalian, M.; Afshar, S.; Rostami, A.; Rashedi, S.; Bahrami, S.H. Fabrication and characterization of chitosan-polycaprolactone core-shell nanofibers containing tetracycline hydrochloride. Colloids Surface. A. 2022, 636, 128163. [Google Scholar] [CrossRef]
- Yu, D.-G.; Wang, M.; Ge, R. Strategies for sustained drug release from electrospun multi-layer nanostructures. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1772. [Google Scholar] [CrossRef] [PubMed]
- Ziyadi, H.; Baghali, M.; Bagherianfar, M.; Mehrali, F.; Faridi-Majidi, R. An investigation of factors affecting the electrospinning of poly (vinyl alcohol)/kefiran composite nanofibers. Adv. Compos. Hybrid Mater. 2021, 4, 768–779. [Google Scholar] [CrossRef]
- Miranda, C.S.; Silva, A.F.G.; Pereira-Lima, S.M.M.A.; Costa, S.P.G.; Homem, N.C.; Felgueiras, H.P. Tunable spun fiber constructs in biomedicine: Influence of processing parameters in the fibers’ architecture. Pharmaceutics 2022, 14, 164. [Google Scholar] [CrossRef]
- Peppas, N. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Liu, Y.; Chen, X.; Yu, D.G.; Liu, H.; Liu, Y.; Liu, P. Electrospun PVP-core/PHBV-shell nanofibers to eliminate tailing off for an improved sustained release of curcumin. Mol. Pharm. 2021, 18, 4170–4178. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhou, W.; Yu, D.-G. Electrospun medical sutures for wound healing: A review. Polymers 2022, 14, 1637. [Google Scholar] [CrossRef]
- Brimo, N.; Serdaroglu, D.C.; Uysal, B. Comparing antibiotic pastes with electrospun nanofibers as modern drug delivery systems for regenerative endodontics. Curr. Drug Deliv. 2021, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Domokos, A.; Farkas, B.; Farkas, A.; Rapi, Z.; Kiss, D.; Nyiri, Z.; Eke, Z.; Szarka, G.; Örkényi, R.; et al. Continuous end-to-end production of solid drug dosage forms: Coupling flow synthesis and formulation by electrospinning. Chem. Eng. J. 2018, 350, 290–299. [Google Scholar] [CrossRef]
- Kiss, K.; Vass, P.; Farkas, A.; Hirsch, E.; Szabo, E.; Mezo, G.; Marosi, G. A solid doxycycline HP-β-CD formulation for reconstitution (iv bolus) prepared by scaled-up electrospinning. Int. J. Pharm. 2020, 586, 119539. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, C.A.; Sosnik, A.; Kalmar, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in drug delivery: From design to application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef] [PubMed]
- Rajzer, I.; Kurowska, A.; Jablonski, A.; Jatteau, S.; Sliwka, M.; Ziabka, M.; Menaszek, E. Layered gelatin/PLLA scaffolds fabricated by electrospinning and 3D printing-for nasal cartilages and subchondral bone reconstruction. Mater. Des. 2020, 155, 297–306. [Google Scholar] [CrossRef]
- Yu, D.G.; Zhu, L.M.; Branford-White, C.; Yang, X.L. Three-dimensional printing in pharmaceutics—Promises and problems. J. Pharm. Sci. 2008, 97, 3666–3690. [Google Scholar] [CrossRef]
- Elkasabgy, N.A.; Mahmoud, A.A.; Maged, A. 3D printing: An appealing route for customized drug delivery systems. Int. J. Pharm. 2020, 588, 119732. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.-G.; Kim, I. Recent advances in poly(α-L-glutamic acid)-based nanomaterials for drug delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef]
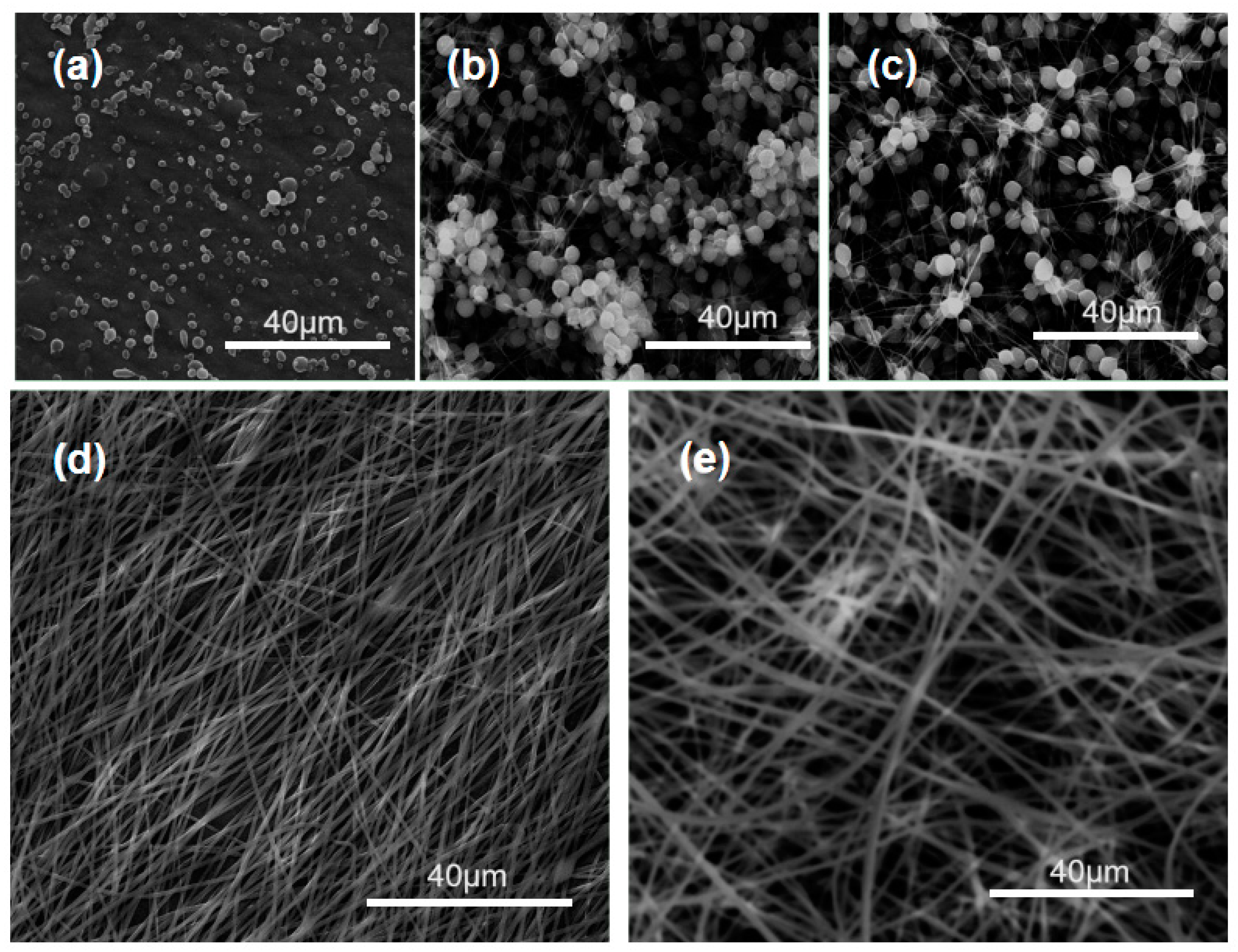

| Denoted | Technique | Working Fluid (w/v%) | Products |
|---|---|---|---|
| F1 | Electro- spraying | 2% PVP | Particles |
| F2 | 4% PVP | Particles | |
| F3 | 5% PVP | Beads-on-a-string | |
| F4 | Electro- spinning | 6% PVP | Nanofibers |
| F5 | 9% PVP | Nanofibers | |
| F6 | 9% PVP & 3% IBU | Nanofibers | |
| F7 | 6% PVP & 2% IBU | Nanofibers | |
| F8 | Casting | 12% EC + 2% IBU | Solid films |
| F9 | Electrospinning//Casting | 2% IBU + 6% PVP//2% IBU + 2% PVP + 12% EC | Double-layer hybrid films (DHFs) |
| No. | Process | CT (wt%) a | CD (wt%) b | EE (%) |
|---|---|---|---|---|
| F7 | Electrospinning | 25 | 25.62 ± 0.25 | 97.6% |
| F8 | Casting | 12.5 | 12.31 ± 0.18 | 101.5% |
| F9 | Combination | 15.80 | 16.01 ± 0.13 | 101.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Jiang, W.; Yang, Z.; Chen, X.; Yu, D.-G.; Shao, J. Hybrid Films Prepared from a Combination of Electrospinning and Casting for Offering a Dual-Phase Drug Release. Polymers 2022, 14, 2132. https://doi.org/10.3390/polym14112132
Liu H, Jiang W, Yang Z, Chen X, Yu D-G, Shao J. Hybrid Films Prepared from a Combination of Electrospinning and Casting for Offering a Dual-Phase Drug Release. Polymers. 2022; 14(11):2132. https://doi.org/10.3390/polym14112132
Chicago/Turabian StyleLiu, Haoran, Wenlai Jiang, Zili Yang, Xiren Chen, Deng-Guang Yu, and Jun Shao. 2022. "Hybrid Films Prepared from a Combination of Electrospinning and Casting for Offering a Dual-Phase Drug Release" Polymers 14, no. 11: 2132. https://doi.org/10.3390/polym14112132
APA StyleLiu, H., Jiang, W., Yang, Z., Chen, X., Yu, D.-G., & Shao, J. (2022). Hybrid Films Prepared from a Combination of Electrospinning and Casting for Offering a Dual-Phase Drug Release. Polymers, 14(11), 2132. https://doi.org/10.3390/polym14112132








