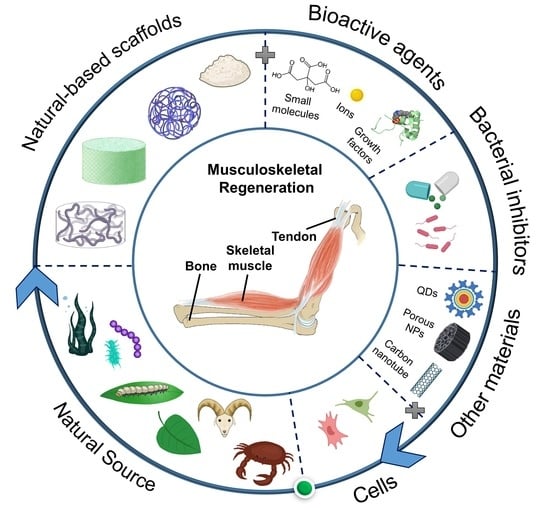
A Review of Recent Advances in Natural Polymer-Based Scaffolds for Musculoskeletal Tissue Engineering
Abstract
:1. Introduction
2. Bone
2.1. Bone Extracellular Matrix
2.2. Bone Structure
2.3. Bone Diseases
2.4. Natural-Based Scaffolds for Bone Tissue Engineering
2.4.1. Collagen
2.4.2. Gelatin
2.4.3. Chitosan
2.4.4. Alginate
2.4.5. Silk Fibroin
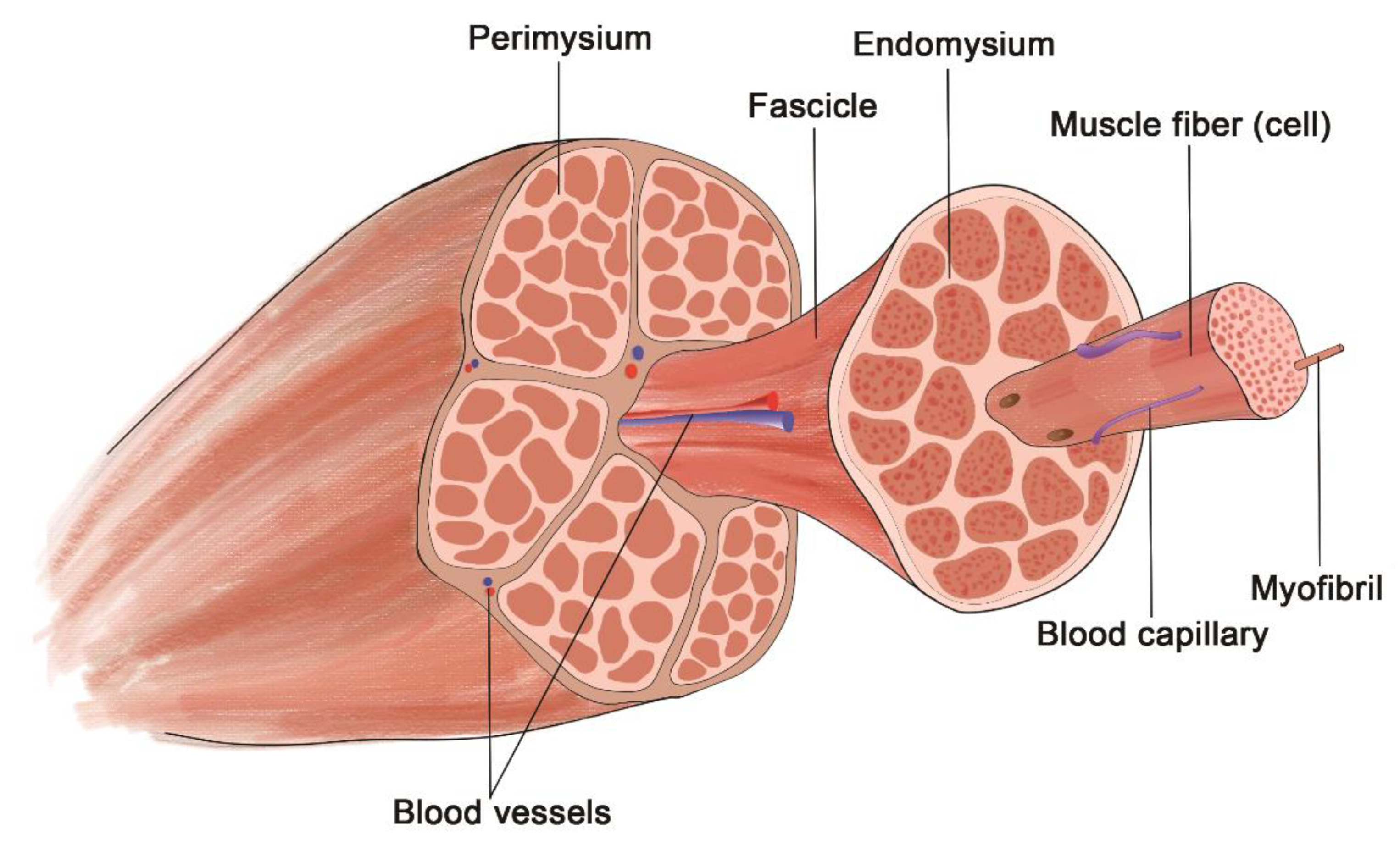
3. Skeletal Muscle
3.1. Skeletal Muscle ECM Structure
3.2. Disorders
3.3. Natural-Based Scaffold for Skeletal Muscle Tissue Engineering
3.3.1. Keratin
3.3.2. Collagen
3.3.3. Alginate
3.3.4. Laminin, Fibrin, and Gelatin
4. Tendon and Ligament
4.1. Tendon and Ligament ECM Structure
4.2. Disorders
4.3. Natural-Based Scaffold for Tendon and Ligament Tissue Engineering
4.3.1. Collagen
4.3.2. Silk
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loebel, C.; Burdick, J.A. Engineering Stem and Stromal Cell Therapies for Musculoskeletal Tissue Repair. Cell Stem Cell 2018, 22, 325–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zumwalt, M.; Reddy, A.P. Stem Cells for Treatment of Musculoskeletal Conditions—Orthopaedic/Sports Medicine Applications. Biochim. et Biophys. Acta Mol. Basis Dis. 2020, 1866, 165624. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Cai, L.; Li, X.; Ye, L.; Xie, J. Stiffness and Topography of Biomaterials Dictate Cell-Matrix Interaction in Musculoskeletal Cells at the Bio-Interface: A Concise Progress Review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2426–2440. [Google Scholar] [CrossRef]
- Madrigal, J.L.; Stilhano, R.; Silva, E.A. Biomaterial-Guided Gene Delivery for Musculoskeletal Tissue Repair. Tissue Eng. Part B Rev. 2017, 23, 347–361. [Google Scholar] [CrossRef]
- Ma, D.; Wang, Y.; Dai, W. Silk Fibroin-Based Biomaterials for Musculoskeletal Tissue Engineering. Mater. Sci. Eng. C 2018, 89, 456–469. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Sikorski, P.; Leach, J.K. Bio-Instructive Materials for Musculoskeletal Regeneration. Acta Biomater. 2019, 96, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.; Jiang, C.; Uzunalli, G.; Thankappan, S.K.; Laurencin, C.T.; Deng, M. Polymeric Electrospinning for Musculoskeletal Regenerative Engineering. Regen. Eng. Transl. Med. 2016, 2, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Nie, X.; Wang, D.A. Decellularized Orthopaedic Tissue-Engineered Grafts: Biomaterial Scaffolds Synthesised by Therapeutic Cells. Biomater. Sci. 2018, 6, 2798–2811. [Google Scholar] [CrossRef]
- Ferrigno, B.; Bordett, R.; Duraisamy, N.; Moskow, J.; Arul, M.R.; Rudraiah, S.; Nukavarapu, S.P.; Vella, A.T.; Kumbar, S.G. Bioactive Polymeric Materials and Electrical Stimulation Strategies for Musculoskeletal Tissue Repair and Regeneration. Bioact. Mater. 2020, 5, 468–485. [Google Scholar] [CrossRef]
- Qazi, T.H.; Mooney, D.J.; Pumberger, M.; Geißler, S.; Duda, G.N. Biomaterials Based Strategies for Skeletal Muscle Tissue Engineering: Existing Technologies and Future Trends. Biomaterials 2015, 53, 502–521. [Google Scholar] [CrossRef]
- Bayrak, E.; Yilgor Huri, P. Engineering Musculoskeletal Tissue Interfaces. Front. Mater. 2018, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- Abalymov, A.; Parakhonskiy, B.; Skirtach, A.G. Polymer-and Hybrid-Based Biomaterials for Interstitial, Connective, Vascular, Nerve, Visceral and Musculoskeletal Tissue Engineering. Polymers 2020, 12, 620. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, M.; Abradelo, C.; San Román, J.; Rojo, L. Bibliographic Review on the State of the Art of Strontium and Zinc Based Regenerative Therapies. Recent Developments and Clinical Applications. J. Mater. Chem. B 2019, 7, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Wheelton, A.; Mace, J.S.; Khan, W.; Anand, S. Biomaterials and Fabrication to Optimise Scaffold Properties for Musculoskeletal Tissue Engineering. Curr. Stem Cell Res. Ther. 2016, 11, 578–584. [Google Scholar] [CrossRef] [Green Version]
- Lim, W.L.; Liau, L.L.; Ng, M.H.; Chowdhury, S.R.; Law, J.X. Current Progress in Tendon and Ligament Tissue Engineering. Tissue Eng. Regen. Med. 2019, 16, 549–571. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural-Origin Polymers as Carriers and Scaffolds for Biomolecules and Cell Delivery in Tissue Engineering Applications. Adv. Drug Deliv. Rev. 2007, 59, 27–207. [Google Scholar] [CrossRef] [Green Version]
- Del Bakhshayesh, A.R.; Asadi, N.; Alihemmati, A.; Tayefi Nasrabadi, H.; Montaseri, A.; Davaran, S.; Saghati, S.; Akbarzadeh, A.; Abedelahi, A. An Overview of Advanced Biocompatible and Biomimetic Materials for Creation of Replacement Structures in the Musculoskeletal Systems: Focusing on Cartilage Tissue Engineering. J. Biol. Eng. 2019, 13, 85. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.J.; Martens, P.J. Engineering Biosynthetic Cell Encapsulation Systems. In Biosynthetic Polymers for Medical Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 205–239. ISBN 9781782421139. [Google Scholar]
- Spicer, C.D. Hydrogel Scaffolds for Tissue Engineering: The Importance of Polymer Choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Bao, W.; Li, M.; Yang, Y.; Wan, Y.; Wang, X.; Bi, N.; Li, C. Advancements and Frontiers in the High Performance of Natural Hydrogels for Cartilage Tissue Engineering. Front. Chem. 2020, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, D.P.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering. Membranes 2018, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, G.Z.; Zhou, Y. Electrospinning of Biomimetic Fibrous Scaffolds for Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 947–960. [Google Scholar] [CrossRef]
- Robb, K.P.; Shridhar, A.; Flynn, L.E. Decellularized Matrices as Cell-Instructive Scaffolds to Guide Tissue-Specific Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 3627–3643. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Koski, C.; Vu, A.A. Additive Manufacturing of Natural Biopolymers and Composites for Bone Tissue Engineering. Mater. Horiz. 2020, 7, 2011–2027. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature- and PH-Responsive Chitosan-Based Injectable Hydrogels for Bone Tissue Engineering. Mater. Sci. Eng. C 2020, 111, 110862. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Ali, A.; Sheikh, J. A Review on Chitosan Centred Scaffolds and Their Applications in Tissue Engineering. Int. J. Biol. Macromol. 2018, 116, 849–862. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate Hydrogels for Bone Tissue Engineering, from Injectables to Bioprinting: A Review. Carbohydr. Polym. 2020, 229, 115514. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate Composites for Bone Tissue Engineering: A Review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Rajesh, R.; Dominic Ravichandran, Y.; Kuo, Y.C. Alginate in Bone Tissue Engineering. In Seaweed Polysaccharides: Isolation, Biological and Biomedical Applications; Venkatesan, J., Anil, S., Kim, S.-K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 349–368. ISBN 9780128098172. [Google Scholar]
- Farokhi, M.; Shariatzadeh, F.J.; Solouk, A.; Mirzadeh, H. Alginate Based Scaffolds for Cartilage Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 230–247. [Google Scholar] [CrossRef]
- Roslan, M.R.; Nasir, N.F.F.M.; Cheng, E.M.; Amin, N.A.M. Tissue Engineering Scaffold Based on Starch: A Review. In Proceedings of the International Conference on Electrical, Electronics, and Optimization Techniques, ICEEOT 2016, Chennai, India, 3–5 March 2016; IEEE: Chennai, India; pp. 1857–1860.
- Robyt, J.F. Starch: Structure, Properties, Chemistry, and Enzymology. In Glycoscience; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1437–1472. [Google Scholar]
- Hemamalini, T.; Giri Dev, V.R. Comprehensive Review on Electrospinning of Starch Polymer for Biomedical Applications. Int. J. Biol. Macromol. 2018, 106, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, A.; Berkland, C. Applications and Emerging Trends of Hyaluronic Acid in Tissue Engineering, as a Dermal Filler and in Osteoarthritis Treatment. Acta Biomater. 2013, 9, 7081–7092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging Roles of Hyaluronic Acid Bioscaffolds in Tissue Engineering and Regenerative Medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Grumezescu, A.M.; Bejenaru, L.E. Hyaluronic Acid-Based Scaffolds for Tissue Engineering. Rom. J. Morphol. Embryol. 2018, 59, 71–76. [Google Scholar] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic Acid Based Scaffolds for Tissue Engineering—A Review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent Advance in Delivery System and Tissue Engineering Applications of Chondroitin Sulfate. Carbohydr. Polym. 2020, 230, 115650. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-Based Biomaterials for Tissue Engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Khang, G.; Lee, S.J.; Kim, M.S.; Lee, H.B. Biomaterials: Tissue Engineering and Scaffolds. In Encyclopedia of Medical Devices and Instrumentation; Major Reference Works; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 366–383. ISBN 9780471732877. [Google Scholar]
- Eslahi, N.; Mahmoodi, A.; Mahmoudi, N.; Zandi, N.; Simchi, A. Processing and Properties of Nanofibrous Bacterial Cellulose-Containing Polymer Composites: A Review of Recent Advances for Biomedical Applications. Polym. Rev. 2020, 60, 144–170. [Google Scholar] [CrossRef]
- Torgbo, S.; Sukyai, P. Bacterial Cellulose-Based Scaffold Materials for Bone Tissue Engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Halib, N.; Ahmad, I.; Grassi, M.; Grassi, G. The Remarkable Three-Dimensional Network Structure of Bacterial Cellulose for Tissue Engineering Applications. Int. J. Pharm. 2019, 566, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Huang, Y.; Meng, F.; Zhuang, Y.; Liu, H.; Du, M.; Ma, Q.; Wang, Q.; Chen, Z.; Chen, L.; et al. Application of Bacterial Cellulose in Skin and Bone Tissue Engineering. Eur. Polym. J. 2020, 122, 109365. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Mao, J.J. Engineering Dextran-Based Scaffolds for Drug Delivery and Tissue Repair. Nanomedicine 2012, 7, 1771–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, J.; Evangelista, M.; Gil, H.; Ferreira, L. Dextran-Based Materials for Biomedical Applications. In Carbohydrates Applications in Medicine; Research Signpost: Kerala, India, 2014; Volume 2, pp. 31–53. ISBN 978-81-308-0523-8. [Google Scholar]
- Varshosaz, J. Dextran Conjugates in Drug Delivery. Expert Opin. Drug Deliv. 2012, 9, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A Review on Synthesis, Properties and Applications of Natural Polymer Based Carrageenan Blends and Composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent Trends on Gellan Gum Blends with Natural and Synthetic Polymers: A Review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.R.; Gilmore, K.J.; Wallace, G.G.; In het Panhuis, M. Tissue Engineering with Gellan Gum. Biomater. Sci. 2016, 4, 1276–1290. [Google Scholar] [CrossRef] [Green Version]
- Mohammadinejad, R.; Kumar, A.; Ranjbar-Mohammadi, M.; Ashrafizadeh, M.; Han, S.S.; Khang, G.; Roveimiab, Z. Recent Advances in Natural Gum-Based Biomaterials for Tissue Engineering and Regenerative Medicine: A Review. Polymers 2020, 12, 176. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of Xanthan Gum as Polysaccharide in Tissue Engineering: A Review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef]
- del Rodriguez-Torres, M.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A. Heparin-Based Nanoparticles: An Overview of Their Applications. J. Nanomater. 2018, 2018, 9780489. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Kiick, K.L. Heparin-Functionalized Polymeric Biomaterials in Tissue Engineering and Drug Delivery Applications. Acta Biomater. 2014, 10, 1588–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-Based Bioinks for Hard Tissue Engineering Applications: A Comprehensive Review. J. Mater. Sci. Mater. Med. 2019, 30, 32. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Jaffe, M.; Shah, R.G. Structure and Behavior of Collagen Fibers. In Handbook of Properties of Textile and Technical Fibres, 2nd ed.; Bunsell, A.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 345–365. ISBN 9780081012727. [Google Scholar]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-Polysaccharide Composite Scaffolds for 3D Cell Culture and Tissue Engineering: Towards Natural Therapeutics. Bioeng. Transl. Med. 2018, 4, 96–115. [Google Scholar] [CrossRef]
- Hoque, M.; Nuge, T.; Yeow, T.; Nordin, N.; Prasad, R. Gelatin Based Scaffolds for Tissue Engineering—A Review. Polym. Res. J. 2015, 9, 15. [Google Scholar]
- Kuttappan, S.; Mathew, D.; Nair, M.B. Biomimetic Composite Scaffolds Containing Bioceramics and Collagen/Gelatin for Bone Tissue Engineering—A Mini Review. Int. J. Biol. Macromol. 2016, 93, 1390–1401. [Google Scholar] [CrossRef]
- Tungkavet, T.; Pattavarakorn, D.; Sirivat, A. Bio-Compatible Gelatins (Ala-Gly-Pro-Arg-Gly-Glu-4Hyp-Gly-Pro-) and Electromechanical Properties: Effects of Temperature and Electric Field. J. Polym. Res. 2012, 19, 9759. [Google Scholar] [CrossRef]
- Melke, J.; Midha, S.; Ghosh, S.; Ito, K.; Hofmann, S. Silk Fibroin as Biomaterial for Bone Tissue Engineering. Acta Biomater. 2016, 31, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, Mechanical Properties and Applications of Silk Fibroin Materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.-J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem Cell-Based Tissue Engineering with Silk Biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.-H.; Le, T.-H.; Huynh, V.Q.N.; Vo, D.-V.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q. Van Silk Fibroin-Based Biomaterials for Biomedical Applications: A Review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasoju, N.; Bora, U. Silk Fibroin in Tissue Engineering. Adv. Healthc. Mater. 2012, 1, 393–412. [Google Scholar] [CrossRef]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E.D.A. Keratin: Dissolution, Extraction and Biomedical Application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.-Y.; Huang, W.-C.; Chen, Y.; Baskaran, N.; Yu, J.; Wei, Y. Effect of Varied Hair Protein Fractions on the Gel Properties of Keratin/Chitosan Hydrogels for the Use in Tissue Engineering. Colloids Surf. B Biointerfaces 2020, 195, 111258. [Google Scholar] [CrossRef]
- Costa, F.; Silva, R.; Boccaccini, A.R. Fibrous Protein-Based Biomaterials (Silk, Keratin, Elastin, and Resilin Proteins) for Tissue Regeneration and Repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Cristina, M., Martins, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 175–204. ISBN 978-0-08-100803-4. [Google Scholar]
- Rouse, J.G.; Van Dyke, M.E. A Review of Keratin-Based Biomaterials for Biomedical Applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Chiti, M.C.; Dolmans, M.M.; Donnez, J.; Amorim, C.A. Fibrin in Reproductive Tissue Engineering: A Review on Its Application as a Biomaterial for Fertility Preservation. Ann. Biomed. Eng. 2017, 45, 1650–1663. [Google Scholar] [CrossRef]
- Bujoli, B.; Scimeca, J.-C.; Verron, E. Fibrin as a Multipurpose Physiological Platform for Bone Tissue Engineering and Targeted Delivery of Bioactive Compounds. Pharmaceutics 2019, 11, 556. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin Gel as an Injectable Biodegradable Scaffold and Cell Carrier for Tissue Engineering. Sci. World J. 2015, 2015, 685690. [Google Scholar] [CrossRef]
- de la Puente, P.; Ludeña, D. Cell Culture in Autologous Fibrin Scaffolds for Applications in Tissue Engineering. Exp. Cell Res. 2014, 322, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yeo, G.C.; Mithieux, S.M.; Weiss, A.S. The Elastin Matrix in Tissue Engineering and Regeneration. Curr. Opin. Biomed. Eng. 2018, 6, 27–32. [Google Scholar] [CrossRef]
- Daamen, W.F.; Veerkamp, J.H.; van Hest, J.C.M.; van Kuppevelt, T.H. Elastin as a Biomaterial for Tissue Engineering. Biomaterials 2007, 28, 4378–4398. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, R.; Jaworski, Ł.; Czubacka, P. The Structural and Mechanical Properties of the Bone. J. Technol. Exploit. Mech. Eng. 2017, 3, 43–50. [Google Scholar] [CrossRef]
- Miller, M.D.; Thompson, S.R. Miller’s Review of Orthopaedics; Elsevier: Amsterdam, The Netherlands, 2016; Volume 53, ISBN 9788578110796. [Google Scholar]
- Khurana, J.S. Bone Pathology; Humana Press: Totowa, NJ, USA, 2009; ISBN 9781588297662. [Google Scholar]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The Development of Collagen Based Composite Scaffolds for Bone Regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef]
- Ishimoto, T.; Sato, B.; Lee, J.W.; Nakano, T. Co-Deteriorations of Anisotropic Extracellular Matrix Arrangement and Intrinsic Mechanical Property in c-Src Deficient Osteopetrotic Mouse Femur. Bone 2017, 103, 216–223. [Google Scholar] [CrossRef]
- Viswanath, B.; Raghavan, R.; Ramamurty, U.; Ravishankar, N. Mechanical Properties and Anisotropy in Hydroxyapatite Single Crystals. Scr. Mater. 2007, 57, 361–364. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kubota, A.; Matsusaki, M.; Duncan, T.; Hatakeyama, Y.; Fukuyama, K.; Quantock, A.J.; Yamato, M.; Akashi, M.; Nishida, K. Anisotropic Mechanical Properties of Collagen Hydrogels Induced by Uniaxial-Flow for Ocular Applications. J. Biomater.Sci. Polym. Ed. 2011, 22, 1427–1442. [Google Scholar] [CrossRef]
- Fan, J.; Jahed, V.; Klavins, K. Metabolomics in Bone Research. Metabolites 2021, 11, 434. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of HHS Bone Health and Osteoporosis: A Report of the Surgeon General. US Health Human Service; Office of the Surgeon General (US): Washington, DC, USA, 2004; p. 437. [CrossRef]
- Monterde-Cruz, L.; Ramírez-Salazar, E.G.; Rico-Martínez, G.; Linares-González, L.M.; Guzmán-González, R.; Delgado-Cedillo, E.; Estrada-Villaseñor, E.; Valdés-Flores, M.; Velázquez-Cruz, R.; Hidalgo-Bravo, A. MicroRNA Expression in Relation with Clinical Evolution of Osteosarcoma. Pathol. Res. Pract. 2020, 216, 153038. [Google Scholar] [CrossRef]
- Lu, Y.; Li, M.; Long, Z.; Yang, D.; Guo, S.; Li, J.; Liu, D.; Gao, P.; Chen, G.; Lu, X.; et al. Collagen/β-TCP Composite as a Bone-Graft Substitute for Posterior Spinal Fusion in Rabbit Model: A Comparison Study. Biomed. Mater. 2019, 14, 045009. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Bai, Y.; Shih, M.S.; Hoffmann, C.; Peters, F.; Waldner, C.; Hübner, W.D. Effect of a β-TCP Collagen Composite Bone Substitute on Healing of Drilled Bone Voids in the Distal Femoral Condyle of Rabbits. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Gironés, J.; Alcaina-Lorente, A.; Ortiz-Ruiz, C.; Ortiz-Ruiz, E.; Pecci-Lloret, M.P.; Ortiz-Ruiz, A.J.; Rodríguez-Lozano, F.J.; Pecci-Lloret, M.R. Biocompatibility of a Ha/Β-tcp/c Scaffoldas a Pulp-capping Agent for Vital Pulp Treatment: An in Vivo Study in Rat Molars. Int. J. Environ. Res. Public Health 2021, 18, 3936. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Botelho, M.G.; Dorozhkin, S.V. Biphasic Calcium Phosphates Bioceramics (HA/TCP): Concept, Physicochemical Properties and the Impact of Standardization of Study Protocols in Biomaterials Research. Mater. Sci. Eng. C 2017, 71, 1293–1312. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.; Rahamana; Daya, D.E.; Balb, B.S.; Fuc, Q.; Junga, S.B.; Lynda, F.; Bonewalde, A.P.T. Bioactive Glass in Tissue Engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Gerhardt, L.C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.A.; Young, G.; Jones, J.R.; Rankin, S. Bioglass/Carbonate Apatite/Collagen Composite Scaffold Dissolution Products Promote Human Osteoblast Differentiation. Mater. Sci. Eng. C 2021, 118, 111393. [Google Scholar] [CrossRef]
- Hamzah, M.S.A.; Ng, C.; Zulkarnain, N.I.S.; Majid, H.A.; Razak, S.I.A.; Nayan, N.H.M. Entrapment of Collagen on Polylactic Acid 3D Scaffold Surface as a Potential Artificial Bone Replacement. Mater. Today Proc. 2021, 46, 1668–1673. [Google Scholar] [CrossRef]
- Dewey, M.J.; Nosatov, A.V.; Subedi, K.; Shah, R.; Jakus, A.; Harley, B.A.C. Inclusion of a 3D-Printed Hyperelastic Bone Mesh Improves Mechanical and Osteogenic Performance of a Mineralized Collagen Scaffold. Acta Biomater. 2021, 121, 224–236. [Google Scholar] [CrossRef]
- Oh, G.W.; Nguyen, V.T.; Heo, S.Y.; Ko, S.C.; Kim, C.S.; Park, W.S.; Choi, I.W.; Jung, W.K. 3D PCL/Fish Collagen Composite Scaffolds Incorporating Osteogenic Abalone Protein Hydrolysates for Bone Regeneration Application: In Vitro and in Vivo Studies. J. Biomater. Sci. Polym. Ed. 2021, 32, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Soufdoost, R.S.; Yazdanian, M.; Tahmasebi, E.; Yazdanian, A.; Tebyanian, H.; Karami, A.; Nourani, M.R.; Panahi, Y. In Vitro and in Vivo Evaluation of Novel Tadalafil/β-TCP/Collagen Scaffold for Bone Regeneration: A Rabbit Critical-Size Calvarial Defect Study. Biocybern. Biomed. Eng. 2019, 39, 789–796. [Google Scholar] [CrossRef]
- Maier, J. High Concentrations of Magnesium Modulate Vascular Endothelial Cell Behaviour In Vitro. Biochim. Et Biophys. Acta BBA Mol. Basis Dis. 2004, 1689, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhou, Y.; Zhou, Y.; Qu, H.; Chen, F.; Zhu, Y.; Chang, J. Biomimetic Hydroxyapatite Porous Microspheres with Co-Substituted Essential Trace Elements: Surfactant-Free Hydrothermal Synthesis, Enhanced Degradation and Drug Release. J. Mater. Chem. 2011, 21, 16558–16565. [Google Scholar] [CrossRef]
- Minardi, S.; Taraballi, F.; Cabrera, F.J.; Van Eps, J.; Wang, X.; Gazze, S.A.; Fernandez-Mourev, J.S.; Tampieri, A.; Francis, L.; Weiner, B.K.; et al. Biomimetic Hydroxyapatite/Collagen Composite Drives Bone Niche Recapitulation in a Rabbit Orthotopic Model. Mater. Today Bio 2019, 2, 100005. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Antoniac, A.; Vasile, E.; Tecu, C.; Fosca, M.; Yankova, V.G.; Rau, J.V. In Vitro Characterization of Novel Nanostructured Collagen-Hydroxyapatite Composite Scaffolds Doped with Magnesium with Improved Biodegradation Rate for Hard Tissue Regeneration. Bioact. Mater. 2021, 6, 3383–3395. [Google Scholar] [CrossRef]
- Ryan, E.J.; Ryan, A.J.; González-Vázquez, A.; Philippart, A.; Ciraldo, F.E.; Hobbs, C.; Nicolosi, V.; Boccaccini, A.R.; Kearney, C.J.; O’Brien, F.J. Collagen Scaffolds Functionalised with Copper-Eluting Bioactive Glass Reduce Infection and Enhance Osteogenesis and Angiogenesis Both In Vitro and In Vivo. Biomaterials 2019, 197, 405–416. [Google Scholar] [CrossRef]
- Jing, Z.; Wu, Y.; Su, W.; Tian, M.; Jiang, W.; Cao, L.; Zhao, L.; Zhao, Z. Carbon Nanotube Reinforced Collagen/Hydroxyapatite Scaffolds Improve Bone Tissue Formation In Vitro and In Vivo. Ann. Biomed. Eng. 2017, 45, 2075–2087. [Google Scholar] [CrossRef]
- Ju, T.; Zhao, Z.; Ma, L.; Li, W.; Li, S.; Zhang, J. Cyclic Adenosine Monophosphate-Enhanced Calvarial Regeneration by Bone Marrow-Derived Mesenchymal Stem Cells on a Hydroxyapatite/Gelatin Scaffold. ACS Omega 2021, 6, 13684–13694. [Google Scholar] [CrossRef]
- Rezaei, H.; Shahrezaee, M.; Jalali Monfared, M.; Ghorbani, F.; Zamanian, A.; Sahebalzamani, M. Mussel-Inspired Polydopamine Induced the Osteoinductivity to Ice-Templating PLGA–Gelatin Matrix for Bone Tissue Engineering Application. Biotechnol. Appl. Biochem. 2021, 68, 185–196. [Google Scholar] [CrossRef]
- Jahangir, S.; Hosseini, S.; Mostafaei, F.; Sayahpour, F.A.; Eslaminejad, M.B. 3D-Porous β-Tricalcium Phosphate–Alginate–Gelatin Scaffold with DMOG Delivery Promotes Angiogenesis and Bone Formation in Rat Calvarial Defects. J. Mater. Sci. Mater. Med. 2019, 30, 1. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Sharma, C.; Purohit, S.D.; Singh, H.; Dinda, A.K.; Potdar, P.D.; Chou, C.F.; Mishra, N.C. Gelatin-Polycaprolactone-Nanohydroxyapatite Electrospun Nanocomposite Scaffold for Bone Tissue Engineering. Mater. Sci. Eng. C 2021, 119, 111588. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Pan, X.; Hu, M.; Zhang, J.; Yu, Y.; Hu, X.; Jiang, K. Fabrication of Graphene/Gelatin/Chitosan/Tricalcium Phosphate 3D Printed Scaffolds for Bone Tissue Regeneration Applications. Appl. Nanosci. 2021, 11, 335–346. [Google Scholar] [CrossRef]
- Qu, L.; Dubey, N.; Ribeiro, J.S.; Bordini, E.A.F.; Ferreira, J.A.; Xu, J.; Castilho, R.M.; Bottino, M.C. Metformin-Loaded Nanospheres-Laden Photocrosslinkable Gelatin Hydrogel for Bone Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2021, 116, 104293. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X.; Ma, M.; Lu, W.; Zhang, B.; Guo, Y. A GelMA-PEGDA-NHA Composite Hydrogel for Bone Tissue Engineering. Materials 2020, 13, 3735. [Google Scholar] [CrossRef]
- Ramírez Rodríguez, G.; Patrício, T.; Delgado López, J. Natural Polymers for Bone Repair. In Bone Repair Biomaterials; Woodhead: Cambridge, UK, 2019; pp. 199–232. [Google Scholar]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Damien, O.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Biodental Eng. V 2019, 24, 3009. [Google Scholar] [CrossRef] [Green Version]
- Brun, P.; Zamuner, A.; Battocchio, C.; Cassari, L.; Todesco, M.; Graziani, V.; Iucci, G.; Marsotto, M.; Tortora, L.; Secchi, V.; et al. Bio-Functionalized Chitosan for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 5916. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Trivedi, R.; Vairamani, M.; Selvamurugan, N. Sinapic Acid-Loaded Chitosan Nanoparticles in Polycaprolactone Electrospun Fibers for Bone Regeneration in Vitro and in Vivo. Carbohydr. Polym. 2019, 216, 1–16. [Google Scholar] [CrossRef]
- Radwan, N.H.; Nasr, M.; Ishak, R.A.H.; Abdeltawab, N.F.; Awad, G.A.S. Chitosan-Calcium Phosphate Composite Scaffolds for Control of Post-Operative Osteomyelitis: Fabrication, Characterization, and In Vitro–In Vivo Evaluation. Carbohydr. Polym. 2020, 244, 116482. [Google Scholar] [CrossRef]
- Bari, E.; Scocozza, F.; Perteghella, S.; Sorlini, M.; Auricchio, F.; Torre, M.L.; Conti, M. 3d Bioprinted Scaffolds Containing Mesenchymal Stem/Stromal Lyosecretome: Next Generation Controlled Release Device for Bone Regenerative Medicine. Pharmaceutics 2021, 13, 515. [Google Scholar] [CrossRef]
- Zhang, J.; Eyisoylu, H.; Qin, X.H.; Rubert, M.; Müller, R. 3D Bioprinting of Graphene Oxide-Incorporated Cell-Laden Bone Mimicking Scaffolds for Promoting Scaffold Fidelity, Osteogenic Differentiation and Mineralization. Acta Biomater. 2021, 121, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Alizadeh, P. On the Role of Alginate Coating on the Mechanical and Biological Properties of 58S Bioactive Glass Scaffolds. Int. J. Biol. Macromol. 2021, 167, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, Q.; Ma, R.; Huang, W.; Zhang, X.; Liu, Y.; Xu, Z.; Zhang, L.; Li, M.; Zhu, C. Modified Alginate-Based Hydrogel as a Carrier of the CB2 Agonist JWH133 for Bone Engineering. ACS Omega 2021, 6, 6861–6870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cai, Q.; Zhu, Y.; Zhu, W. A Simple Hydrogel Scaffold with Injectability, Adhesivity and Osteogenic Activity for Bone Regeneration. Biomater. Sci. 2021, 9, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Garske, D.S.; Schmidt-Bleek, K.; Ellinghaus, A.; Dienelt, A.; Gu, L.; Mooney, D.J.; Duda, G.N.; Cipitria, A. Alginate Hydrogels for In Vivo Bone Regeneration: The Immune Competence of the Animal Model Matters. Tissue Eng. Part A 2020, 26, 852–862. [Google Scholar] [CrossRef]
- Erickson, C.B.; Newsom, J.P.; Fletcher, N.A.; Feuer, Z.M.; Yu, Y.; Rodriguez-Fontan, F.; Hadley Miller, N.; Krebs, M.D.; Payne, K.A. In Vivo Degradation Rate of Alginate–Chitosan Hydrogels Influences Tissue Repair Following Physeal Injury. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2484–2494. [Google Scholar] [CrossRef]
- Ke, X.; Dong, Z.; Tang, S.; Chu, W.; Zheng, X.; Zhen, L.; Chen, X.; Ding, C.; Luo, J.; Li, J. A Natural Polymer Based Bioadhesive with Self-Healing Behavior and Improved Antibacterial Properties. Biomater. Sci. 2020, 8, 4346–4357. [Google Scholar] [CrossRef]
- Yang, N.; Moore, M.J.; Michael, P.L.; Santos, M.; Lam, Y.T.; Bao, S.; Ng, M.K.C.; Rnjak-Kovacina, J.; Tan, R.P.; Wise, S.G. Silk Fibroin Scaffold Architecture Regulates Inflammatory Responses and Engraftment of Bone Marrow-Mononuclear Cells. Adv. Healthc. Mater. 2021, 2100615, e2100615. [Google Scholar] [CrossRef]
- Teramoto, H.; Shirakawa, M.; Tamada, Y. Click Decoration of Bombyx Mori Silk Fibroin for Cell Adhesion Control. Molecules 2020, 25, 4106. [Google Scholar] [CrossRef]
- Akrami-Hasan-Kohal, M.; Eskandari, M.; Solouk, A. Silk Fibroin Hydrogel/Dexamethasone Sodium Phosphate Loaded Chitosan Nanoparticles as a Potential Drug Delivery System. Colloids Surf. B Biointerfaces 2021, 205, 111892. [Google Scholar] [CrossRef]
- Zheng, H.; Zuo, B. Functional Silk Fibroin Hydrogels: Preparation, Properties and Applications. J. Mater. Chem. B 2021, 9, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Han, Z.; Liu, W.; Yao, J.; Zhao, B.; Shao, Z.; Chen, X. Silk-Based Hybrid Microfibrous Mats as Guided Bone Regeneration Membranes. J. Mater. Chem. B 2021, 9, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ke, X.; Yu, P.; Wang, D.; Pan, S.; Yang, J.; Ding, C.; Xiao, S.; Luo, J.; Li, J. A Facile Strategy to Construct Silk Fibroin Based GTR Membranes with Appropriate Mechanical Performance and Enhanced Osteogenic Capacity. J. Mater. Chem. B 2020, 8, 10407–10415. [Google Scholar] [CrossRef] [PubMed]
- McNamara, S.L.; McCarthy, E.M.; Schmidt, D.F.; Johnston, S.P.; Kaplan, D.L. Rheological Characterization, Compression, and Injection Molding of Hydroxyapatite-Silk Fibroin Composites. Biomaterials 2021, 269, 120643. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Rasheed, S.; Yougen, C. Silk Fibroin/Hydroxyapatite Scaffold: A Highly Compatible Material for Bone Regeneration. Sci. Technol. Adv. Mater. 2020, 21, 242–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Wei, D.; Huang, L.; Zhu, M.; Zhang, Y.; Zhu, Y. 3D Printing of Mesoporous Bioactive Glass/Silk Fibroin Composite Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 103, 109731. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wu, M.; Yan, F.; Xie, Y.; Liu, Z.; Huang, H.; Yang, Z.; Yao, S.; Cai, L. A Radial 3D Polycaprolactone Nanofiber Scaffold Modified by Biomineralization and Silk Fibroin Coating Promote Bone Regeneration In Vivo. Int. J. Biol. Macromol. 2021, 172, 19–29. [Google Scholar] [CrossRef]
- Chen, P.; Liu, L.; Pan, J.; Mei, J.; Li, C.; Zheng, Y. Biomimetic Composite Scaffold of Hydroxyapatite/Gelatin-Chitosan Core-Shell Nanofibers for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 97, 325–335. [Google Scholar] [CrossRef]
- Purohit, S.D.; Bhaskar, R.; Singh, H.; Yadav, I.; Gupta, M.K.; Mishra, N.C. Development of a Nanocomposite Scaffold of Gelatin–Alginate–Graphene Oxide for Bone Tissue Engineering. Int. J. Biol. Macromol. 2019, 133, 592–602. [Google Scholar] [CrossRef]
- Thomas, A.; Bera, J. Preparation and Characterization of Gelatin-Bioactive Glass Ceramic Scaffolds for Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2019, 30, 561–579. [Google Scholar] [CrossRef]
- Sharifi, F.; Atyabi, S.M.; Norouzian, D.; Zandi, M.; Irani, S.; Bakhshi, H. Polycaprolactone/Carboxymethyl Chitosan Nanofibrous Scaffolds for Bone Tissue Engineering Application. Int. J. Biol. Macromol. 2018, 115, 243–248. [Google Scholar] [CrossRef]
- Dai, C.; Li, Y.; Pan, W.; Wang, G.; Huang, R.; Bu, Y.; Liao, X.; Guo, K.; Gao, F. Three-Dimensional High-Porosity Chitosan/Honeycomb Porous Carbon/Hydroxyapatite Scaffold with Enhanced Osteoinductivity for Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 575–586. [Google Scholar] [CrossRef]
- Shi, D.; Shen, J.; Zhang, Z.; Shi, C.; Chen, M.; Gu, Y.; Liu, Y. Preparation and Properties of Dopamine-Modified Alginate/Chitosan–Hydroxyapatite Scaffolds with Gradient Structure for Bone Tissue Engineering. J. Biomed. Mater. Res. Part A 2019, 107, 1615–1627. [Google Scholar] [CrossRef]
- Saini, R.K.; Bagri, L.P.; Bajpai, A.K. Nano-Silver Hydroxyapatite Based Antibacterial 3D Scaffolds of Gelatin/Alginate/Poly (Vinyl Alcohol) for Bone Tissue Engineering Applications. Colloids Surf. B Biointerfaces 2019, 177, 211–218. [Google Scholar] [CrossRef]
- Echave, M.C.; Pimenta-Lopes, C.; Pedraz, J.L.; Mehrali, M.; Dolatshahi-Pirouz, A.; Ventura, F.; Orive, G. Enzymatic Crosslinked Gelatin 3D Scaffolds for Bone Tissue Engineering. Int. J. Pharm. 2019, 562, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Rajzer, I.; Kurowska, A.; Jabłoński, A.; Jatteau, S.; Śliwka, M.; Ziąbka, M.; Menaszek, E. Layered Gelatin/PLLA Scaffolds Fabricated by Electrospinning and 3D Printing- for Nasal Cartilages and Subchondral Bone Reconstruction. Mater. Des. 2018, 155, 297–306. [Google Scholar] [CrossRef]
- Panzavolta, S.; Torricelli, P.; Casolari, S.; Parrilli, A.; Fini, M.; Bigi, A. Strontium-Substituted Hydroxyapatite-Gelatin Biomimetic Scaffolds Modulate Bone Cell Response. Macromol. Biosci. 2018, 18, e1800096. [Google Scholar] [CrossRef]
- Sattary, M.; Rafienia, M.; Kazemi, M.; Salehi, H.; Mahmoudzadeh, M. Promoting Effect of Nano Hydroxyapatite and Vitamin D3 on the Osteogenic Differentiation of Human Adipose-Derived Stem Cells in Polycaprolactone/Gelatin Scaffold for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 97, 141–155. [Google Scholar] [CrossRef]
- Sun, J.L.; Jiao, K.; Niu, L.-N.; Jiao, Y.; Song, Q.; Shen, L.-J.; Tay, F.R.; Chen, J.-H. 5Intrafibrillar Silicified Collagen Scaffold Modulates Monocyte to Promote Cell Homing, Angiogenesis and Bone Regeneration. Biomaterials 2017, 113, 203–216. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, M.; Xie, Q.; Sun, H.; Huang, Y.; Zhang, D.D.; Yu, Z.; Bi, X.; Chen, J.; Wang, J.; et al. Electrospun Silk Fibroin/Poly(Lactide-Co-ε-Caprolactone) Nanofibrous Scaffolds for Bone Regeneration. Int. J. Nanomed. 2016, 11, 1483–1500. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Wang, Y.; Ma, W.; Dong, W.; Zhang, M.; Sun, D. Dual-Drug-Loaded Silk Fibroin/PLGA Scaffolds for Potential Bone Regeneration Applications. J. Nanomater. 2019, 2019, 8050413. [Google Scholar] [CrossRef] [Green Version]
- He, J.X.; Tan, W.L.; Han, Q.M.; Cui, S.Z.; Shao, W.; Sang, F. Fabrication of Silk Fibroin/Cellulose Whiskers–Chitosan Composite Porous Scaffolds by Layer-by-Layer Assembly for Application in Bone Tissue Engineering. J. Mater. Sci. 2016, 51, 4399–4410. [Google Scholar] [CrossRef]
- Luetchford, K.A.; Chaudhuri, J.B.; De Bank, P.A. Silk Fibroin/Gelatin Microcarriers as Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2020, 106, 110116. [Google Scholar] [CrossRef]
- Benedini, L.; Laiuppa, J.; Santillán, G.; Baldini, M.; Messina, P. Antibacterial Alginate/Nano-Hydroxyapatite Composites for Bone Tissue Engineering: Assessment of Their Bioactivity, Biocompatibility, and Antibacterial Activity. Mater. Sci. Eng. C 2020, 115, 111101. [Google Scholar] [CrossRef]
- Zheng, A.; Cao, L.; Liu, Y.; Wu, J.; Zeng, D.; Hu, L.; Zhang, X.; Jiang, X. Biocompatible Silk/Calcium Silicate/Sodium Alginate Composite Scaffolds for Bone Tissue Engineering. Carbohydr. Polym. 2018, 199, 244–255. [Google Scholar] [CrossRef]
- Wang, P.; Song, Y.; Weir, M.D.; Sun, J.; Zhao, L.; Simon, C.G.; Xu, H.H.K. A Self-Setting IPSMSC-Alginate-Calcium Phosphate Paste for Bone Tissue Engineering. Dent. Mater. 2016, 32, 252–263. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Li, Y.; Qin, X.; Wa, Q. 3D Printing of Concentrated Alginate/Gelatin Scaffolds with Homogeneous Nano Apatite Coating for Bone Tissue Engineering. Mater. Des. 2018, 146, 12–19. [Google Scholar] [CrossRef]
- Scott, W.; Stevens, J.; Binder-Macleod, S.A. Human Skeletal Muscle Fiber Type Classifications. Phys. Ther. 2001, 81, 1810–1816. [Google Scholar] [CrossRef]
- Relaix, F.; Bencze, M.; Borok, M.J.; Der Vartanian, A.; Gattazzo, F.; Mademtzoglou, D.; Perez-Diaz, S.; Prola, A.; Reyes-Fernandez, P.C.; Rotini, A.; et al. Perspectives on Skeletal Muscle Stem Cells. Nat. Commun. 2021, 12, 692. [Google Scholar] [CrossRef]
- Ostrovidov, S.; Salehi, S.; Costantini, M.; Suthiwanich, K.; Ebrahimi, M.; Sadeghian, R.B.; Fujie, T.; Shi, X.; Cannata, S.; Gargioli, C.; et al. 3D Bioprinting in Skeletal Muscle Tissue Engineering. Small 2019, 15, e1805530. [Google Scholar] [CrossRef]
- Fischer, K.M.; Scott, T.E.; Browe, D.P.; McGaughey, T.A.; Wood, C.; Wolyniak, M.J.; Freeman, J.W. Hydrogels for Skeletal Muscle Regeneration. Regen. Eng. Transl. Med. 2020, 7, 353–361. [Google Scholar] [CrossRef]
- Liu, J.; Saul, D.; Böker, K.O.; Ernst, J.; Lehman, W.; Schilling, A.F. Current Methods for Skeletal Muscle Tissue Repair and Regeneration. BioMed Res. Int. 2018, 2018, 1984879. [Google Scholar] [CrossRef]
- Gholobova, D.; Terrie, L.; Gerard, M.; Declercq, H.; Thorrez, L. Vascularization of Tissue-Engineered Skeletal Muscle Constructs. Biomaterials 2020, 235, 119708. [Google Scholar] [CrossRef]
- Zhuang, P.; An, J.; Chua, C.K.; Tan, L.P. Bioprinting of 3D in Vitro Skeletal Muscle Models: A Review. Mater. Des. 2020, 193, 108794. [Google Scholar] [CrossRef]
- del Carmen Ortuño-Costela, M.; García-López, M.; Cerrada, V.; Gallardo, M.E. IPSCs: A Powerful Tool for Skeletal Muscle Tissue Engineering. J. Cell. Mol. Med. 2019, 23, 3784–3794. [Google Scholar] [CrossRef]
- Gillies, A.R.; Lieber, R.L. Structure and Function of the Skeletal Muscle Extracellular Matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef] [Green Version]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix—What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef] [Green Version]
- Takala, T.E.; Virtanen, P. Biochemical Composition of Muscle Extracellular Matrix: The Effect of Loading. Scand. J. Med. Sci. Sports 2000, 10, 321–325. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Deries, M.; Cachaço, A.S.; Bajanca, F. The Extracellular Matrix Dimension of Skeletal Muscle Development. Dev. Biol. 2011, 354, 191–207. [Google Scholar] [CrossRef] [Green Version]
- Baker, H.B.; Passipieri, J.A.; Siriwardane, M.; Ellenburg, M.D.; Vadhavkar, M.; Bergman, C.R.; Saul, J.M.; Tomblyn, S.; Burnett, L.; Christ, G.J. Cell and Growth Factor-Loaded Keratin Hydrogels for Treatment of Volumetric Muscle Loss in a Mouse Model. Tissue Eng. Part A 2017, 23, 572–581. [Google Scholar] [CrossRef]
- Tomblyn, S.; Kneller, E.L.P.; Walker, S.J.; Ellenburg, M.D.; Kowalczewski, C.J.; Van Dyke, M.; Burnett, L.; Saul, J.M. Keratin Hydrogel Carrier System for Simultaneous Delivery of Exogenous Growth Factors and Muscle Progenitor Cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 864–879. [Google Scholar] [CrossRef] [Green Version]
- Passipieri, J.A.; Baker, H.B.; Siriwardane, M.; Ellenburg, M.D.; Vadhavkar, M.; Saul, J.M.; Tomblyn, S.; Burnett, L.; Christ, G.J. Keratin Hydrogel Enhances In Vivo Skeletal Muscle Function in a Rat Model of Volumetric Muscle Loss. Tissue Eng. Part A 2017, 23, 556–571. [Google Scholar] [CrossRef]
- Wan, X.; Li, P.; Jin, X.; Su, F.; Shen, J.; Yuan, J. Poly(ε-Caprolactone)/Keratin/Heparin/VEGF Biocomposite Mats for Vascular Tissue Engineering. J. Biomed. Mater. Res. Part A 2020, 108, 292–300. [Google Scholar] [CrossRef]
- Dou, J.; Wang, Y.; Jin, X.; Li, P.; Wang, L.; Yuan, J.; Shen, J. PCL/Sulfonated Keratin Mats for Vascular Tissue Engineering Scaffold with Potential of Catalytic Nitric Oxide Generation. Mater. Sci. Eng. C 2020, 107, 110246. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Jin, X.; Dou, J.; Han, X.; Wan, X.; Yuan, J.; Shen, J. Fabrication of PCL/Keratin Composite Scaffolds for Vascular Tissue Engineering with Catalytic Generation of Nitric Oxide Potential. J. Mater. Chem. B 2020, 8, 6092–6099. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Bohacz, J. Biodegradation of Keratin Waste: Theory and Practical Aspects. Waste Manag. 2011, 31, 1689–1701. [Google Scholar] [CrossRef]
- Panayi, A.C.; Smit, L.; Hays, N.; Udeh, K.; Endo, Y.; Li, B.; Sakthivel, D.; Tamayol, A.; Neppl, R.L.; Orgill, D.P.; et al. A Porous Collagen-GAG Scaffold Promotes Muscle Regeneration Following Volumetric Muscle Loss Injury. Wound Repair Regen. 2020, 28, 61–74. [Google Scholar] [CrossRef]
- Wang, H.D.; Lough, D.M.; Kurlander, D.E.; Lopez, J.; Quan, A.; Kumar, A.R. Muscle-Derived Stem Cell-Enriched Scaffolds Are Capable of Enhanced Healing of a Murine Volumetric Muscle Loss Defect. Plast. Reconstr. Surg. 2019, 143, 329e–339e. [Google Scholar] [CrossRef]
- Quarta, M.; Cromie, M.; Chacon, R.; Blonigan, J.; Garcia, V.; Akimenko, I.; Hamer, M.; Paine, P.; Stok, M.; Shrager, J.B.; et al. Bioengineered Constructs Combined with Exercise Enhance Stem Cell-Mediated Treatment of Volumetric Muscle Loss. Nat. Commun. 2017, 8, 15613. [Google Scholar] [CrossRef]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and Characterization of Conductive Polypyrrole/Chitosan/Collagen Electrospun Nanofiber Scaffold for Tissue Engineering Application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef]
- Hwangbo, H.; Kim, W.J.; Kim, G.H. Lotus-Root-Like Microchanneled Collagen Scaffold. ACS Appl. Mater. Interfaces 2021, 13, 12656–12667. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Wieringa, P.; Yuste, Y.; de la Portilla, F.; Guererro, A.; Romero, A.; Moroni, L. Fabrication of Hybrid Scaffolds Obtained from Combinations of PCL with Gelatin or Collagen via Electrospinning for Skeletal Muscle Tissue Engineering. J. Biomed. Mater. Res. Part A 2021, 109, 1600–1612. [Google Scholar] [CrossRef]
- Basurto, I.M.; Mora, M.A.; Christ, G.J.; Caliari, S.R. Aligned and Conductive 3D Collagen Scaffolds for Skeletal Muscle Tissue Engineering. In Proceedings of the Transactions of the Annual Meeting of the Society for Biomaterials and the Annual International Biomaterials Symposium, Seattle, WA, USA, 3–6 April 2019; Volume 40, p. 370. [Google Scholar]
- Nakayama, K.H.; Quarta, M.; Paine, P.; Alcazar, C.; Karakikes, I.; Garcia, V.; Abilez, O.J.; Calvo, N.S.; Simmons, C.S.; Rando, T.A.; et al. Treatment of Volumetric Muscle Loss in Mice Using Nanofibrillar Scaffolds Enhances Vascular Organization and Integration. Commun. Biol. 2019, 2, s42003–s42019. [Google Scholar] [CrossRef]
- Quigley, A.F.; Cornock, R.; Mysore, T.; Foroughi, J.; Kita, M.; Razal, J.M.; Crook, J.; Moulton, S.E.; Wallace, G.G.; Kapsa, R.M.I. Wet-Spun Trojan Horse Cell Constructs for Engineering Muscle. Front. Chem. 2020, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Distler, T.; Solisito, A.A.; Schneidereit, D.; Friedrich, O.; Detsch, R.; Boccaccini, A.R. 3D Printed Oxidized Alginate-Gelatin Bioink Provides Guidance for C2C12 Muscle Precursor Cell Orientation and Differentiation via Shear Stress During Bioprinting. IOP Publ. Biofabr. J. Biofabr. 2020, 12, 045005. [Google Scholar] [CrossRef]
- Ansari, S.; Chen, C.; Xu, X.; Annabi, N.; Zadeh, H.H.; Wu, B.M.; Khademhosseini, A.; Shi, S.; Moshaverinia, A. Muscle Tissue Engineering Using Gingival Mesenchymal Stem Cells Encapsulated in Alginate Hydrogels Containing Multiple Growth Factors. Ann. Biomed. Eng. 2016, 44, 1908–1920. [Google Scholar] [CrossRef] [Green Version]
- Ciriza, J.; Rodríguez-Romano, A.; Nogueroles, I.; Gallego-Ferrer, G.; Cabezuelo, R.M.; Pedraz, J.L.; Rico, P. Borax-Loaded Injectable Alginate Hydrogels Promote Muscle Regeneration in Vivo after an Injury. Mater. Sci. Eng. C 2021, 123, 112003. [Google Scholar] [CrossRef]
- Pęziński, M.; Daszczuk, P.; Pradhan, B.S.; Lochmüller, H.; Prószyński, T.J. An Improved Method for Culturing Myotubes on Laminins for the Robust Clustering of Postsynaptic Machinery. Sci. Rep. 2020, 10, 4524. [Google Scholar] [CrossRef]
- Marcinczyk, M.; Dunn, A.; Haas, G.; Madsen, J.; Scheidt, R.; Patel, K.; Talovic, M.; Garg, K. The Effect of Laminin-111 Hydrogels on Muscle Regeneration in a Murine Model of Injury. Tissue Eng. Part A 2019, 25, 1001–1012. [Google Scholar] [CrossRef]
- Gilbert-Honick, J.; Ginn, B.; Zhang, Y.; Salehi, S.; Wagner, K.R.; Mao, H.Q.; Grayson, W.L. Adipose-Derived Stem/Stromal Cells on Electrospun Fibrin Microfiber Bundles Enable Moderate Muscle Reconstruction in a Volumetric Muscle Loss Model. Cell Transplant. 2018, 27, 1644–1656. [Google Scholar] [CrossRef] [Green Version]
- Russell, C.S.; Mostafavi, A.; Quint, J.P.; Panayi, A.C.; Baldino, K.; Williams, T.J.; Daubendiek, J.G.; Sánchez, V.H.; Bonick, Z.; Trujillo-Miranda, M.; et al. In Situ Printing of Adhesive Hydrogel Scaffolds for the Treatment of Skeletal Muscle Injuries. ACS Appl. Bio Mater. 2020, 3, 1568–1579. [Google Scholar] [CrossRef]
- Hanjun, H.; Hyeongjin, L.; Eun-Ju, J.; JaeYoon, L.; Yunju, J.; Dongryeol, R.; GeunHyung, K. Bio-printing of aligned GelMa-based cell-laden structure for muscle tissue regeneration. Bioact. Mater. 2022, 8, 57–70. [Google Scholar] [CrossRef]
- Basurto, I.M.; Mora, M.A.; Christ, G.J.; Caliari, S.R. Aligned and Conductive 3D Collagen Scaffolds for Skeletal Muscle Tissue Engineering. Trans. Annu. Meet. Soc. Biomater. Annu. Int. Biomater. Symp. 2019, 40, 370. [Google Scholar] [CrossRef]
- Aguilar-Agon, K.W.; Capel, A.J.; Martin, N.R.W.; Player, D.J.; Lewis, M.P. Mechanical Loading Stimulates Hypertrophy in Tissue-Engineered Skeletal Muscle: Molecular and Phenotypic Responses. J. Cell. Physiol. 2019, 234, 23547–23558. [Google Scholar] [CrossRef] [Green Version]
- Yi, H.; Forsythe, S.; He, Y.; Liu, Q.; Xiong, G.; Wei, S.; Li, G.; Atala, A.; Skardal, A.; Zhang, Y. Tissue-Specific Extracellular Matrix Promotes Myogenic Differentiation of Human Muscle Progenitor Cells on Gelatin and Heparin Conjugated Alginate Hydrogels. Acta Biomater. 2017, 62, 222–233. [Google Scholar] [CrossRef]
- Pumberger, M.; Qazi, T.H.; Ehrentraut, M.C.; Textor, M.; Kueper, J.; Stoltenburg-Didinger, G.; Winkler, T.; von Roth, P.; Reinke, S.; Borselli, C.; et al. Synthetic Niche to Modulate Regenerative Potential of MSCs and Enhance Skeletal Muscle Regeneration; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 99, ISBN 4930450659. [Google Scholar]
- Poveda-Reyes, S.; Moulisova, V.; Sanmartín-Masiá, E.; Quintanilla-Sierra, L.; Salmerón-Sánchez, M.; Ferrer, G.G. Gelatin—Hyaluronic Acid Hydrogels with Tuned Stiffness to Counterbalance Cellular Forces and Promote Cell Differentiation. Macromol. Biosci. 2016, 16, 1311–1324. [Google Scholar] [CrossRef]
- Guo, Y.; Gilbert-Honick, J.; Somers, S.M.; Mao, H.Q.; Grayson, W.L. Modified Cell-Electrospinning for 3D Myogenesis of C2C12s in Aligned Fibrin Microfiber Bundles. Biochem. Biophys. Res. Commun. 2019, 516, 558–564. [Google Scholar] [CrossRef]
- Acosta, F.M.; Jia, U.T.A.; Stojkova, K.; Pacelli, S.; Brey, E.M.; Rathbone, C. Divergent Effects of Myogenic Differentiation and Diabetes on the Capacity for Muscle Precursor Cell Adipogenic Differentiation in a Fibrin Matrix. Biochem. Biophys. Res. Commun. 2020, 526, 21–28. [Google Scholar] [CrossRef]
- Marcinczyk, M.; Elmashhady, H.; Talovic, M.; Dunn, A.; Bugis, F.; Garg, K. Laminin-111 Enriched Fibrin Hydrogels for Skeletal Muscle Regeneration. Biomaterials 2017, 141, 233–242. [Google Scholar] [CrossRef]
- Somers, S.M.; Zhang, N.Y.; Morrissette-McAlmon, J.B.F.; Tran, K.; Mao, H.Q.; Grayson, W.L. Myoblast Maturity on Aligned Microfiber Bundles at the Onset of Strain Application Impacts Myogenic Outcomes. Acta Biomater. 2019, 94, 232–242. [Google Scholar] [CrossRef]
- Carnes, M.E.; Pins, G.D. Etching Anisotropic Surface Topography onto Fibrin Microthread Scaffolds for Guiding Myoblast Alignment. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2308–2319. [Google Scholar] [CrossRef]
- Thorrez, L.; DiSano, K.; Shansky, J.; Vandenburgh, H. Engineering of Human Skeletal Muscle with an Autologous Deposited Extracellular Matrix. Front. Physiol. 2018, 9, 1076. [Google Scholar] [CrossRef]
- Chen, X.; Du, W.; Cai, Z.; Ji, S.; Dwivedi, M.; Chen, J.; Zhao, G.; Chu, J. Uniaxial Stretching of Cell-Laden Microfibers for Promoting C2C12 Myoblasts Alignment and Myofibers Formation. ACS Appl. Mater. Interfaces 2020, 12, 2162–2170. [Google Scholar] [CrossRef]
- Hejbøl, E.K.; Sellathurai, J.; Nair, P.D.; Schrøder, H.D. Injectable Scaffold Materials Differ in Their Cell Instructive Effects on Primary Human Myoblasts. J. Tissue Eng. 2017, 8, 2041731417717677. [Google Scholar] [CrossRef] [Green Version]
- Attalla, R.; Puersten, E.; Jain, N.; Selvaganapathy, P.R. 3D Bioprinting of Heterogeneous Bi- and Tri-Layered Hollow Channels within Gel Scaffolds Using Scalable Multi-Axial Microfluidic Extrusion Nozzle. Biofabrication 2019, 11, 015012. [Google Scholar] [CrossRef]
- Witt, R.; Weigand, A.; Boos, A.M.; Cai, A.; Dippold, D.; Boccaccini, A.R.; Schubert, D.W.; Hardt, M.; Lange, C.; Arkudas, A.; et al. Mesenchymal Stem Cells and Myoblast Differentiation under HGF and IGF-1 Stimulation for 3D Skeletal Muscle Tissue Engineering. BMC Cell Biol. 2017, 18, 15. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Li, Y.; Zhang, C.; Chen, H.; Liu, L.; Chen, S. Effects of SW033291 on the Myogenesis of Muscle-Derived Stem Cells and Muscle Regeneration. Stem Cell Res. Ther. 2020, 11, 76. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.J.; Lee, H.; Lee, J.U.; Atala, A.; Yoo, J.J.; Lee, S.J.; Kim, G.H. Efficient Myotube Formation in 3D Bioprinted Tissue Construct by Biochemical and Topographical Cues. Biomaterials 2020, 230, 119632. [Google Scholar] [CrossRef]
- Williams, N.P.; Rhodehamel, M.; Yan, C.; Smith, A.S.T.; Jiao, A.; Murry, C.E.; Scatena, M.; Kim, D.H. Engineering Anisotropic 3D Tubular Tissues with Flexible Thermoresponsive Nanofabricated Substrates. Biomaterials 2020, 240, 119856. [Google Scholar] [CrossRef]
- Fischetti, T.; Celikkin, N.; Negrini, N.C.; Farè, S.; Swieszkowski, W. Tripolyphosphate-Crosslinked Chitosan/Gelatin Biocomposite Ink for 3D Printing of Uniaxial Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 400. [Google Scholar] [CrossRef]
- Seyedmahmoud, R.; Çelebi-Saltik, B.; Barros, N.; Nasiri, R.; Banton, E.; Shamloo, A.; Ashammakhi, N.; Dokmeci, R.M.; Ahadian, S. Three-Dimensional Bioprinting of Functional Skeletal Muscle Tissue Using Gelatin. Micromachines 2019, 10, 679. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.H.; Cakal, S.D.; Li, J.; Pless, C.J.; Radeke, C.; Jepsen, M.L.; Jensen, T.E.; Dufva, M.; Lind, J.U. Large-Scale Spontaneous Self-Organization and Maturation of Skeletal Muscle Tissues on Ultra-Compliant Gelatin Hydrogel Substrates. Sci. Rep. 2020, 10, 13305. [Google Scholar] [CrossRef]
- Simsa, R.; Yuen, J.; Stout, A.; Rubio, N.; Fogelstrand, P.; Kaplan, D.L. Extracellular Heme Proteins Influence Bovine Myosatellite Cell Proliferation and the Color of Cell-Based Meat. Foods 2019, 8, 521. [Google Scholar] [CrossRef] [Green Version]
- Denes, L.T.; Riley, L.A.; Mijares, J.R.; Arboleda, J.D.; McKee, K.; Esser, K.A.; Wang, E.T. Culturing C2C12 Myotubes on Micromolded Gelatin Hydrogels Accelerates Myotube Maturation. Skelet. Muscle 2019, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Asahara, H.; Inui, M.; Lotz, M.K. Tendons and Ligaments: Connecting Developmental Biology to Musculoskeletal Disease Pathogenesis. J. Bone Miner. Res. 2017, 32, 1773–1782. [Google Scholar] [CrossRef]
- Beldjilali-Labro, M.; Garcia Garcia, A.; Farhat, F.; Bedoui, F.; Grosset, J.-F.; Dufresne, M.; Legallais, C. Biomaterials in Tendon and Skeletal Muscle Tissue Engineering: Current Trends and Challenges. Materials 2018, 11, 1116. [Google Scholar] [CrossRef] [Green Version]
- Uquillas, J.A.; Pacelli, S.; Kobayashi, S.; Uquillas, S. Musculoskeletal Tissue Engineering: Tendon, Ligament, and Skeletal Muscle Replacement and Repair. In Tissue Engineering for Artificial Organs; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Volume 2, pp. 465–523. ISBN 9783527689934. [Google Scholar]
- Govoni, M.; Muscari, C.; Lovecchio, J.; Guarnieri, C.; Giordano, E. Mechanical Actuation Systems for the Phenotype Commitment of Stem Cell-Based Tendon and Ligament Tissue Substitutes. Stem Cell Rev. Rep. 2016, 12, 189–201. [Google Scholar] [CrossRef]
- Smith, R.; Carr, A.; Dakin, S.; Snelling, S.; Yapp, C.; Hakimi, O. The Response of Tenocytes to Commercial Scaffolds Used for Rotator Cuff Repair. Eur. Cells Mater. 2016, 31, 107–118. [Google Scholar] [CrossRef]
- Wunderli, S.L.; Blache, U.; Snedeker, J.G. Tendon Explant Models for Physiologically Relevant In Vitro Study of Tissue Biology—A Perspective. Connect. Tissue Res. 2020, 61, 262–277. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Webster, T.J. Toxicity and Biocompatibility Properties of Nanocomposites for Musculoskeletal Tissue Regeneration. In Nanocomposites for Musculoskeletal Tissue Regeneration; Elsevier: Amsterdam, The Netherlands, 2016; pp. 95–122. ISBN 9781782424758. [Google Scholar]
- Anjana, J.; Deepthi, S.; Shalumon, K.T.; Mony, U.; Chen, J.P.; Jayakumar, R. Nanoengineered Biomaterials for Tendon/Ligament Regeneration. In Nanoengineered Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 73–93. ISBN 9780128133552. [Google Scholar]
- Sensini, A.; Gualandi, C.; Focarete, M.L.; Belcari, J.; Zucchelli, A.; Boyle, L.; Reilly, G.C.; Kao, A.P.; Tozzi, G.; Cristofolini, L. Multiscale Hierarchical Bioresorbable Scaffolds for the Regeneration of Tendons and Ligaments. Biofabrication 2019, 11, 35026. [Google Scholar] [CrossRef]
- Gereke, T.; Döbrich, O.; Aibibu, D.; Nowotny, J.; Cherif, C. Approaches for Process and Structural Finite Element Simulations of Braided Ligament Replacements. J. Ind. Text. 2017, 47, 408–425. [Google Scholar] [CrossRef]
- Higashiyama, R.; Sekiguchi, H.; Takata, K.; Endo, T.; Takamori, Y.; Takaso, M. Arthroscopic Reconstruction of the Anterior Tibiotalar Ligament Using a Free Tendon Graft. Arthrosc. Tech. 2020, 9, e541–e547. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.I.; Lee, J.S.; Kang, K.T.; Shim, Y.B.; Kim, Y.S.; Jang, J.W.; Moon, S.H.; D’Lima, D.D. In Vitro and In Vivo Performance of Tissue-Engineered Tendons for Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2018, 46, 1641–1649. [Google Scholar] [CrossRef]
- Pauly, H.M.; Kelly, D.J.; Popat, K.C.; Trujillo, N.A.; Dunne, N.J.; McCarthy, H.O.; Haut Donahue, T.L. Mechanical Properties and Cellular Response of Novel Electrospun Nanofibers for Ligament Tissue Engineering: Effects of Orientation and Geometry. J. Mech. Behav. Biomed. Mater. 2016, 61, 258–270. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-H.; Chen, S.-H.; Kuo, C.-Y.; Li, M.-L.; Chen, J.-P. Response of Dermal Fibroblasts to Biochemical and Physical Cues in Aligned Polycaprolactone/Silk Fibroin Nanofiber Scaffolds for Application in Tendon Tissue Engineering. Nanomaterials 2017, 7, 219. [Google Scholar] [CrossRef] [Green Version]
- Sundararaj, S.; Slusarewicz, P.; Brown, M.; Hedman, T. Genipin Crosslinker Releasing Sutures for Improving the Mechanical/Repair Strength of Damaged Connective Tissue. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2199–2205. [Google Scholar] [CrossRef]
- Atluri, K.; Chinnathambi, S.; Mendenhall, A.; Martin, J.A.; Sander, E.A.; Salem, A.K. Targeting Cell Contractile Forces: A Novel Minimally Invasive Treatment Strategy for Fibrosis. Ann. Biomed. Eng. 2020, 48, 1850–1862. [Google Scholar] [CrossRef]
- Gaut, L.; Duprez, D.; Gaut, L.; Duprez, D.; Reviews, I. Tendon Development and Diseases. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 5–23. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, P.J.; Hodgkinson, T.; Amado, I.; Sadowska, J.M.; Ryan, A.J.; Romanazzo, S.; Carroll, S.; Cryan, S.A.; Kelly, D.J.; O’Brien, F.J. Development of Collagen-Poly (Caprolactone)-Based Core-Shell Scaffolds Supplemented with Proteoglycans and Glycosaminoglycans for Ligament Repair. Mater. Sci. Eng. C 2021, 120, 111657. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Xu, P.; Luo, Q.; Song, G. Bin Proliferation and Tenogenic Differentiation of Bone Marrow Mesenchymal Stem Cells in a Porous Collagen Sponge Scaffold. World J. Stem Cells 2021, 13, 115–127. [Google Scholar] [CrossRef]
- Chen, P.; Li, L.; Dong, L.; Wang, S.; Huang, Z.; Qian, Y.; Wang, C.; Liu, W.; Yang, L. Gradient Biomineralized Silk Fibroin Nanofibrous Scaffold with Osteochondral Inductivity for Integration of Tendon to Bone. ACS Biomater. Sci. Eng. 2021, 7, 841–851. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Chen, Y.; Mo, X.; Fan, C. Tenogenic Adipose-Derived Stem Cell Sheets with Nanoyarn Scaffolds for Tendon Regeneration. Mater. Sci. Eng. C 2021, 119, 111506. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Guo, Y.Z.; Nonoyama, T.; Nakajima, T.; Kurokawa, T.; Gong, J.P. A Facile Method to Fabricate Anisotropic Hydrogels with Perfectly Aligned Hierarchical Fibrous Structures. Adv. Mater. 2018, 30, 1704937. [Google Scholar] [CrossRef]
- Laranjeira, M.; Domingues, R.M.A.; Costa-Almeida, R.; Reis, R.L.; Gomes, M.E. 3D Mimicry of Native-Tissue-Fiber Architecture Guides Tendon-Derived Cells and Adipose Stem Cells into Artificial Tendon Constructs. Small 2017, 13, 1700689. [Google Scholar] [CrossRef]
- Zheng, Z.; Ran, J.; Chen, W.; Hu, Y.; Zhu, T.; Chen, X.; Yin, Z.; Heng, B.C.; Feng, G.; Le, H.; et al. Alignment of Collagen Fiber in Knitted Silk Scaffold for Functional Massive Rotator Cuff Repair. Acta Biomater. 2017, 51, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Choi, Y.; Kim, J. Anisotropic Hybrid Hydrogels with Superior Mechanical Properties Reminiscent of Tendons or Ligaments. Adv. Funct. Mater. 2019, 29, 1904342. [Google Scholar] [CrossRef]
- Bottagisio, M.; Lopa, S.; Granata, V.; Talò, G.; Bazzocchi, C.; Moretti, M.; Barbara Lovati, A. Different Combinations of Growth Factors for the Tenogenic Differentiation of Bone Marrow Mesenchymal Stem Cells in Monolayer Culture and in Fibrin-Based Three-Dimensional Constructs. Differentiation 2017, 95, 44–53. [Google Scholar] [CrossRef]
- Younesi, M.; Akkus, A.; Akkus, O. Microbially-Derived Nanofibrous Cellulose Polymer for Connective Tissue Regeneration. Mater. Sci. Eng. C 2019, 99, 96–102. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Chiera, S.; Gershovich, P.; Motta, A.; Reis, R.L.; Gomes, M.E. Enhancing the Biomechanical Performance of Anisotropic Nanofibrous Scaffolds in Tendon Tissue Engineering: Reinforcement with Cellulose Nanocrystals. Adv. Healthc. Mater. 2016, 5, 1364–1375. [Google Scholar] [CrossRef]
- Green, E.C.; Zhang, Y.; Li, H.; Minus, M.L. Gel-Spinning of Mimetic Collagen and Collagen/Nano-Carbon Fibers: Understanding Multi-Scale Influences on Molecular Ordering and Fibril Alignment. J. Mech. Behav. Biomed. Mater. 2017, 65, 552–564. [Google Scholar] [CrossRef] [Green Version]
- Sensini, A.; Cristofolini, L.; Focarete, M.L.; Belcari, J.; Zucchelli, A.; Kao, A.; Tozzi, G. High-Resolution X-Ray Tomographic Morphological Characterisation of Electrospun Nanofibrous Bundles for Tendon and Ligament Regeneration and Replacement. J. Microsc. 2018, 272, 196–206. [Google Scholar] [CrossRef]
- Sharifi-Aghdam, M.; Faridi-Majidi, R.; Derakhshan, M.A.; Chegeni, A.; Azami, M. Preparation of Collagen/Polyurethane/Knitted Silk as a Composite Scaffold for Tendon Tissue Engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 652–662. [Google Scholar] [CrossRef]
- Mozafari, M.; Kargozar, S.; de Santiago, G.T.; Mohammadi, M.R.; Milan, P.B.; Koudehi, M.F.; Aghabarari, B.; Nourani, M.R. Synthesis and Characterisation of Highly Interconnected Porous Poly(ε-Caprolactone)-Collagen Scaffolds: A Therapeutic Design to Facilitate Tendon Regeneration. Mater. Technol. 2018, 33, 29–37. [Google Scholar] [CrossRef]
- Grier, W.K.; Iyoha, E.M.; Harley, B.A.C. The influence of pore size and stiffness on tenocyte bioactivity and transcriptomic stability in collagen-GAG scaffolds. J. Mech. Behav. Biomed. Mater. 2017, 65, 295–305. [Google Scholar] [CrossRef] [Green Version]
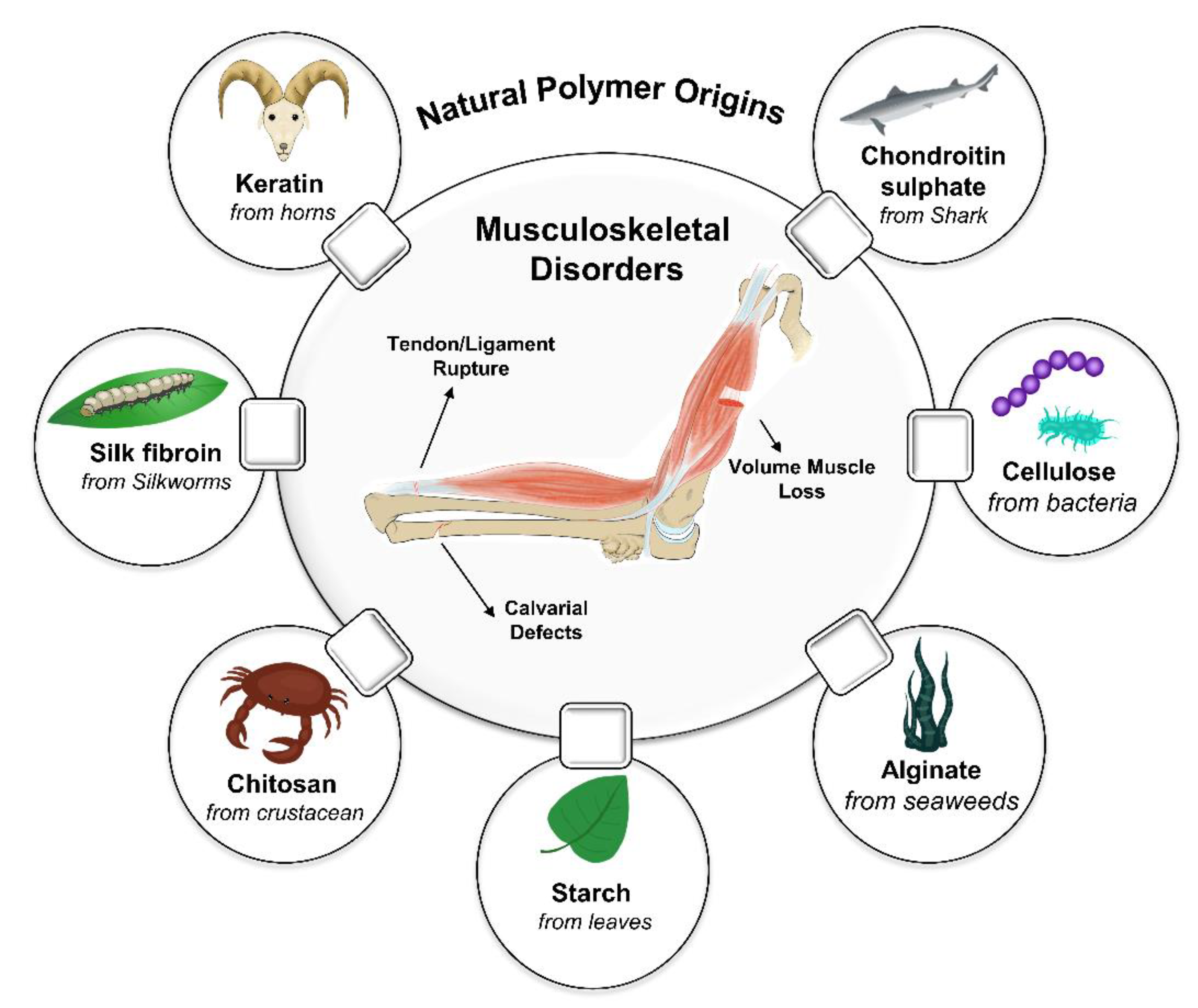


| Materials | Structure | Sources | Key Features | Ref |
|---|---|---|---|---|
| Chitosan | Linear polysaccharide | The shell of crustaceans (crabs, lobsters, shrimps, crayfish, and king crabs) as well as mollusks (e.g., squids), cuticles of insects, and cell walls of fungi | Second most abundant natural polymer, Biocompatible, Biodegradable, Bioadhesive, Biologically renewable, Antimicrobial, Hemostatic nature, Non-antigenic, Antioxidant, pH-sensitive | [27,28,29] |
| Alginate | Linear polysaccharide | Seaweeds and typically extracted from brown algae | Biocompatible, Biodegradable, Cytocompatible, Non-immunogenic, Mucoadhesive, Source abundance, Low cost, Water-soluble, pH-sensitive, in situ gelation | [30,31,32,33] |
| Starch | Composed of two kinds of polysaccharides, amylose, and amylopectin | The leaves of all green plants and in the seeds, fruits, stems, roots, and tubers of most plants and also in algae | Biocompatible, Biodegradable, Biorenewable, Low cost, Semicrystalline, High mechanical strength | [34,35,36] |
| Hyaluronic acid | Linear polysaccharide | A major macromolecular component of the ECM in the most connective tissues | Biocompatible, Biodegradable, Bioresorbable, Limited immunogenicity, Recognized by cell surface receptors, Flexible, Unique viscoelasticity | [37,38,39,40] |
| Chondroitin sulfate | Unbranched polysaccharide | A major component of ECM | Biocompatible, Biodegradable, Easily available, Immune-enhancing activity, Anti-inflammatory, Antioxidant, Antitumor, Anti-coagulation | [41] |
| Agarose | Liner polysaccharide | Marine red algae and also found as a support structure of cell wall for marine algae | Biocompatible, Non-immunogenic, Water solubility, pH-sensitive, Electro-responsive activity, Thermoreversible gelation behavior | [42,43] |
| Bacterial Cellulose | Linear polysaccharide | Microorganisms belonging to the Gluconacetobacter xylinum | Biocompatible, Biodegradable, High water-holding capacity, High mechanical strength, Porous structure, High crystallinity | [44,45,46,47,48] |
| Dextran | Branched polysaccharide | Lactic-acid bacteria | Biocompatible, Low cost, Easy to modify, Stable under mild acidic/basic conditions, Slowly degraded | [49,50,51] |
| Carrageenans | Linear polysaccharide | Marine red algae | Viscoelastic and gelling properties, Anti-inflammatory, Antitumor | [52] |
| Gellan gum | Linear polysaccharide | Sphingomonas elodea or Pseudomonas elodea bacteria | Minimal cytotoxicity, Ability to form hard and translucent gels which are stable at low pH, Thermally reversible gel in the presence of metallic ions | [53,54,55] |
| Xanthan gum | Branched polysaccharide | Xanthomonas bacteria | Biocompatible, Non-toxicity, Biodegradable, Stabile under a broad spectrum of pH, Shear-thinning | [56] |
| Heparin | Linear polysaccharide | Mucosal tissues such as the porcine intestine or bovine lungs | Antitumor, Anti-viral, Angiogenesis regulatory activities | [57,58] |
| Collagen | Fibrous protein | A major ECM component of most connective tissues within the mammalian body | Biocompatible, Biodegradable, Low-immunogenic, Hemostatic, High swelling ability, Low antigenicity, Capacity to facilitate cellular attachment | [59,60,61,62] |
| Gelatin | Protein | A hydrolysis derivative of collagen | Biocompatible, Biodegradable, Non-immunogenic, Elastic, Lower antigenicity, More accessible functional groups | [63,64,65,66] |
| Silk fibroin | Protein | Silkworms and spiders | Biocompatible, Biodegradable, Great mechanical properties, Versatile processability | [67,68,69,70,71] |
| Keratin | Polypeptide | A major component in nail, skin, hair, horns hooves, wool, feathers | Biocompatible, Biodegradable, Possesses cellular interaction sites Low-immunogenic, Intrinsic ability to self-assemble into three-dimensional structures | [72,73,74,75] |
| Fibrin | Glycoprotein | Fibrinogen | Biocompatible, Biodegradable, Ability of monomers to self-assemble into a gel | [76,77,78,79] |
| Elastin | Structural protein | A component in the ECMs of connective tissues (e.g., blood vessels, esophagus, skin) | Biocompatible, Biodegradable, Elasticity, Self-assembly, Long-term stability | [80,81] |
| Ref | Applied Materials | Cell Type | Structure/Production Method | Benefits |
|---|---|---|---|---|
| [140] | HA/gelatin/chitosan | Human osteoblast-like cell line (MG-63) | Core–shell nanofibers/freeze-drying method and calcium ion crosslinking | Biomimetic porous 3D scaffold with gradient and layered microstructure |
| [141] | Gelatin–alginate graphene oxide | Human osteoblast-like cell line (MG-63) | Nanocomposite scaffold/freeze drying technique | Enhanced compressive strength, 700% swelling ratio, slow biodegradation (≈30% in 28 days) |
| [142] | Gelatin-bioactive glass-ceramic | Human osteoblast-like cell line (MG-63) | Macroporous composite/lyophilization | Controlled degradation of gelatin scaffold and enhanced mechanical strength by incorporation of bioactive glass particles |
| [143] | Carboxymethyl chitosan/PCL | Human osteoblast-like cell line (MG-63) | Nanofibrous scaffold/electrospinning | Ultrafine and splitting fibers, reduced water contact angle |
| [144] | Chitosan/honeycomb porous carbon/HA | Bone marrow mesenchymal stem cells | Hierarchical porous structures/vacuum freeze-dried | Suitable pore size and high porosity for cell viability, mineralization, proliferation, and osteoinduction |
| [145] | Alginate/chitosan-HA | Human chondrocytes and fibroblasts | Porous gradient scaffold/freeze-drying and crosslinking by calcium ions | High compression modules and porosity |
| [146] | Gelatin/alginate/polyvinyl alcohol | MC3T3-E1 pre-osteoblast cells | Macroporous 3D spongy scaffold/cryogelation technique | Anti-bacterial scaffold for bone regeneration |
| [147] | Gelatin | L-929 fibroblasts, D1 MSC and MG63 osteoblasts | Fiber scaffold/freeze-dried | Enzymatically crosslinked scaffold for bone regeneration |
| [148] | Gelatin/PLLA | L929 fibroblasts | Multifunctional layered scaffold/electrospinning and 3D printing | Nasal cartilages and subchondral bone reconstruction |
| [149] | Strontium-Substituted HA/Gelatin | Coculture of osteoblasts and osteoclasts | Porous 3D scaffold/freeze-drying | Useful for local delivery of strontium and excessive bone resorption ability |
| [150] | Gelatin/PCL/nanoHA/vitamin D3 | Human adipose-derived stem cells | Nanocomposite scaffold/electrospinning | nHA and vitamin D3 have a synergistic effect on the osteogenic differentiation of hADSCs |
| [151] | Collagen/silica | Lymphocytes | Collagen fibrils with deposition of intrafibrillar amorphous silica | Promoting bone regeneration and angiogenesis via monocyte immunomodulation. Differentiation of blood-derived monocytes into TRAP-positive cells due to sustained release of silicic acid |
| [152] | Fibroin/poly(lactide-co-ε-caprolactone) | Human adipose-derived stem cells | Hybrid nanofibrous scaffold | Inducing cell adhesion and proliferation, favorable tensile strength, and surface roughness |
| [153] | Fibroin/PLGA | Rat bone marrow mesenchymal stem cells | Core–shell nanofibers | Enhancing cell adhesion, diffusion, and proliferation, promoting the osteogenic differentiation |
| [154] | SF/cellulose/chitosan | Human osteoblast cell line | Composite Porous scaffold | Supporting cell proliferation and promoting biomineralization |
| [155] | Fibroin/gelatin | Rat mesenchymal stem cell | Composite microcarrier | Supporting cell adhesion, proliferation, and elastic modulus |
| [156] | Alginate/nano-HA | Rat calvaria osteoblast | Composites | Good bioactivity, high biocompatibility, antibacterial activity |
| [157] | Silk/calcium silicate/sodium alginate | Bone marrow stromal cells | Hydrogel | Good biodegradation, cytocompatibility, bioactivity, and the proliferation of bone marrow stromal cells |
| [158] | Alginate/calcium phosphate paste | Stem cells | Injectable microbeads | Enhancing cell viability, proliferation, osteogenic differentiation, and bone regeneration |
| [159] | Alginate/gelatin/apatite coating | Rat bone marrow stem cells | 3D printed composite scaffold | Higher proliferation, osteogenic differentiation, surface protein adsorption, and Young’s modulus for apatite-coated scaffold |
| Ref | Applied Materials | Cell Type | Structure/Production Method | Advantages |
|---|---|---|---|---|
| [196] | Collagen/PPy | C2C12 mouse myoblast | 3D, highly aligned, and electrically conductive collagen scaffold via directional lyophilization of a polypyrrole-doped collagen suspension | Increasing electrical conductivity by using polypyrrole (PPy) |
| [197] | Collagen | C2C12 murine skeletal muscle myoblast cell | Fused deposition modeling (FDM) | Increased IGF1 mRNA and, Akt, p70S6K, and 4EBP1 phosphorylation, along with myotube hypertrophy and improved designed muscle functionality |
| [198] | Alginate/Gelatin/Heparin | Human skeletal muscle progenitor cells (hSMPCs) | Hydrogel | Cost-effective and an alternative for commercial biomaterials |
| [199] | Alginate | Mesenchymal stromal cells (MSCs) | Hydrogel | IGF-1 and VEGF165 had significant effects on muscle progenitor cells |
| [188] | Alginate/Gelatin | C2C12 | Extrusion-bioprinting of hydrogel | Alginate–gelatin hydrogel is a simple and cost-efficient biodegradable bio-ink |
| [200] | Gelatin/Hyaluronic acid | C2C12 | Hydrogel | Myotube production was established throughout the hydrogel when both gelatin and hyaluronic acid were present, and no shrinkage occurred |
| [201] | Fibrin/Polyethylene oxide (PEO) | C2C12 | C2C12s are encapsulated and electrospun into fibrin/polyethylene oxide (PEO) microfiber bundles with aqueous solution electrospinning. | Loading C2C12s as cellular aggregates increasing cell viability |
| [202] | Fibrin | Muscle progenitor cells (MPCs) adipogenic | Hydrogel | Adipogenic differentiation was decreased by myogenic differentiation but not prevented, and MPCs produced from diabetic animals had a higher capacity for adipogenic differentiation. |
| [203] | Fibrin/Laminin | C2C12 | Hydrogel | Integrating laminin-111 into fibrin hydrogels is possible |
| [204] | Fibrin/Alginate | C2C12 | Three-dimensional engineering of skeletal muscle tissue using electrospun fibrin microfiber bundles | To promote tissue formation, myoblasts should undergo biophysical stimulation |
| [205] | Fibrin/Thrombin | C2C12 | 3D printing, co-extruding fibrinogen and thrombin | Enhancing the regeneration of functional muscle tissue by tuning the topographic features of scaffolds |
| [206] | Fibrin/Collagen | Primary human skeletal muscle cells | Hydrogels | The Young’s modulus increased twofold, maximum strain decreased 2.5 times, and collagen deposition increased 1.6 times |
| [207] | Gelatin methacrylate (GelMA) | C2C12 | Under single UV exposure, silicone tubes-based coagulant produces cell-laden GelMA microfibers | Increased uniaxial strain ratio of up to 35–45% and significantly improved myotube contractility |
| [208] | Fibrin + Alginate | Primary human myoblasts | Injectable gel | Optimization of myoblast transplantation can include consideration of cell state |
| [209] | Fibrin/Alginate/Collagen | Human umbilical vein endothelial cells (HUVEC) | The use of 3D printing to create scaffolds composed of multiple gel layers and hollow channels | They developed a very cost-effective 3D printing system |
| [210] | Fibrin/Collagen-I | Mesenchymal stem cells (MSCs) | Parallel nanofiber electrospinning | When myogenic differentiation occurs, IGFBPs play a role, varying based on culture and stimulation conditions. |
| [211] | Fibrin | Muscle-derived stem cells (MDSCs) | Gel | SW033291 increased MDSC myogenic differentiation and myotube creation in a significant way. |
| [212] | Gelatin | C2C12 | Cell-based 3D bioprinting | The dECM components accelerated myogenic differentiation, while topographical cues caused cellular alignment |
| [213] | Gelatin | C2C12 | Cryogel | Myoblasts organize themselves around this pore structure and colonize the entire three-dimensional structure |
| [214] | Gelatin/Chitosan | L929 fibroblasts cell line | Hydrogel–3D printing | Increased cell viability |
| [215] | Gelatin/Alginate | C2C12 | Hydrogel–3D printing | Adding calcium peroxide (CPO) as an oxygen-generating source to bio-ink can improve cell metabolic activity in Gelma bio-ink |
| [216] | Gelatin | C2C12 | Hydrogel | Soft substrates can support longer-term cell culture |
| [217] | Fibrin | Bovine satellite cells (BSCs) | Hydrogel | Up to a 15-fold increase in myoglobin expression in vascular smooth muscle cells |
| [218] | Gelatin | C2C12 | Hydrogel | An increase in sarcomere formation in myotube cultures using micropatterned gelatin hydrogels |
| Ref. | Applied Materials | Cell Type | Structure/Production Method | Advantages |
|---|---|---|---|---|
| [241] | Silk/Collagen Polyurethane | L929 fibroblast cell line | Knitted silk covered by electrospun collagen/polyurethane | ___ |
| [240] | Collagen/Silk | Tendon stem progenitor cells (TSPCs) | Knitted silk scaffold dipped in collagen solution (in vivo study) | Macroporous structure |
| [242] | Alginate/Polyacrylamide Silica Microparticles | ____ | Hydrogel scaffolds dried under stretch | Scaffold production under tension |
| [243] | Alginate/Cellulose | ____ | Aligned fibrous hydrogels dried under stretch | Scaffold production under tension |
| [244] | Fibrin | Rabbit bone marrow-derived mesenchymal stem cells (BMSCs) | 2D and 3D fiber based structures | Use of different growth factors |
| [245] | Collagen/Nanocarbon fibers | ___ | Electrospun collagen/nanocarbon fibers | Use of nanocarbon fibers |
| [246] | Bacterial Cellulose | Human mesenchymal stem cells (hMSCs) | Bacterial cellulose sheets | Use of invaluable bacterial cellulose |
| [247] | PCL/CHT/CNC (Cellulose Nanocrystals) | Tendon-derived cells and adipose stem cells | Aligned electrospun nanofiber threads, braided and woven scaffolds | Reinforcement of mechanical properties by CNC |
| [248] | PCL/CHT CNCs | Human tendon-derived cells (hTDCs) | Electrospun nanofibrous scaffolds | Reinforcement of mechanical properties by CNC |
| [249] | PLLA/Collagen | ___ | Electrospun fibrous structure | CT scans of fiber to compare the morphology with native tendon |
| [250] | Collagen/PCL | C2C12 cells | Scaffold production using solvent casting and freeze drying including a subsequent crosslinking | Highly interconnected porous scaffold |
| [251] | Collagen–GAG | Equine tenocytes | Directional solidification of scaffolds | Investigation of scaffold pore size and crosslinking density |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Abedi-Dorcheh, K.; Sadat Vaziri, A.; Kazemi-Aghdam, F.; Rafieyan, S.; Sohrabinejad, M.; Ghorbani, M.; Rastegar Adib, F.; Ghasemi, Z.; Klavins, K.; et al. A Review of Recent Advances in Natural Polymer-Based Scaffolds for Musculoskeletal Tissue Engineering. Polymers 2022, 14, 2097. https://doi.org/10.3390/polym14102097
Fan J, Abedi-Dorcheh K, Sadat Vaziri A, Kazemi-Aghdam F, Rafieyan S, Sohrabinejad M, Ghorbani M, Rastegar Adib F, Ghasemi Z, Klavins K, et al. A Review of Recent Advances in Natural Polymer-Based Scaffolds for Musculoskeletal Tissue Engineering. Polymers. 2022; 14(10):2097. https://doi.org/10.3390/polym14102097
Chicago/Turabian StyleFan, Jingzhi, Keyvan Abedi-Dorcheh, Asma Sadat Vaziri, Fereshteh Kazemi-Aghdam, Saeed Rafieyan, Masoume Sohrabinejad, Mina Ghorbani, Fatemeh Rastegar Adib, Zahra Ghasemi, Kristaps Klavins, and et al. 2022. "A Review of Recent Advances in Natural Polymer-Based Scaffolds for Musculoskeletal Tissue Engineering" Polymers 14, no. 10: 2097. https://doi.org/10.3390/polym14102097
APA StyleFan, J., Abedi-Dorcheh, K., Sadat Vaziri, A., Kazemi-Aghdam, F., Rafieyan, S., Sohrabinejad, M., Ghorbani, M., Rastegar Adib, F., Ghasemi, Z., Klavins, K., & Jahed, V. (2022). A Review of Recent Advances in Natural Polymer-Based Scaffolds for Musculoskeletal Tissue Engineering. Polymers, 14(10), 2097. https://doi.org/10.3390/polym14102097