Relating Amorphous Structure to the Tear Strength of Polylactic Acid Films
Abstract
:1. Introduction
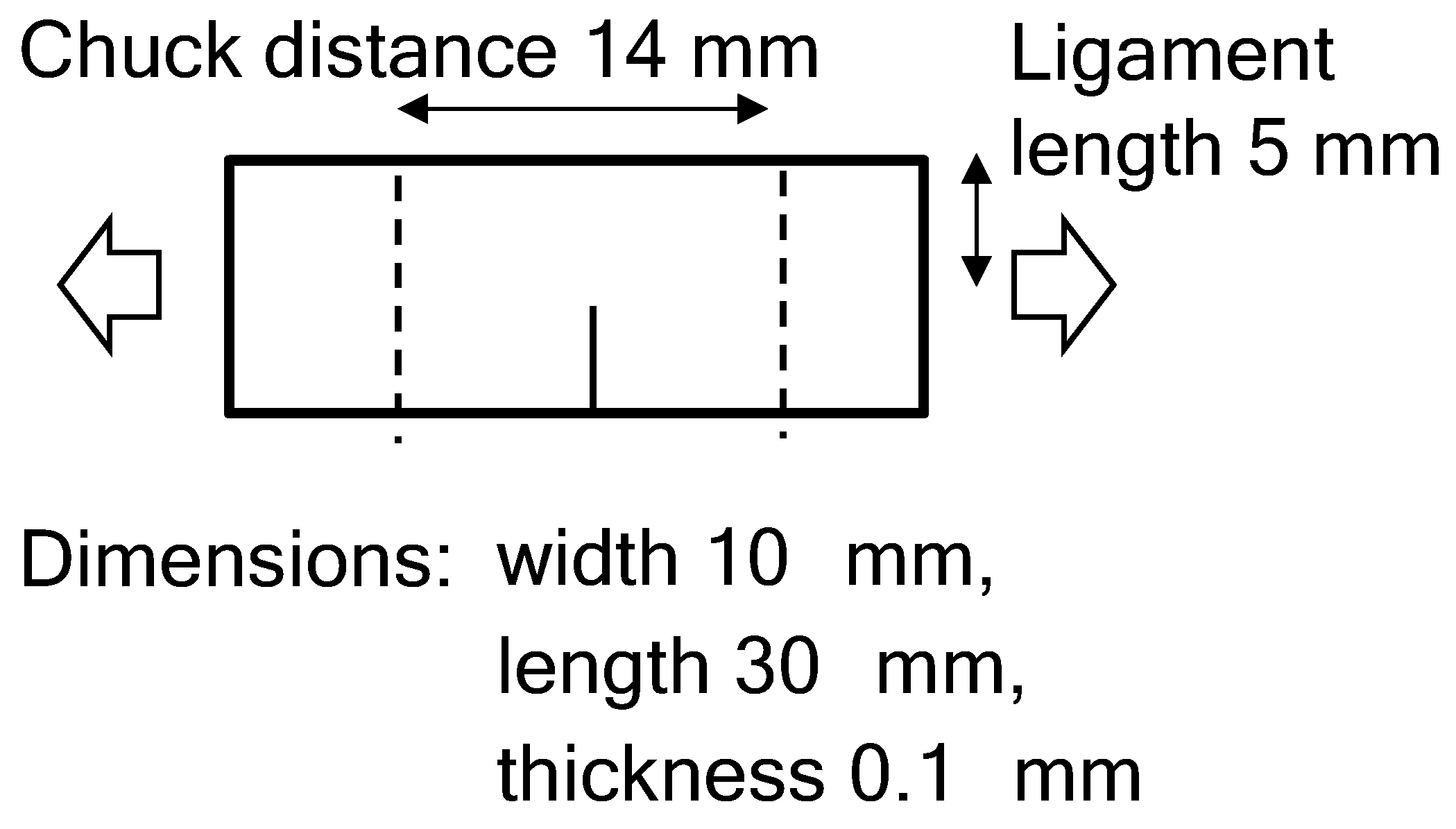
2. Experimental Setup
3. Results and Discussion
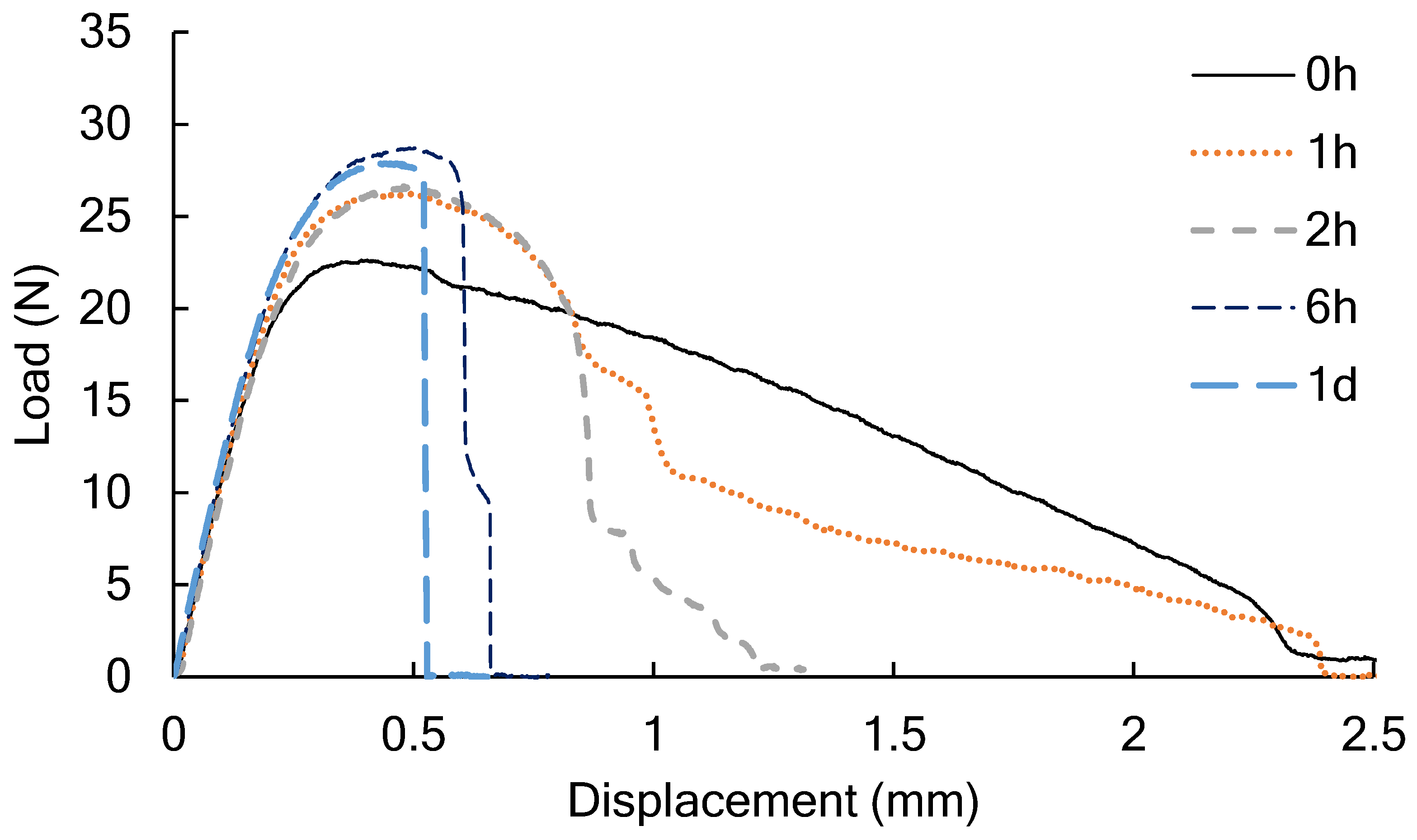
3.1. The Tear Strength of PLA after Conditioning in a Humid Chamber or an Oven
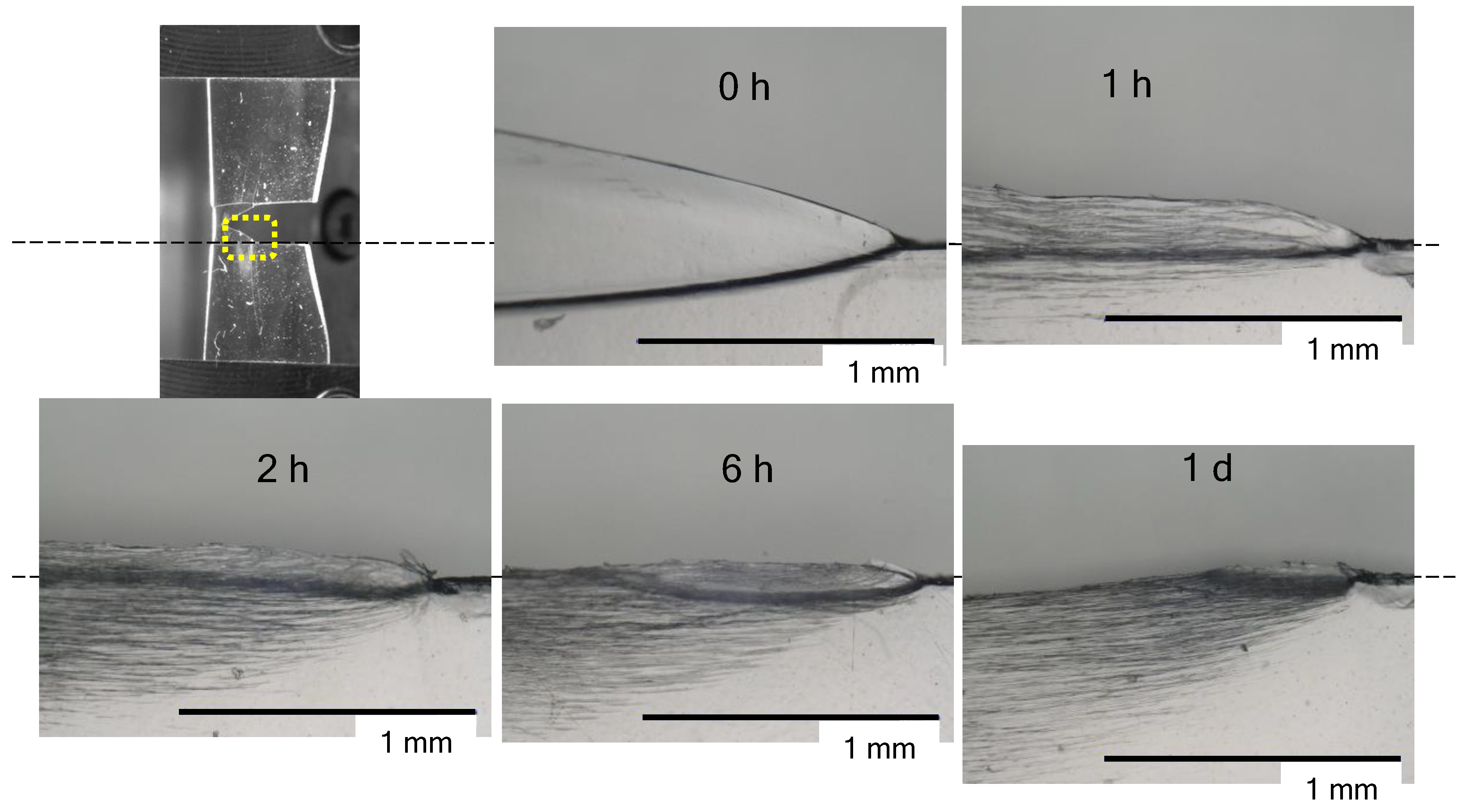
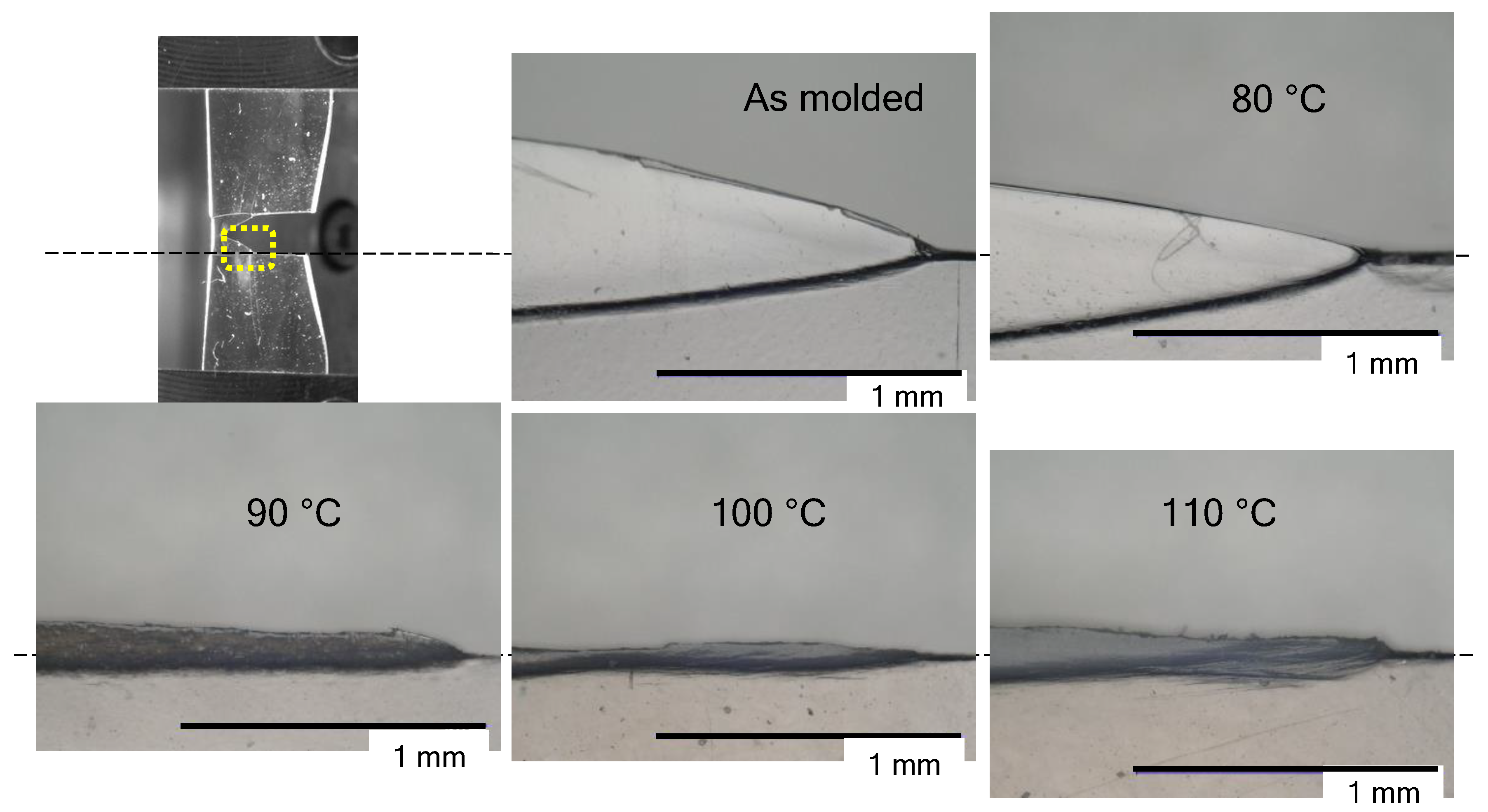
3.2. The Appearance of Films after the Tear Test
3.3. Morphological Changes at the Tip of the Tear
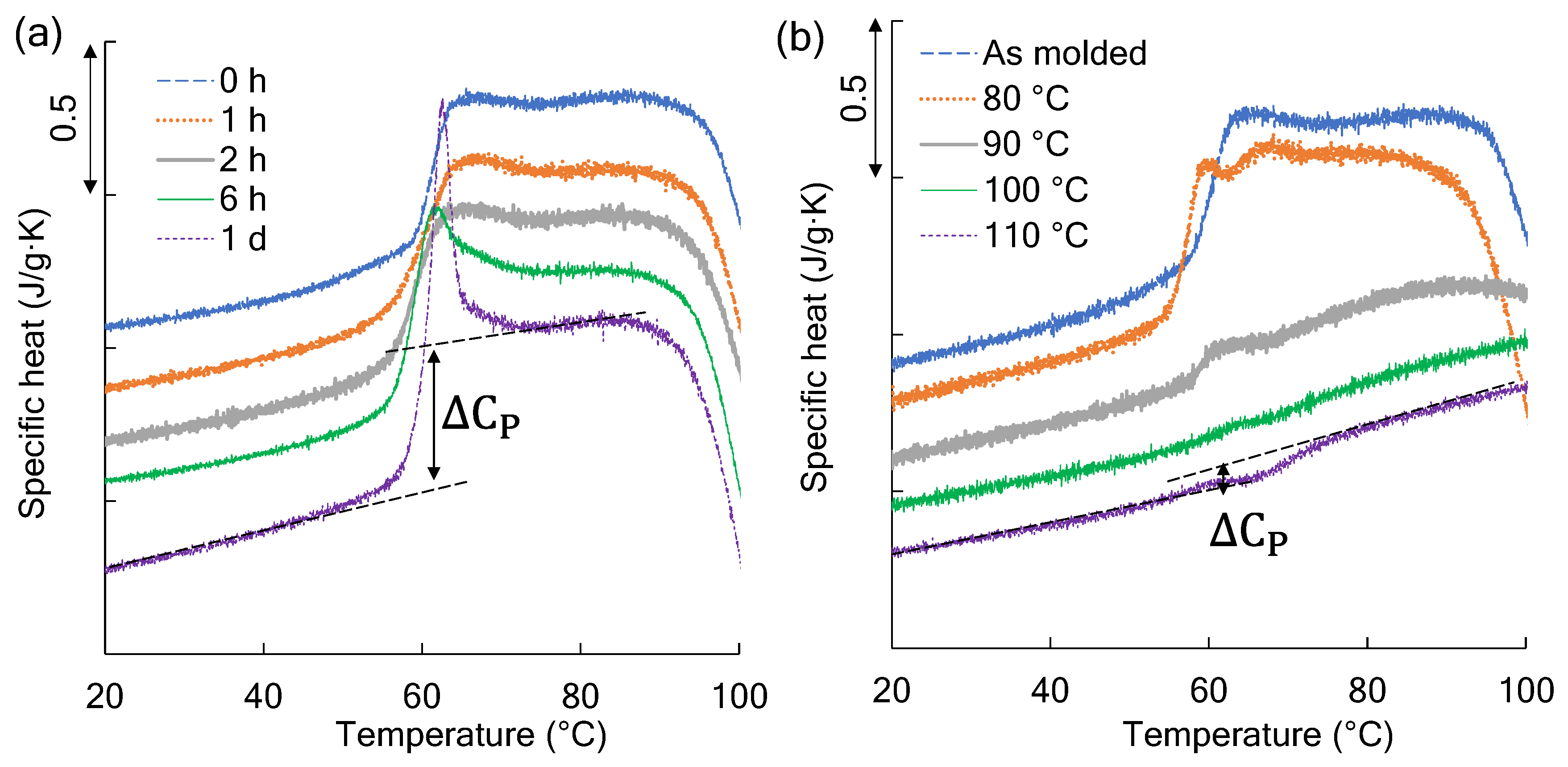
3.4. Changes in Heat Capacity at the Glass Transition Temperature
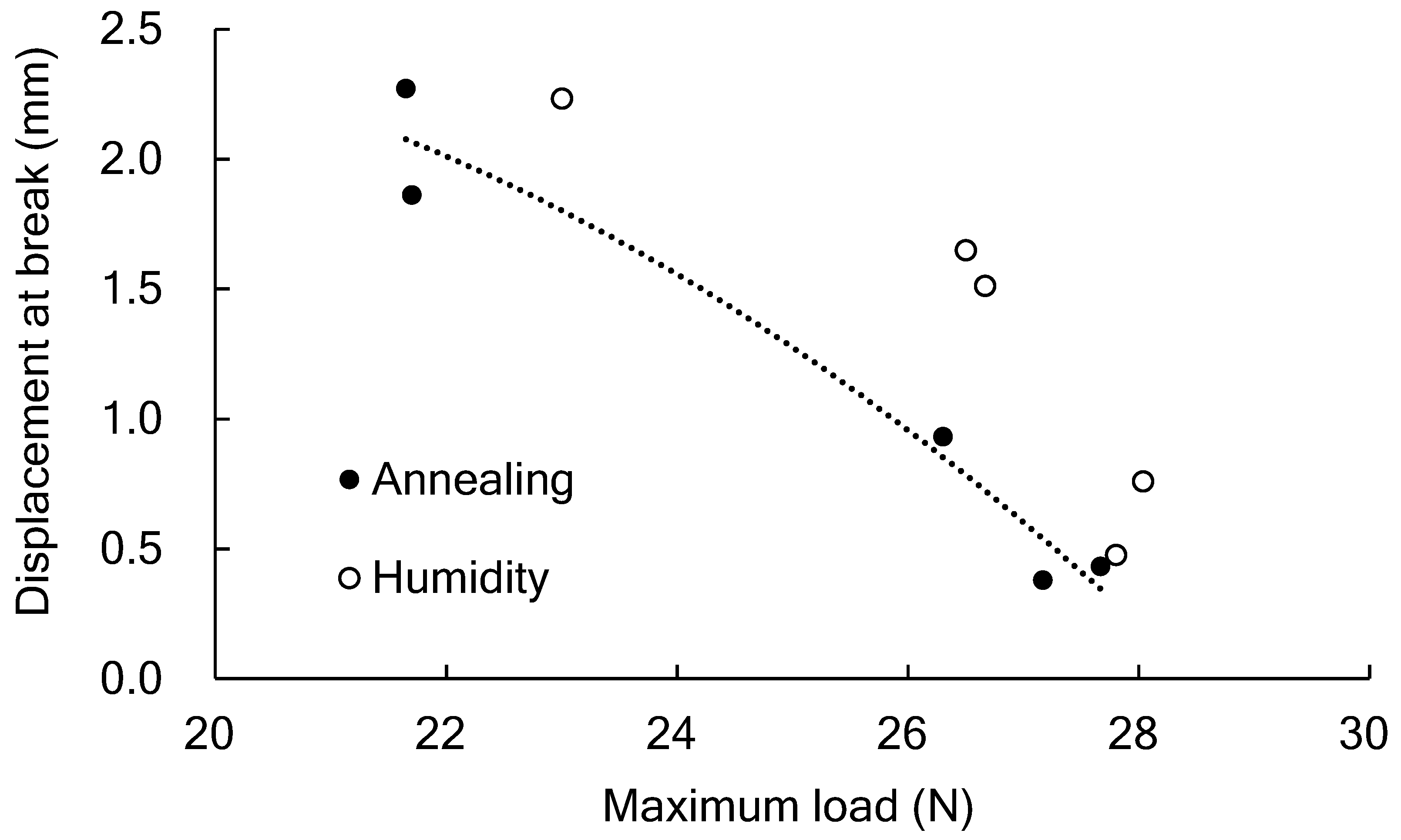
3.5. Effect of Water Absorption on the Tear Test
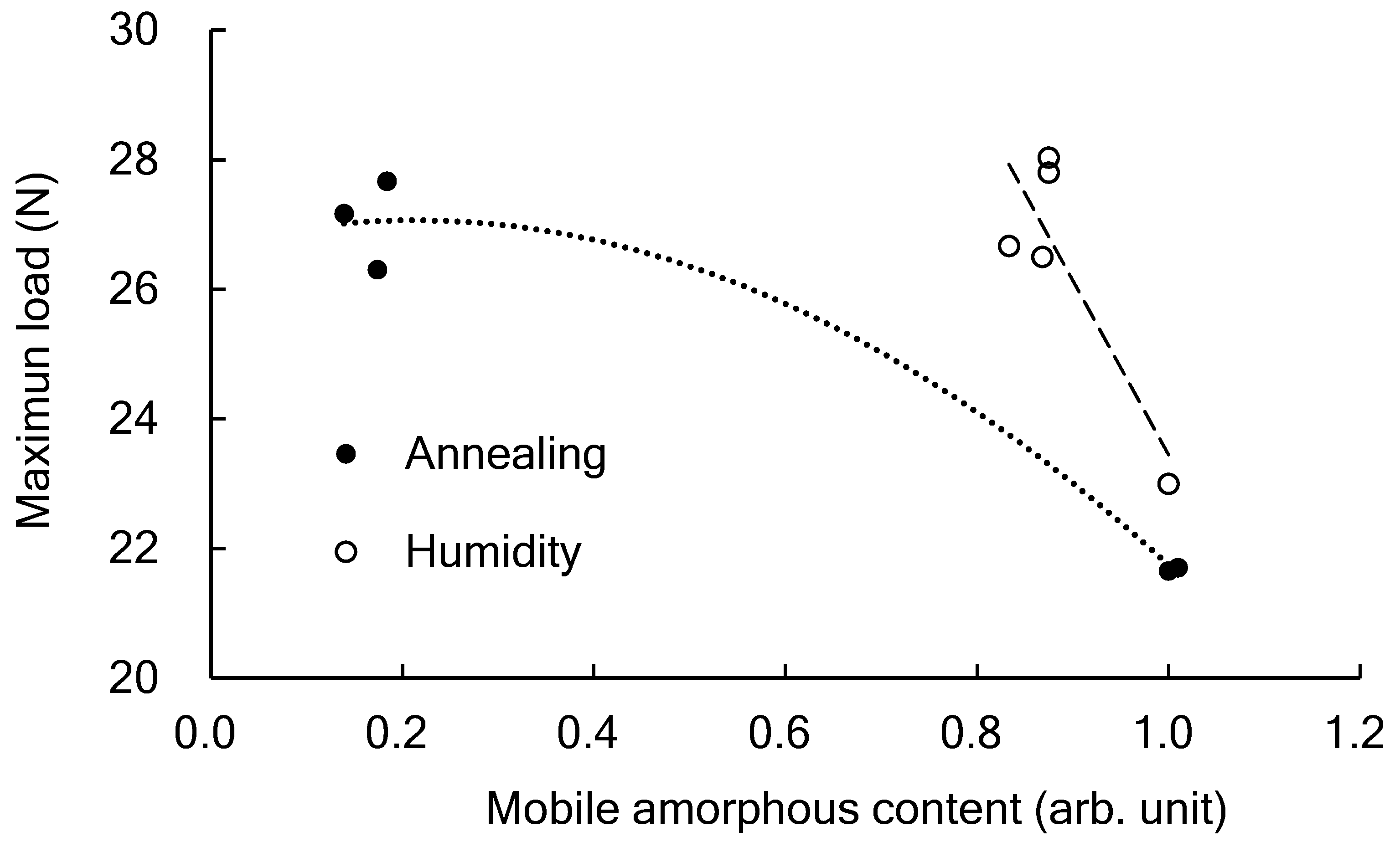
3.6. Influence of Mobile Amorphous on the Fracture Generation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masry, M.; Rossignol, S.; Gardette, J.-L.; Therias, S.; Bussière, P.-O.; Wong-Wah-Chung, P. Characteristics, Fate, and Impact of Marine Plastic Debris Exposed to Sunlight: A Review. Mar. Pollut. Bull. 2021, 171, 112701. [Google Scholar] [CrossRef] [PubMed]
- Freeland, B.; McCarthy, E.; Balakrishnan, R.; Fahy, S.; Boland, A.; Rochfort, K.D.; Dabros, M.; Marti, R.; Kelleher, S.M.; Gaughran, J. A Review of Polylactic Acid as a Replacement Material for Single-Use Laboratory Components. Materials 2022, 15, 2989. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Environmental Deterioration of Biodegradable, Oxo-Biodegradable, Compostable, and Conventional Plastic Carrier Bags in the Sea, Soil, and Open-Air Over a 3-Year Period. Environ. Sci. Technol. 2019, 53, 4775–4783. [Google Scholar] [CrossRef] [PubMed]
- Manfra, L.; Marengo, V.; Libralato, G.; Costantini, M.; De Falco, F.; Cocca, M. Biodegradable Polymers: A Real Opportunity to Solve Marine Plastic Pollution? J. Hazard. Mater. 2021, 416, 125763. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-X.; Huang, D.; Ji, J.-H.; Völker, C.; Wurm, F.R. Seawater-Degradable Polymers—Fighting the Marine Plastic Pollution. Adv. Sci. 2021, 8, 2001121. [Google Scholar] [CrossRef]
- Oyama, H.T.; Abe, S. Stereocomplex Poly(Lactic Acid) Alloys with Superb Heat Resistance and Toughness. ACS Sustain. Chem. Eng. 2015, 3, 3245–3252. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (Lactic Acid) Blends: Processing, Properties and Applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(Lactic Acid) Stereocomplexes: A Decade of Progress. Adv. Drug Deliv. Rev. 2016, 107, 97–135. [Google Scholar] [CrossRef]
- Gupta, P.; Wilkes, G.L.; Sukhadia, A.M.; Krishnaswamy, R.K.; Lamborn, M.J.; Wharry, S.M.; Tso, C.C.; DesLauriers, P.J.; Mansfield, T.; Beyer, F.L. Does the Length of the Short Chain Branch Affect the Mechanical Properties of Linear Low Density Polyethylenes? An Investigation Based on Films of Copolymers of Ethylene/1-Butene, Ethylene/1-Hexene and Ethylene/1-Octene Synthesized by a Single Site Metallocene Catalyst. Polymer 2005, 46, 8819–8837. [Google Scholar] [CrossRef]
- Guichon, O.; Séguéla, R.; David, L.; Vigier, G. Influence of the Molecular Architecture of Low-Density Polyethylene on the Texture and Mechanical Properties of Blown Films. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 327–340. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, H.; Wang, X.; Yu, X.; Zhou, W.; Peng, S. Super Tough Poly(Lactic Acid) Blends: A Comprehensive Review. RSC Adv. 2020, 10, 13316–13368. [Google Scholar] [CrossRef] [Green Version]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic Acid Blends: The Future of Green, Light and Tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(Lactic Acid) Modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Mahboubi, A.; Taherzadeh, M.J.; Åkesson, D.; Lennartsson, P.R. Application of Fungal Biomass for the Development of New Polylactic Acid-Based Biocomposites. Polymers 2022, 14, 1738. [Google Scholar] [CrossRef]
- Gigante, V.; Bosi, L.; Parlanti, P.; Gemmi, M.; Aliotta, L.; Lazzeri, A. Analysis of the Damage Mechanism around the Crack Tip for Two Rubber-Toughened PLA-Based Blends. Polymers 2021, 13, 53. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Canesi, I.; Cinelli, P.; Coltelli, M.-B.; Lazzeri, A. Poly(Lactic Acid) (PLA)/Poly(Butylene Succinate-Co-Adipate) (PBSA) Compatibilized Binary Biobased Blends: Melt Fluidity, Morphological, Thermo-Mechanical and Micromechanical Analysis. Polymers 2021, 13, 218. [Google Scholar] [CrossRef]
- Rocca-Smith, J.R.; Whyte, O.; Brachais, C.-H.; Champion, D.; Piasente, F.; Marcuzzo, E.; Sensidoni, A.; Debeaufort, F.; Karbowiak, T. Beyond Biodegradability of Poly(Lactic Acid): Physical and Chemical Stability in Humid Environments. ACS Sustain. Chem. Eng. 2017, 5, 2751–2762. [Google Scholar] [CrossRef]
- Limsukon, W.; Auras, R.; Smith, T. Effects of the Three-Phase Crystallization Behavior on the Hydrolysis of Amorphous and Semicrystalline Poly(Lactic Acid)S. ACS Appl. Polym. Mater. 2021, 3, 5920–5931. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ueda, T.; Ishigami, A.; Ito, H. Changes in Crystal Structure and Accelerated Hydrolytic Degradation of Polylactic Acid in High Humidity. Polymers 2021, 13, 4324. [Google Scholar] [CrossRef]
- Henricks, J.; Boyum, M.; Zheng, W. Crystallization Kinetics and Structure Evolution of a Polylactic Acid during Melt and Cold Crystallization. J. Therm. Anal. Calorim. 2015, 120, 1765–1774. [Google Scholar] [CrossRef]
- Uetani, K.; Koga, H.; Nogi, M. Estimation of the Intrinsic Birefringence of Cellulose Using Bacterial Cellulose Nanofiber Films. ACS Macro Lett. 2019, 8, 250–254. [Google Scholar] [CrossRef]
- Sato, T.; Araki, T.; Sasaki, Y.; Tsuru, T.; Tadokoro, T.; Kawakami, S. Compact Ellipsometer Employing a Static Polarimeter Module with Arrayed Polarizer and Wave-Plate Elements. Appl. Opt. 2007, 46, 4963–4967. [Google Scholar] [CrossRef]
- Jabarin, S.A.; Lofgren, E.A. Effects of Water Absorption on Physical Properties and Degree of Molecular Orientation of Poly (Ethylene Terephthalate). Polym. Eng. Sci. 1986, 26, 620–625. [Google Scholar] [CrossRef]
- Rubin, J.; Andrews, R.D. Effect of Solvent Treatments on the Mechanical Properties of Nylon 6. Polym. Eng. Sci. 1968, 8, 302–309. [Google Scholar] [CrossRef]
- Ohara, A.; Kodama, H. Correlation between Enthalpy Relaxation and Mechanical Response on Physical Aging of Polycarbonate in Relation to the Effect of Molecular Weight on Ductile-Brittle Transition. Polymer 2019, 181, 121720. [Google Scholar] [CrossRef]
- Pan, P.; Zhu, B.; Inoue, Y. Enthalpy Relaxation and Embrittlement of Poly(l-Lactide) during Physical Aging. Macromolecules 2007, 40, 9664–9671. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, Y.; Ishigami, A.; Ito, H. Relating Amorphous Structure to the Tear Strength of Polylactic Acid Films. Polymers 2022, 14, 1965. https://doi.org/10.3390/polym14101965
Kobayashi Y, Ishigami A, Ito H. Relating Amorphous Structure to the Tear Strength of Polylactic Acid Films. Polymers. 2022; 14(10):1965. https://doi.org/10.3390/polym14101965
Chicago/Turabian StyleKobayashi, Yutaka, Akira Ishigami, and Hiroshi Ito. 2022. "Relating Amorphous Structure to the Tear Strength of Polylactic Acid Films" Polymers 14, no. 10: 1965. https://doi.org/10.3390/polym14101965
APA StyleKobayashi, Y., Ishigami, A., & Ito, H. (2022). Relating Amorphous Structure to the Tear Strength of Polylactic Acid Films. Polymers, 14(10), 1965. https://doi.org/10.3390/polym14101965







