Crystallization of Isotactic Polypropylene Nanocomposites with Fibrillated Poly(tetrafluoroethylene) under Elevated Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
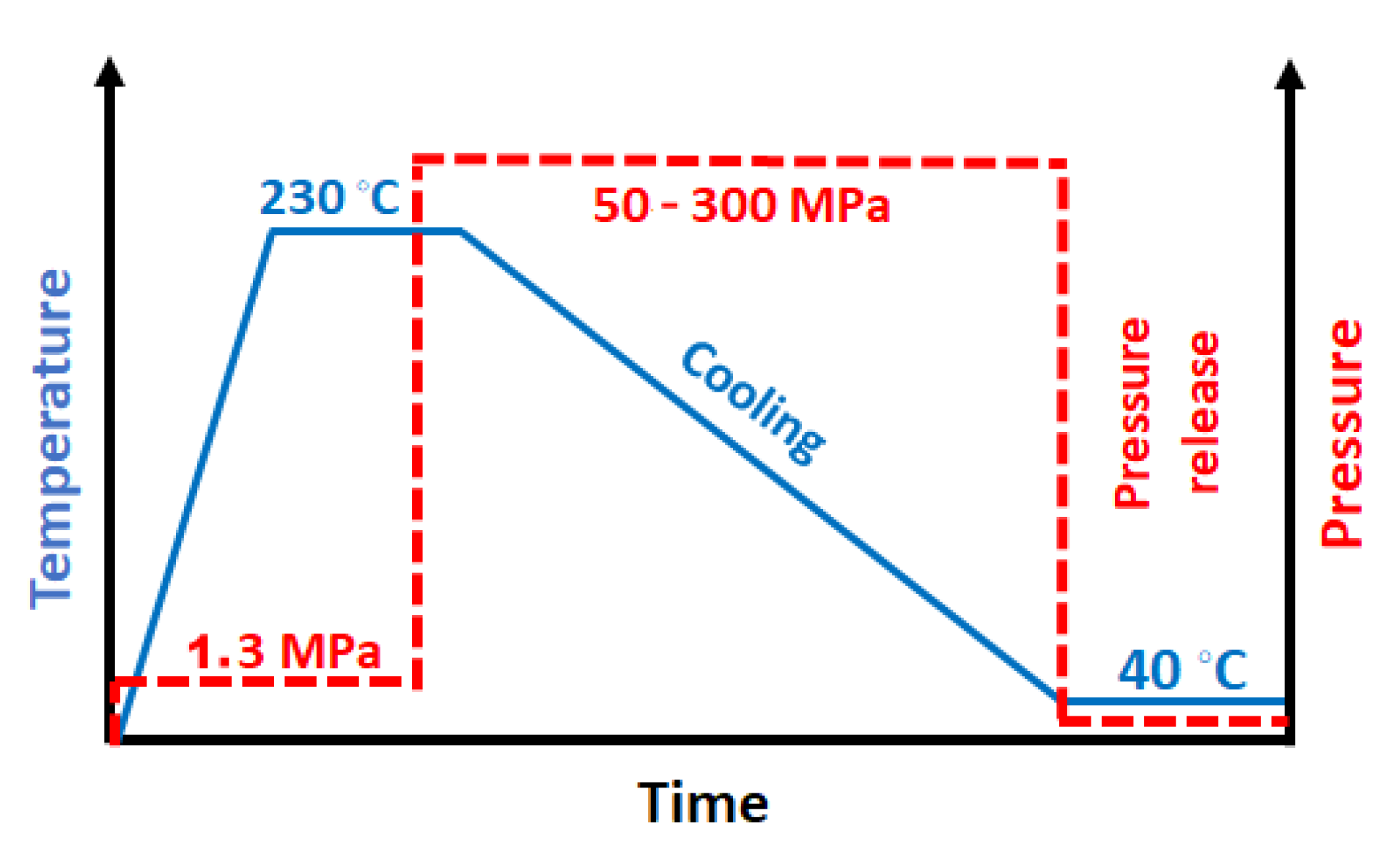
2.2. Crystallization under High Pressure
2.3. Characterization
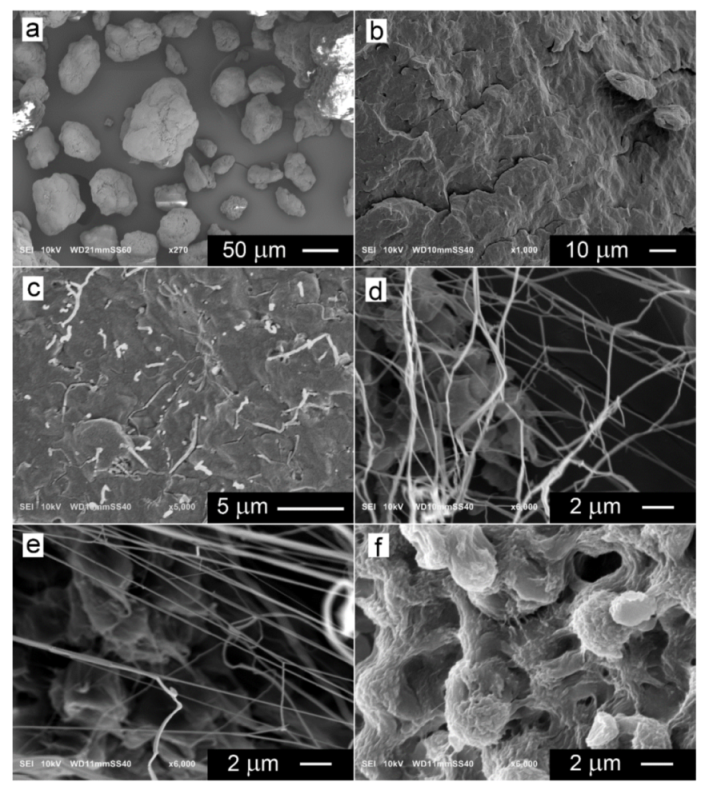
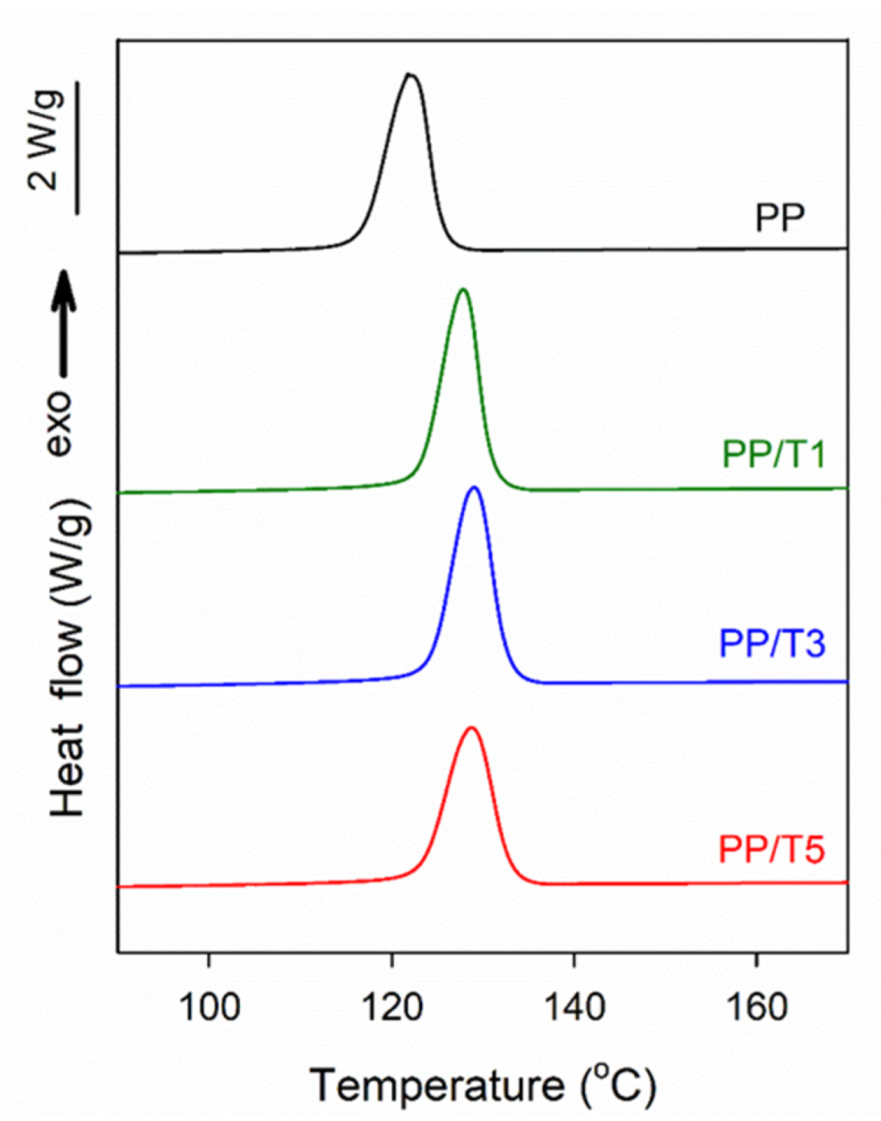
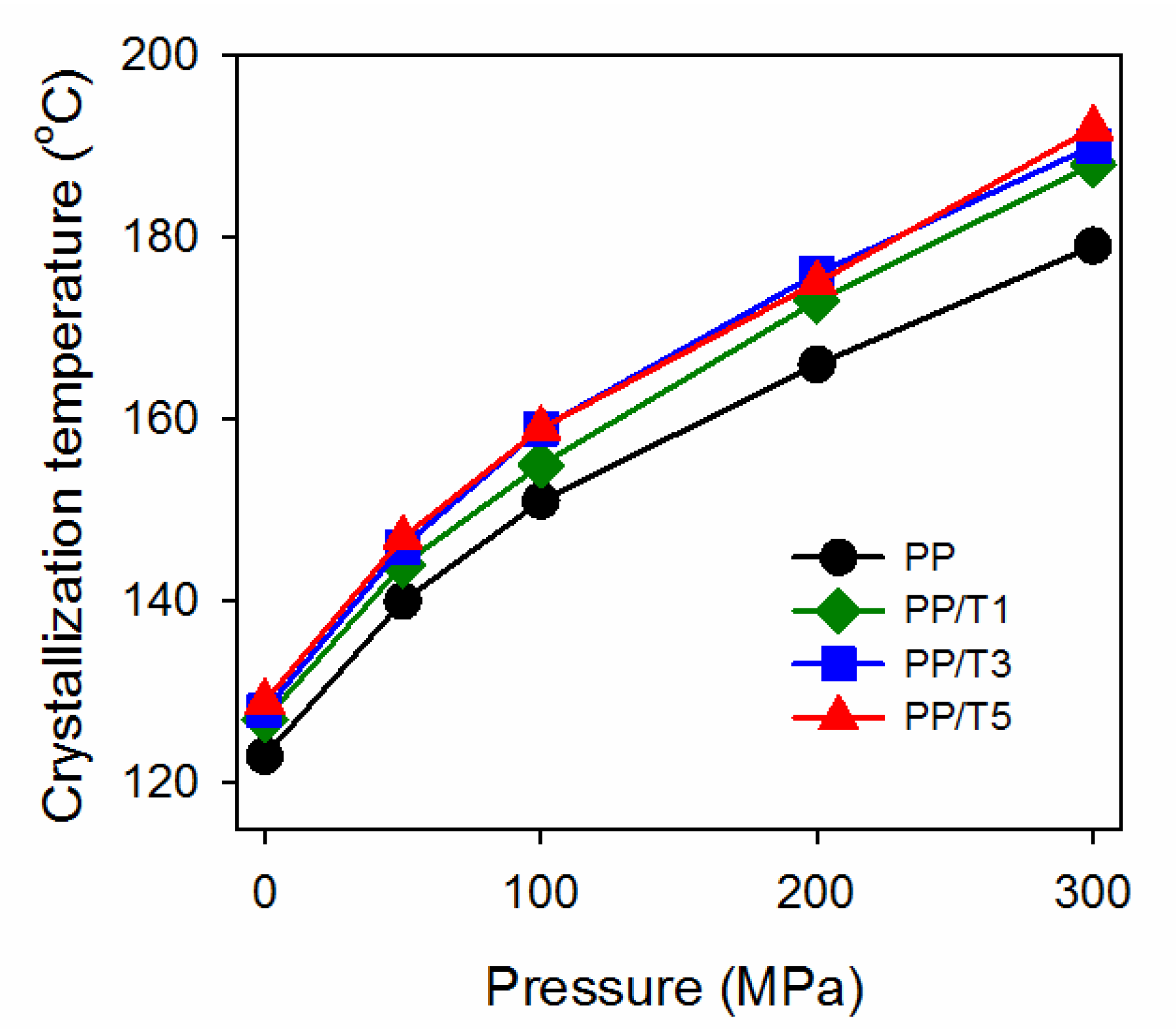
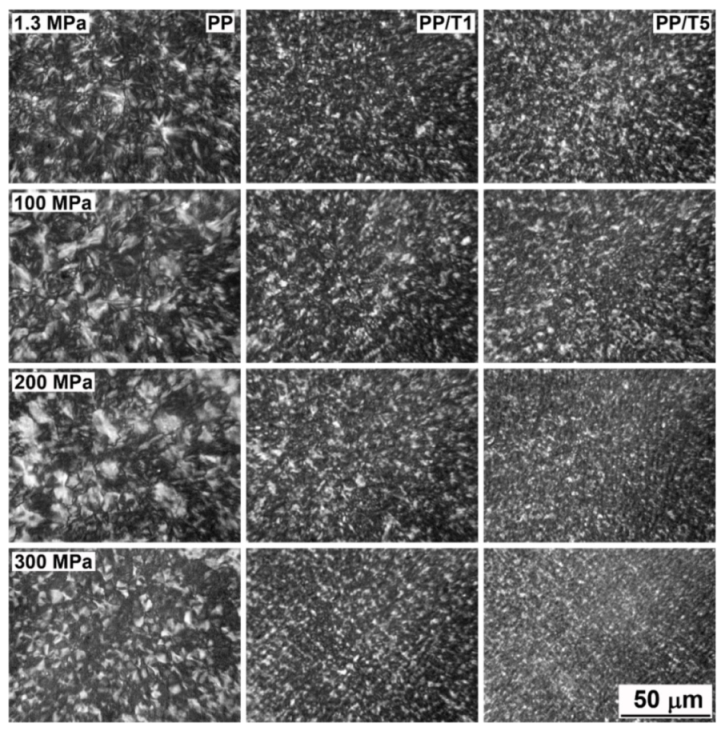
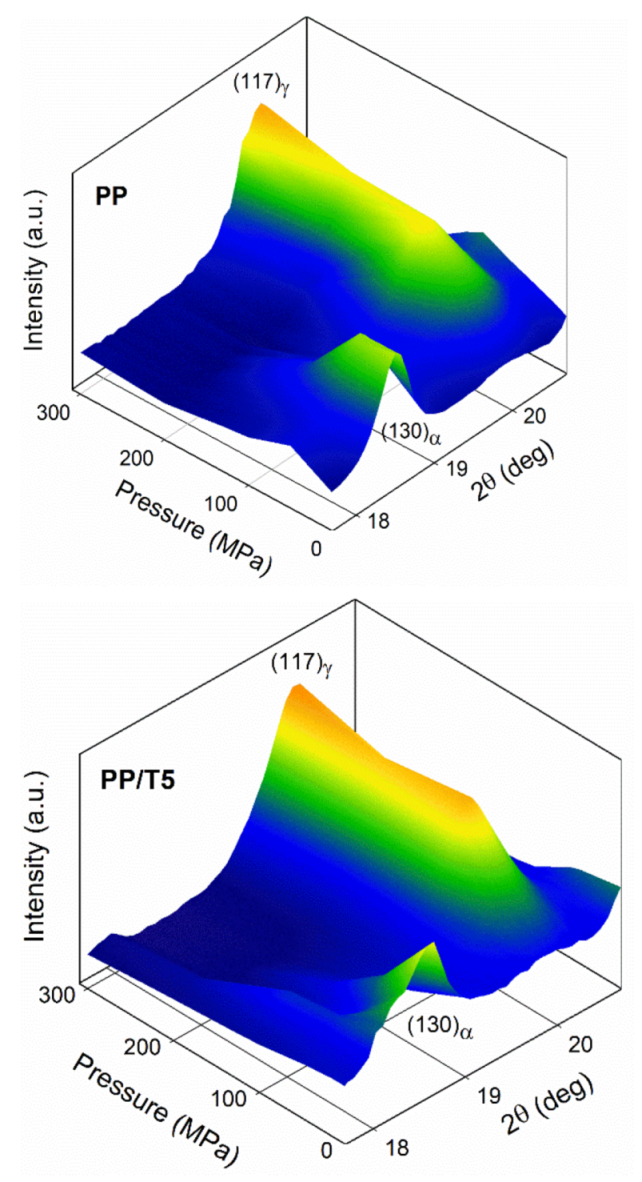
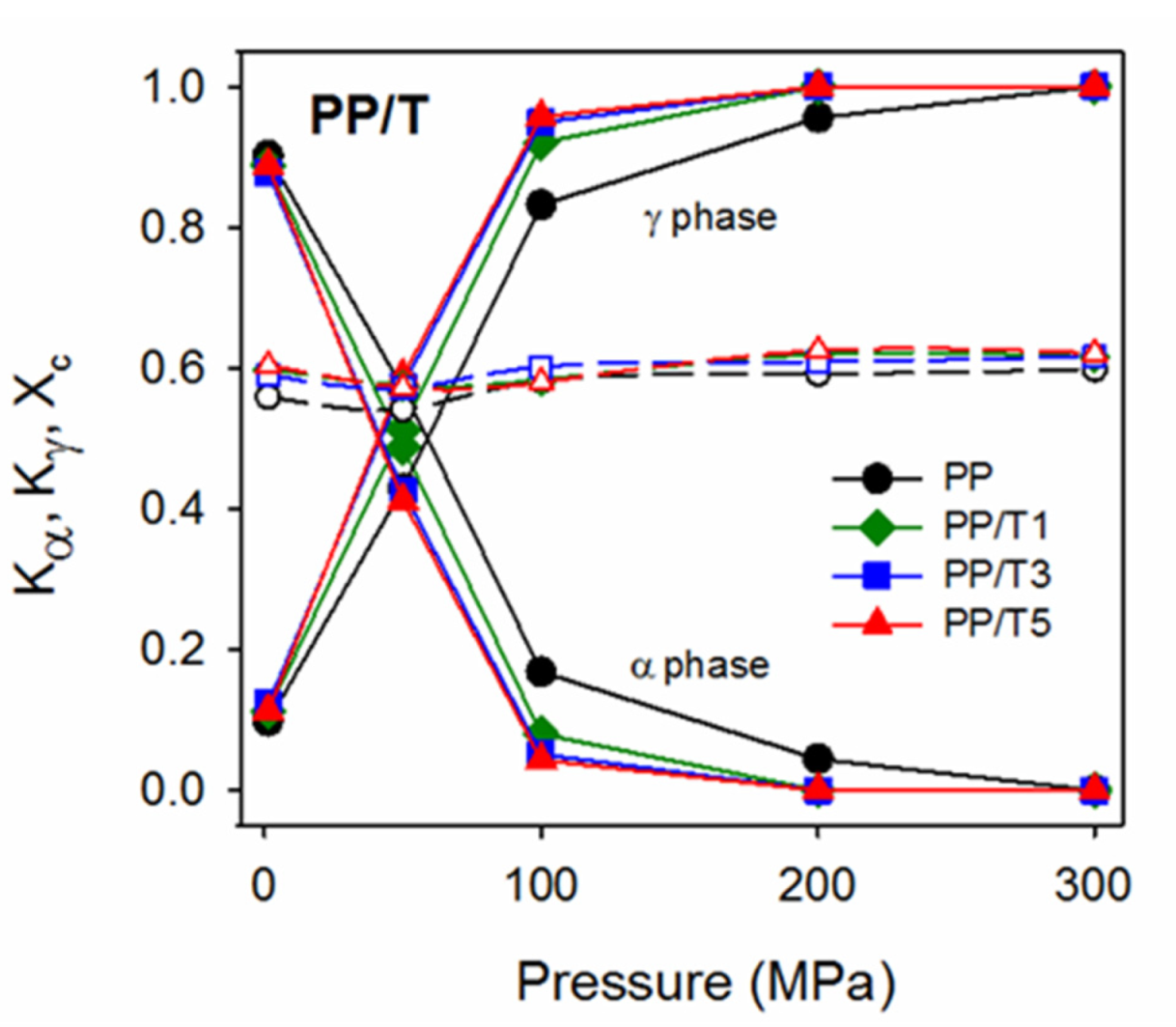
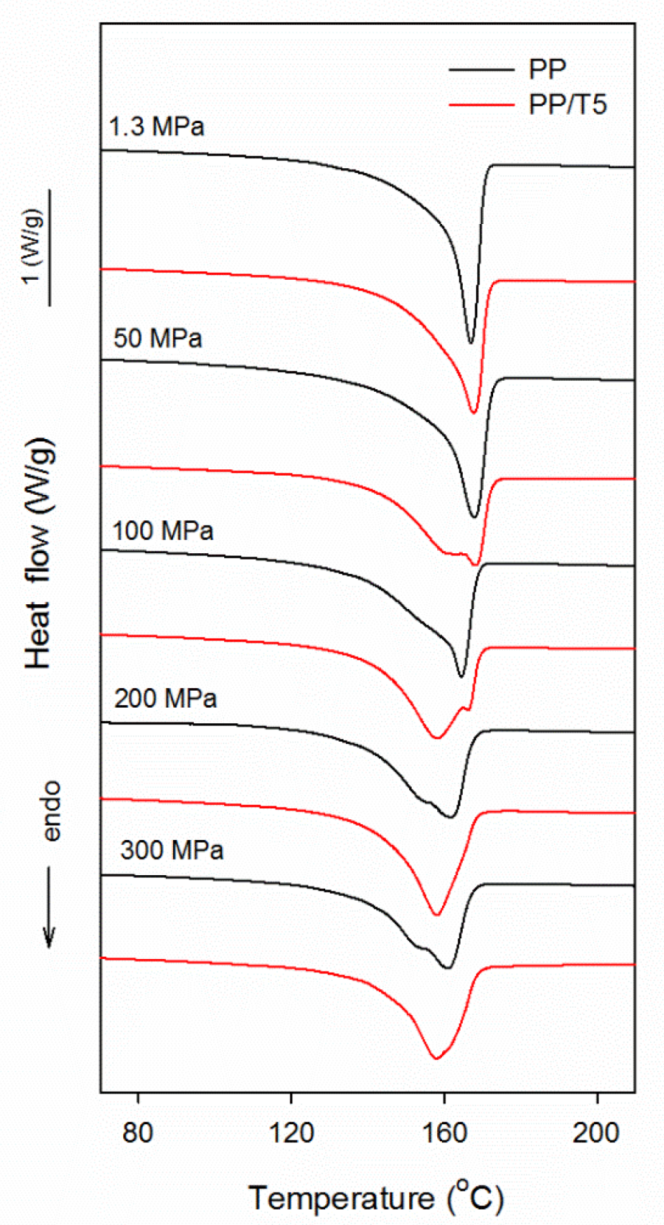
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, C.; Yu, L.; Liu, H.; Chen, L. Development of self-reinforced polymer composites. Prog. Polym. Sci. 2012, 37, 767–780. [Google Scholar] [CrossRef]
- Kmetty, Á.; Bárány, T.; Karger-Kocsis, J. Self-reinforced polymeric materials: A review. Prog. Polym. Sci. 2010, 35, 1288–1310. [Google Scholar] [CrossRef]
- Jurczuk, K.; Galeski, A.; Piorkowska, E. All-polymer nanocomposites with nanofibrillar inclusions generated in situ during compounding. Polymer 2013, 54, 4617–4628. [Google Scholar] [CrossRef]
- Bernland, K.; Smith, P. Nucleating polymer crystallization with poly(tetrafluoroethylene) nanofibrils. J. Appl. Polym. Sci. 2009, 114, 281–287. [Google Scholar] [CrossRef]
- Jurczuk, K.; Galeski, A.; Piorkowska, E. Strain hardening of molten thermoplastic polymers reinforced with poly(tetrafluoroethylene) nanofibers. J. Rheol. 2014, 58, 589–605. [Google Scholar] [CrossRef]
- Krajenta, J.; Pawlak, A.; Galeski, A. Deformation of disentangled polypropylene crystalline grains into nanofibers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1983–1994. [Google Scholar] [CrossRef]
- Li, L.W.; Li, W.; Geng, L.H.; Chen, B.Y.; Mi, H.Y.; Hong, K.L.; Peng, X.F.; Kuang, T.R. Formation of stretched fibrils and nanohybrid shish-kebabs in isotactic polypropylene-based nanocomposites by application of a dynamic oscillatory shear. Chem. Eng. J. 2018, 348, 546–556. [Google Scholar] [CrossRef]
- Jurczuk, K.; Galeski, A. Thermoplastic elastomers reinforced with poly(tetrafluoroethylene) nanofibers. Eur. Polym. J. 2016, 80, 58–69. [Google Scholar] [CrossRef]
- Van der Meer, D.W.; Milazzo, D.; Sanguineti, A.; Vancso, G.J. Oriented crystallization and mechanical properties of polypropylene nucleated on fibrillated polytetrafluoroethylene scaffolds. Polym. Eng. Sci. 2005, 45, 458–468. [Google Scholar] [CrossRef]
- Galeski, S.; Piorkowska, E.; Rozanski, A.; Regnier, G.; Galeski, A.; Jurczuk, K. Crystallization kinetics of polymer fibrous nanocomposites. Eur. Polym. J. 2016, 83, 181–201. [Google Scholar] [CrossRef][Green Version]
- Qiao, Y.; Jalali, A.; Yang, J.; Chen, Y.; Wang, S.; Jiang, Y.; Hou, J.; Jiang, J.; Li, Q.; Park, C.B. Non-isothermal crystallization kinetics of polypropylene/polytetrafluoroethylene fibrillated composites. J. Mater. Sci. 2021, 56, 3562–3575. [Google Scholar] [CrossRef]
- Masirek, R.; Piorkowska, E. Nucleation of crystallization in isotactic polypropylene and polyoxymethylene with poly(tetrafluoroethylene) particles. Eur. Polym. J. 2010, 46, 1436–1445. [Google Scholar] [CrossRef]
- Lovinger, A.J.; Chua, J.O.; Gryte, C.C. Studies on the α and β forms of isotactic polypropylene by crystallization in a temperature gradient. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 641–656. [Google Scholar] [CrossRef]
- Mollova, A.; Androsch, R.; Mileva, D.; Gahleitner, M.; Funari, S.S. Crystallization of isotactic polypropylene containing beta-phase nucleating agent at rapid cooling. Eur. Polym. J. 2013, 49, 1057–1065. [Google Scholar] [CrossRef]
- Varga, J. β-modification of isotactic polypropylene: Preparation, structure, processing, properties, and application. J Macromol. Sci. Part B Phys. 2002, B41, 1121–1171. [Google Scholar] [CrossRef]
- Lotz, B.; Graff, S.; Wittmann, J.C. Crystal morphology of the γ-(triclinic) phase of isotactic polypropylene and its relation to the α-phase. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 2017–2032. [Google Scholar] [CrossRef]
- Morrow, D.R.; Newman, B.A. Crystallization of low-molecular-weight polypropylene fractions. J. Appl. Phys. 1968, 39, 4944–4950. [Google Scholar] [CrossRef]
- Kojima, M. Solution-grown lamellar crystals of thermally decomposed isotactic polypropylene. J. Polym. Sci. Part B Polym. Lett. 1967, 5, 245–250. [Google Scholar] [CrossRef]
- Turner-Jones, A. Development of the γ-crystal form in random copolymers of propylene and their analysis by dsc and X-ray methods. Polymer 1971, 12, 487–508. [Google Scholar] [CrossRef]
- Guidetti, G.P.; Busi, P.; Giulianelli, I.; Zannetti, R. Structure properties relationships in some random co-polymers of propylene. Eur. Polym. J. 1983, 19, 757–759. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Della Volpe, G.; Segre, A.; Rossi, E.; Simonazzi, T. Composition-properties relationships in propene-ethene random copolymers obtained with high-yield Ziegler-Natta supported catalysts. Makromol. Chem. 1986, 187, 1927–1943. [Google Scholar] [CrossRef]
- Marigo, A.; Marega, C.; Zannetti, R.; Paganetto, G.; Canossa, E.; Coletta, F.; Gottardi, F. Crystallization of the γ-form of isotactic poly(propylene). Makromol. Chem. 1989, 190, 2805–2813. [Google Scholar] [CrossRef]
- Fischer, D.; Mulhaupt, R. The influence of regio- and stereoirregularities on the crystallization behavior of isotactic poly(propylene) prepared with homogeneous group IVa metallocene/methylaluminoxane Ziegler-Natta catalysts. Macromol. Chem. Phys. 1994, 195, 1433–1441. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Paolillo, M.; Resconi, L.; Camurati, I. Crystallization behavior and mechanical properties of regiodefective, highly stereoregular isotactic polypropylene: Effect of regiodefects versus stereodefects and influence of the molecular mass. Macromolecules 2005, 38, 9143–9154. [Google Scholar] [CrossRef]
- Thomann, R.; Semke, H.; Maier, R.D.; Thomann, Y.; Scherble, J.; Mulhaupt, R.; Kressler, J. Influence of stereoirregularities on the formation of the γ-phase in isotactic polypropene. Polymer 2001, 42, 4597–4603. [Google Scholar] [CrossRef]
- Mezghani, K.; Phillips, P.J. The γ-phase of high molecular weight isotactic polypropylene. II: The morphology of the γ-form crystallized at 200 MPa. Polymer 1997, 38, 5725–5733. [Google Scholar] [CrossRef]
- Kardos, J.L.; Christiansen, A.W.; Baer, E. Structure of pressure-crystallized polypropylene. J. Polym. Sci. Part A2 Polym. Phys. 1966, 4, 777–788. [Google Scholar] [CrossRef]
- Kalay, G.; Zhong, Z.; Allan, P.; Bevis, M.J. The occurrence of the γ-phase in injection moulded polypropylene in relation to the processing conditions. Polymer 1996, 37, 2077–2085. [Google Scholar] [CrossRef]
- Mezghani, K.; Phillips, P.J. The γ-phase of high molecular weight isotactic polypropylene: III. The equilibrium melting point and the phase diagram. Polymer 1998, 39, 3735–3744. [Google Scholar] [CrossRef]
- Lotz, B.; Ruan, J.; Thierry, A.; Alfonso, G.C.; Hiltner, A.; Baer, E.; Piorkowska, E.; Galeski, A. A structure of copolymers of propene and hexene isomorphous to isotactic poly(1-butene) form I. Macromolecules 2006, 39, 5777–5781. [Google Scholar] [CrossRef]
- De Rosa, C.; Auriemma, F.; Corradini, P.; Tarallo, O.; Dello Iacono, S.; Ciaccia, E.; Resconi, L. Crystal structure of the trigonal form of isotactic polypropylene as an example of density-driven polymer structure. J. Am. Chem. Soc. 2006, 128, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Lotz, B. A new ε crystal modification found in stereodefective isotactic polypropylene samples. Macromolecules 2014, 47, 7612–7624. [Google Scholar] [CrossRef]
- Piccarolo, S.; Saiu, M.; Brucato, V.; Titomanlio, G. Crystallization of polymer melts under fast cooling.II. High-purity iPP. J. Appl. Polym. Sci. 1992, 46, 625–634. [Google Scholar] [CrossRef]
- Sondergaard, K.; Mina, P.; Piccarolo, S. Wide-range cooling characteristics of a selected isotactic polypropylene. J. Macromol. Sci. Part B Phys. 1997, 36, 733–747. [Google Scholar] [CrossRef]
- Meille, S.V.; Brückner, S. Non-parallel chains in crystalline γ-isotactic polypropylene. Nature 1989, 340, 455–457. [Google Scholar] [CrossRef]
- Meille, S.V.; Bruckner, S.; Porzio, W. γ-isotactic polypropylene—A structure with nonparallel chain axes. Macromolecules 1990, 23, 4114–4121. [Google Scholar] [CrossRef]
- Lotz, B.; Graff, S.; Straupe, C.; Wittmann, J.C. Single crystals of γ-phase isotactic polypropylene—combined diffraction and morphological support for a structure with non-parallel chains. Polymer 1991, 32, 2902–2910. [Google Scholar] [CrossRef]
- Foresta, T.; Piccarolo, S.; Goldbeck-Wood, G. Competition between α and γ phases in isotactic polypropylene: Effects of ethylene content and nucleating agents at different cooling rates. Polymer 2001, 42, 1167–1176. [Google Scholar] [CrossRef]
- Lezak, E.; Bartczak, Z.; Galeski, A. Plastic deformation of the γ phase in isotactic polypropylene in plane-strain compression. Macromolecules 2006, 39, 4811–4819. [Google Scholar] [CrossRef]
- von Baeckmann, C.; Wilhelm, H.; Spieckermann, F.; Strobel, S.; Polt, G.; Sowinski, P.; Piorkowska, E.; Bismarck, A.; Zehetbauer, M. The influence of crystallization conditions on the macromolecular structure and strength of γ-polypropylene. Thermochim. Acta 2019, 677, 131–138. [Google Scholar] [CrossRef]
- Sowinski, P.; Piorkowska, E.; Boyer, S.A.E.; Haudin, J.M.; Zapala, K. The role of nucleating agents in high-pressure induced gamma crystallization in isotactic polypropylene. Colloid. Polym. Sci. 2015, 293, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Sowinski, P.; Piorkowska, E.; Boyer, S.A.E.; Haudin, J.M. Nucleation of crystallization of isotactic polypropylene in the gamma form under high pressure in nonisothermal conditions. Eur. Polym. J. 2016, 85, 564–574. [Google Scholar] [CrossRef]
- Sowinski, P.; Piorkowska, E.; Boyer, S.A.E.; Haudin, J.M. On the structure and nucleation mechanism in nucleated isotactic polypropylene crystallized under high pressure. Polymer 2018, 151, 179–186. [Google Scholar] [CrossRef]
- Kazmierczak, T.; Galeski, A. Transforming of polyethylene crystals by high-pressure annealing. J. Appl. Polym. Sci. 2002, 86, 1337–1350. [Google Scholar] [CrossRef]
- Sowinski, P.; Piorkowska, E.; Boyer, S.A.E.; Haudin, J.M. High-pressure crystallization of iPP nucleated with 1,3:2,4-bis(3,4-dimethylbenzylidene)sorbitol. Polymers 2021, 13, 145. [Google Scholar] [CrossRef]
- Rabiej, M. Application of immune and genetic algorithms to the identification of a polymer based on its X-ray diffraction curve. J. Appl. Crystallogr. 2013, 46, 1136–1144. [Google Scholar] [CrossRef]
- Turner Jones, A.; Aizlewood, J.M.; Beckett, D.R. Crystalline forms of isotactic polypropylene. Makromol. Chem. 1964, 75, 134–158. [Google Scholar] [CrossRef]
- Brown, E.N.; Clausen, B.; Brown, D.W. In situ measurement of crystalline lattice strains in phase IV polytetrafluoroethylene. J. Neutron Res. 2007, 15, 139–146. [Google Scholar] [CrossRef]
- Kristiansen, M.; Werner, M.; Tervoort, T.; Smith, P.; Blomenhofer, M.; Schmidt, H.-W. The binary system isotactic polypropylene/bis(3,4-dimethylbenzylidene)sorbitol: Phase behavior, nucleation, and optical properties. Macromolecules 2003, 36, 5150–5156. [Google Scholar] [CrossRef]
- Mathieu, C.; Thierry, A.; Wittmann, J.C.; Lotz, B. “Multiple” nucleation of the (010) contact face of isotactic polypropylene, alpha phase. Polymer 2000, 41, 7241–7253. [Google Scholar] [CrossRef]
- Yan, S.; Katzenberg, F.; Petermann, J.; Yang, D.; Shen, Y.; Straupe, C.; Wittmann, J.C.; Lotz, B. A novel epitaxy of isotactic polypropylene (alpha phase) on PTFE and organic substrates. Polymer 2000, 41, 2613–2625. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowinski, P.; Veluri, S.; Piorkowska, E. Crystallization of Isotactic Polypropylene Nanocomposites with Fibrillated Poly(tetrafluoroethylene) under Elevated Pressure. Polymers 2022, 14, 88. https://doi.org/10.3390/polym14010088
Sowinski P, Veluri S, Piorkowska E. Crystallization of Isotactic Polypropylene Nanocomposites with Fibrillated Poly(tetrafluoroethylene) under Elevated Pressure. Polymers. 2022; 14(1):88. https://doi.org/10.3390/polym14010088
Chicago/Turabian StyleSowinski, Przemyslaw, Sivanjineyulu Veluri, and Ewa Piorkowska. 2022. "Crystallization of Isotactic Polypropylene Nanocomposites with Fibrillated Poly(tetrafluoroethylene) under Elevated Pressure" Polymers 14, no. 1: 88. https://doi.org/10.3390/polym14010088
APA StyleSowinski, P., Veluri, S., & Piorkowska, E. (2022). Crystallization of Isotactic Polypropylene Nanocomposites with Fibrillated Poly(tetrafluoroethylene) under Elevated Pressure. Polymers, 14(1), 88. https://doi.org/10.3390/polym14010088






