Preparation and Characterization of Calcium Cross-Linked Starch Monolithic Cryogels and Their Application as Cost-Effective Green Filters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
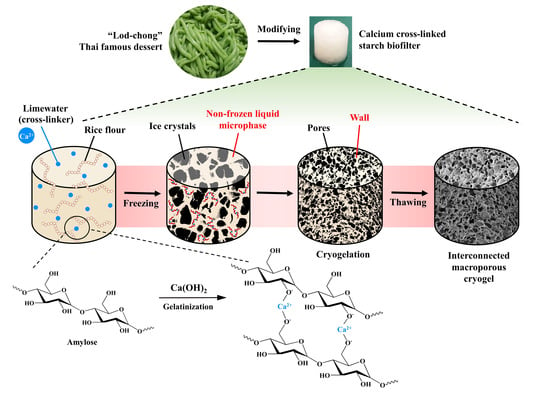
2.2. Preparation of Cryogels
2.3. Characterization of Cryogels
2.4. Swelling Ratio, Water Uptake Capacity, Water Retention, and Porosity of Cryogels
2.5. Application of Cryogels as Biofilters
3. Results and Discussion
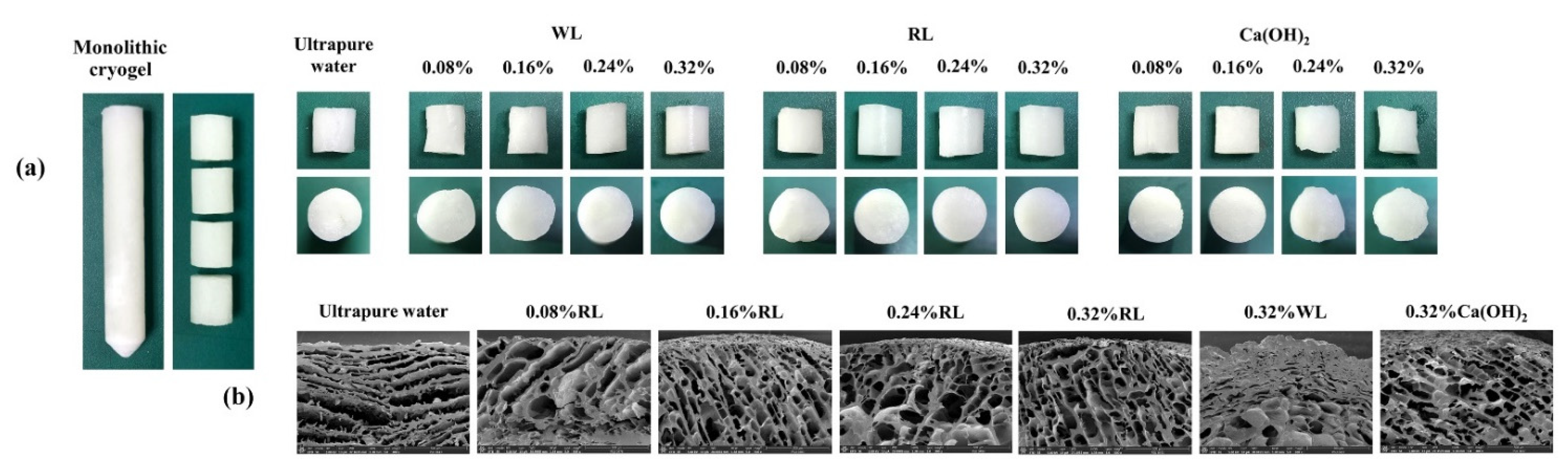
3.1. Preparation of Cryogels
| Type of Cross-Linker | Concentration of Cross-Linker (%w/v) *** | Concentration of Ca2+ (mg/L) * | Recovers from Compression ** | Porosity (%) | Swelling Ratio (Sg/g) |
|---|---|---|---|---|---|
| WL | 0.08 | 117 ± 5 | No | - | - |
| WL | 0.16 | 114 ± 1 | No | - | - |
| WL | 0.24 | 112 ± 0 | No | - | - |
| WL | 0.32 | 119 ± 1 | Yes | 51 ± 4 | 5.9 ± 0.3 |
| RL | 0.08 | 176 ± 0 | No | - | - |
| RL | 0.16 | 145 ± 5 | Yes | 19 ± 2 | 3.3 ± 0.3 |
| RL | 0.24 | 403 ± 12 | Yes | 33 ± 3 | 4.2 ± 0.4 |
| RL | 0.32 | 630 ± 3 | Yes | 52 ± 6 | 6.0 ± 0.3 |
| Ca(OH)2 | 0.08 | 364 ± 5 | Yes | 39 ± 2 | 4.5 ± 0.3 |
| Ca(OH)2 | 0.16 | 419 ± 8 | Yes | 51 ± 2 | 6.4 ± 0.6 |
| Ca(OH)2 | 0.24 | 688 ± 22 | Yes | 32 ± 8 | 4.1 ± 0.6 |
| Ca(OH)2 | 0.32 | 712 ± 8 | Yes | 28 ± 1 | 3.6 ± 0.3 |
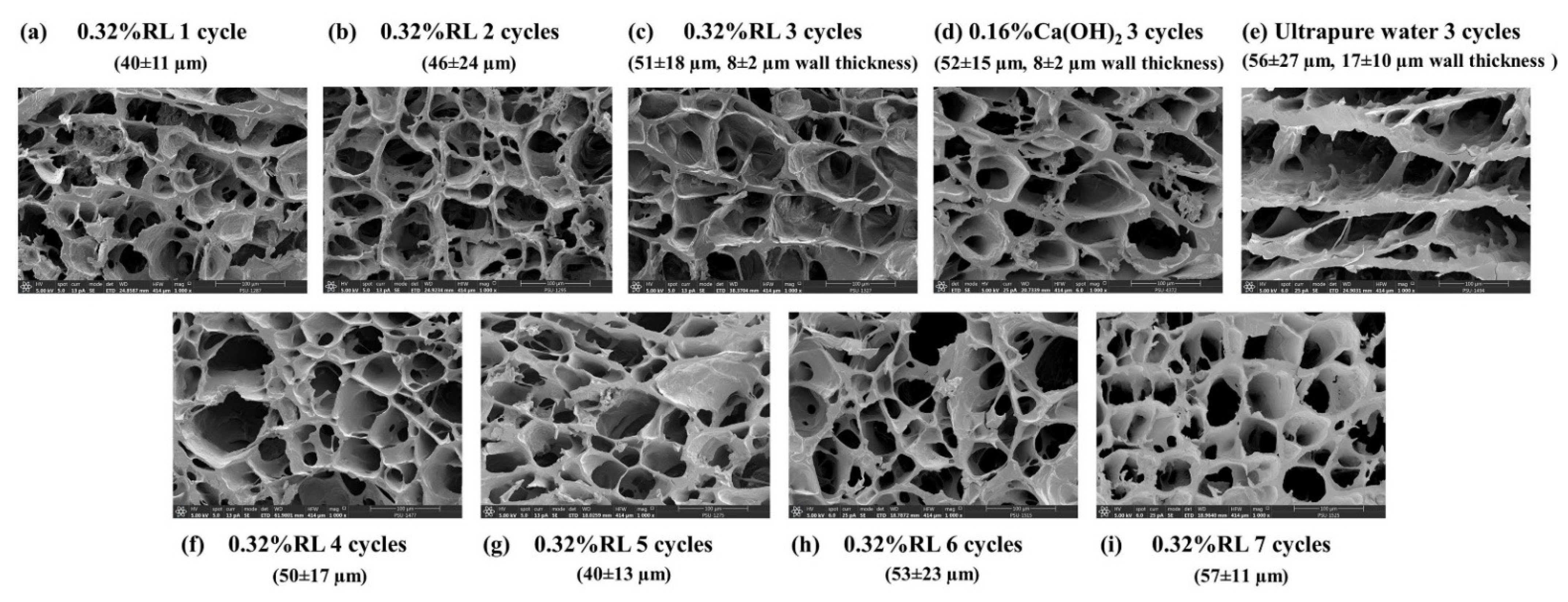
3.2. Characterization of Cryogels
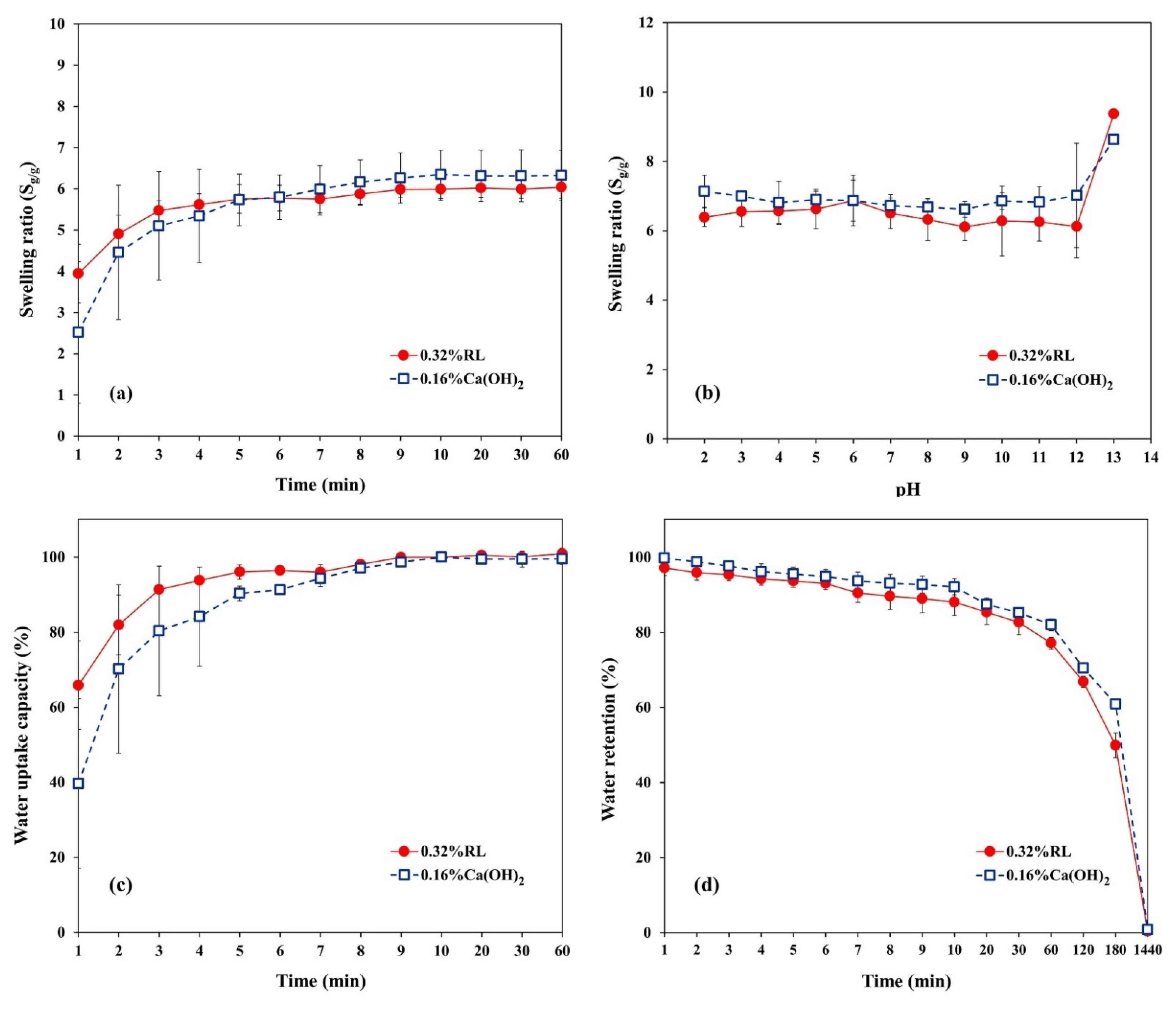
3.3. Water Uptake Capacity, Swelling Capacity, and Water Retention of Cryogels
3.4. Swelling Kinetics of Cryogels
3.5. Application of Cryogels as Filters
3.6. Biodegradation of Cryogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henderson, T.M.A.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682–2695. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, L.J.; Rogers, Z.J.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable cryogels for biomedical applications. Trends Biotechnol. 2020, 38, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Baimenov, A.; Berillo, D.A.; Poulopoulos, S.G.; Inglezakis, V.J. A review of cryogels synthesis, characterization and applications on the removal of heavy metals from aqueous solutions. Adv. Colloid Interface Sci. 2020, 276, 102088. [Google Scholar] [CrossRef] [PubMed]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterisation. Adv. Colloid Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Ertürk, G.; Mattiasson, B. Cryogels-versatile tools in bioseparation. J. Chromatogr. A 2014, 1357, 24–35. [Google Scholar] [CrossRef]
- Dainiak, M.B.; Kumar, A.; Galaev, I.Y.; Mattiasson, B. Detachment of affinity-captured bioparticles by elastic deformation of a macroporous hydrogel. Proc. Natl. Acad. Sci. USA 2006, 103, 849–854. [Google Scholar] [CrossRef] [Green Version]
- Galaev, I.Y.; Dainiak, M.B.; Plieva, F.; Mattiasson, B. Effect of matrix elasticity on affinity binding and release of bioparticles. Elution of bound cells by temperature-induced shrinkage of the smart macroporous hydrogel. Langmuir 2007, 23, 35–40. [Google Scholar] [CrossRef]
- Plieva, F.M.; Karlsson, M.; Aguilar, M.R.; Gomez, D.; Mikhalovsky, S.; Galaev’, I.Y. Pore structure in supermacroporous polyacrylamide based cryogels. Soft Matter 2005, 1, 303–309. [Google Scholar] [CrossRef]
- Nakamatsu, J.; Torres, F.G.; Troncoso, O.P.; Min-Lin, Y.; Boccaccini, A.R. Processing and characterization of porous structures from chitosan and starch for tissue engineering scaffolds. Biomacromolecules 2006, 7, 3345–3355. [Google Scholar] [CrossRef]
- Plieva, F.M.; Galaev, I.Y.; Mattiasson, B. Macroporous gels prepared at subzero temperatures as novel materials for chromatography of particulate-containing fluids and cell culture applications. J. Sep. Sci. 2007, 30, 1657–1671. [Google Scholar] [CrossRef]
- Mattiasson, B.; Kumar, A.; Galaev, I.Y. Macroporous Polymers: Production Properties and Biotechnological/Biomedical Applications. In Macroporous Polymers: Production Properties and Biotechnological/Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Cui, Z. Medical Biotechnology and Healthcare. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Kumar, A. Supermacroporous Cryogels: Biomedical and Biotechnological Applications. In Supermacroporous Cryogels: Biomedical and Biotechnological Applications; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Rac, V.; Lević, S.; Balanč, B.; Graells, B.O.; Bijelic, G. PVA Cryogel as model hydrogel for iontophoretic transdermal drug delivery investigations. Comparison with PAA/PVA and PAA/PVP interpenetrating networks. Colloids Surf. B 2019, 180, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Choodum, A.; Kanatharana, P.; Wongniramaikul, W.; Daéid, N.N. Poly vinyl alcohol cryogel as a selective test kit for pre and post blast trinitrotoluene. Sens. Actuators B Chem. 2016, 222, 654–662. [Google Scholar] [CrossRef]
- Sahiner, N.; Yildiz, S. Preparation of superporous poly(4-vinyl pyridine) cryogel and their templated metal nanoparticle composites for H2 production via hydrolysis reactions. Fuel Process. Technol. 2014, 126, 324–331. [Google Scholar] [CrossRef]
- Erdem, A.; Ngwabebhoh, F.A.; Çetintaş, S.; Bingöl, D.; Yildiz, U. Fabrication and characterization of novel macroporous Jeffamine/diamino hexane cryogels for enhanced Cu(II) metal uptake: Optimization, isotherms, kinetics and thermodynamic studies. Chem. Eng. Res. Des. 2017, 117, 122–138. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. The application of chitosan/collagen/hyaluronic acid sponge cross-linked by dialdehyde starch addition as a matrix for calcium phosphate in situ precipitation. Int. J. Biol. Macromol. 2018, 107, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Liu, F.; Cheng, Q.; Li, H.; Wu, B.; Zhang, G.; Lin, W. Collagen Cryogel Cross-Linked by Dialdehyde Starch. Macromol. Mater. Eng. 2010, 295, 100–107. [Google Scholar] [CrossRef]
- Sionkowska, A.; Michalska-Sionkowska, M.; Walczak, M. Preparation and characterization of collagen/hyaluronic acid/chitosan film crosslinked with dialdehyde starch. Int. J. Biol. Macromol. 2020, 149, 290–295. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Wegrzynowska-Drzymalska, K.; Bajek, A.; Maj, M.; Sionkowska, A. Is dialdehyde starch a valuable cross-linking agent for collagen/elastin based materials? J. Mater. Sci. Mater. Med. 2016, 27, 67. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Wu, B.; Li, C.; Mu, C.; Li, H.; Lin, W. Collagen cryogel cross-linked by naturally derived dialdehyde carboxymethyl cellulose. Carbohydr. Polym. 2015, 129, 17–24. [Google Scholar] [CrossRef]
- Chang, K.H.; Liao, H.T.; Chen, J.P. Preparation and characterization of gelatin/hyaluronic acid cryogels for adipose tissue engineering: In vitro and in vivo studies. Acta Biomater. 2013, 9, 9012–9026. [Google Scholar] [CrossRef] [PubMed]
- Kutlusoy, T.; Oktay, B.; Apohan, N.K.; Süleymanoğlu, M.; Kuruca, S.E. Chitosan-co-Hyaluronic acid porous cryogels and their application in tissue engineering. Int. J. Biol. Macromol. 2017, 103, 366–378. [Google Scholar] [CrossRef]
- Suner, S.S.; Demirci, S.; Yetiskin, B.; Fakhrullin, R.; Naumenko, E.; Okay, O.; Ayyala, R.S.; Sahiner, N. Cryogel composites based on hyaluronic acid and halloysite nanotubes as scaffold for tissue engineering. Int. J. Biol. Macromol. 2019, 130, 627–635. [Google Scholar] [CrossRef]
- Tripathi, A.; Kumar, A. Multi-featured macroporous agarose-alginate cryogel: Synthesis and characterization for bioengineering applications. Macromol. Biosci. 2011, 11, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.; Rudaz, C.; Vasilescu, M.; Budtova, T. Physically and chemically cross-linked cellulose cryogels: Structure, properties and application for controlled release. Carbohydr. Polym. 2016, 151, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhang, B.; Bian, J.; Peng, F.; Sun, R.C. Nanoreinforced hemicellulose-based hydrogels prepared by freeze–thaw treatment. Cellulose 2014, 21, 1709–1721. [Google Scholar] [CrossRef]
- Loghin, D.F.A.; Biliuta, G.; Coseri, S.; Dragan, E.S. Preparation and characterization of oxidized starch/poly(N,N-dimethylaminoethyl methacrylate) semi-IPN cryogels and in vitro controlled release evaluation of indomethacin. Int. J. Biol. Macromol. 2017, 96, 589–599. [Google Scholar] [CrossRef]
- Dragan, E.S.; Loghin, D.F.A. Enhanced sorption of methylene blue from aqueous solutions by semi-IPN composite cryogels with anionically modified potato starch entrapped in PAAm matrix. Chem. Eng. Sci. 2013, 234, 211–222. [Google Scholar] [CrossRef]
- Reis, A.V.; Guilherme, M.R.; Moia, T.A.; Mattoso, L.H.C.; Muniz, E.C.; Tambourgi, E.B. Synthesis and characterization of a starch-modified hydrogel as potential carrier for drug delivery system. J. Polym. Sci. A Polym. Chem. 2008, 46, 2567–2574. [Google Scholar] [CrossRef]
- Junlapong, K.; Maijan, P.; Chaibundit, C.; Chantarak, S. Effective adsorption of methylene blue by biodegradable superabsorbent cassava starch-based hydrogel. Int. J. Biol. Macromol. 2020, 158, 258–264. [Google Scholar] [CrossRef]
- Zou, F.; Budtova, T. Tailoring the morphology and properties of starch aerogels and cryogels via starch source and process parameter. Carbohydr. Polym. 2021, 255, 117344. [Google Scholar] [CrossRef] [PubMed]
- Coria-Hernández, J.; Méndez-Albores, A.; Meléndez-Pérez, R.; Rosas-Mendoza, M.E.; Arjona-Román, J.L. Thermal, Structural, and Rheological Characterization of Waxy Starch as a Cryogel for Its Application in Food Processing. Polymers 2018, 10, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pookidakan, P.; Jangchud, K. Preference Mapping of Pandan Noodles for Thai Lod-Chong. In Proceedings of the 16th Food Innovation Asia Conference 2014: Science and Innovation for Quality of Life, Bangkok, Thailand, 12–13 June 2014; pp. 779–787. [Google Scholar]
- Jayaramudu, T.; Ko, H.U.; Kim, H.C.; Kim, J.W.; Kim, J. Swelling Behavior of Polyacrylamide-Cellulose Nanocrystal Hydrogels: Swelling Kinetics, Temperature, and pH Effects. Materials 2019, 12, 2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, W.; Champ, S.; Huglin, M.B.; Jones, T.G.J. Rapid swelling and deswelling in cryogels of crosslinked poly(N-isopropylacrylamide-co-acrylic acid). Eur. Polym. J. 2004, 40, 467–476. [Google Scholar] [CrossRef]
- Bryant, C.; Hamaker, B. Effect of Lime on Gelatinization of Corn Flour and Starch. Cereal Chem. 1997, 74, 171–175. [Google Scholar] [CrossRef]
- Chanjarujit, W.; Hongsprabhas, P.; Chaiseri, S. Physicochemical properties and flavor retention ability of alkaline calcium hydroxide-mungbean starch film. Carbohydr. Polym. 2018, 198, 473–480. [Google Scholar] [CrossRef]
- Israkarn, K.; Hongsprabhas, P. Effect of CaCl2 on the formation of Ca-induced starch aggregates and spherulitic structure in dried starch film. Dry. Technol. 2017, 35, 1552–1560. [Google Scholar] [CrossRef]
- Cornejo-Villegas, M.Á.; Rincón-Londoño, N.; Real-López, A.D.; Rodríguez-García, M.E. The effect of Ca2+ ions on the pasting, morphological, structural, vibrational, and mechanical properties of corn starch–water system. J. Cereal Sci. 2018, 79, 174–182. [Google Scholar] [CrossRef]
- Chau, M.; De France, K.J.; Kopera, B.; Machado, V.R.; Rosenfeldt, S.; Reyes, L.; Chan, K.J.W.; Förster, S.; Cranston, E.D.; Hoare, T.; et al. Composite Hydrogels with Tunable Anisotropic Morphologies and Mechanical Properties. Chem. Mater. 2016, 28, 3406–3415. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shrivastava, M. Swelling kinetics of a hydrogel of poly(ethylene glycol) and poly(acrylamide-co-styrene). J. Appl. Polym. Sci. 2002, 85, 1419–1428. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Yu, J.; Copeland, L.; Wang, S. Phase transition and swelling behaviour of different starch granules over a wide range of water content. LWT-Food Sci. Technol. 2014, 59, 597–604. [Google Scholar] [CrossRef]
- Lozinsky, V.; Okay, O. Basic Principles of Cryotropic Gelation. Adv. Polym. Sci. 2014, 263, 49–101. [Google Scholar] [CrossRef]
- Baudron, V.; Gurikov, P.; Smirnova, I.; Whitehouse, S. Porous Starch Materials via Supercritical- and Freeze-Drying. Gels 2019, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Xu, T.; Wang, Z.; Peng, X. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of Starch Composition and Molecular Weight on Physicochemical Properties of Biodegradable Films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, X.; Wang, S.; Copeland, L. Changes of multi-scale structure during mimicked DSC heating reveal the nature of starch gelatinization. Sci. Rep. 2016, 6, 28271. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Jiang, T.; Gan, L.; Zhang, X.; Dai, H.; Zhang, X. The plasticizing mechanism and effect of calcium chloride on starch/poly(vinyl alcohol) films. Carbohydr. Polym. 2012, 90, 1677–1684. [Google Scholar] [CrossRef]
- Boonkanon, C.; Phatthanawiwat, K.; Wongniramaikul, W.; Choodum, A. Curcumin nanoparticle doped starch thin film as a green colorimetric sensor for detection of boron. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 224, 117351. [Google Scholar] [CrossRef]
- Wongniramaikul, W.; Limsakul, W.; Choodum, A. A biodegradable colorimetric film for rapid low-cost field determination of formaldehyde contamination by digital image colorimetry. Food Chem. 2018, 249, 154–161. [Google Scholar] [CrossRef]
- Gupta, N.V.; Shivakumar, H.G. Investigation of Swelling Behavior and Mechanical Properties of a pH-Sensitive Superporous Hydrogel Composite. Iran. J. Pharm. Res. 2012, 11, 481–493, PMCID:PMC3832170. [Google Scholar] [PubMed]
- Basavarajappa, S.; Perea-Lowery, L.; Aati, S.; Al-Kheraif, A.A.A.; Ramakrishnaiah, R.; Matinlinna, J.P.; Vallittu, P.K. The effect of ethanol on surface of semi-interpenetrating polymer network (IPN) polymer matrix of glass-fibre reinforced composite. J. Mech. Behav. Biomed. Mater. 2019, 98, 1–10. [Google Scholar] [CrossRef] [PubMed]





| Sample | Type | Absorbance at λMAX (290 nm) | %Reduction | |||
|---|---|---|---|---|---|---|
| before | after 0.32%RL | after 0.16%Ca(OH)2 | 0.32%RL | 0.16%Ca(OH)2 | ||
| 1 | Surface water | 0.192 ± 0.005 | 0.069 ± 0.005 | 0.078 ± 0.008 | 64 ± 3 | 59 ± 5 |
| 2 | Surface water | 0.111 ± 0.006 | 0.004 ± 0.005 | 0.007 ± 0.006 | 96 ± 5 | 94 ± 6 |
| 3 | Surface water | 0.193 ± 0.006 | 0.098 ± 0.003 | 0.100 ± 0.002 | 49 ± 1 | 48 ± 2 |
| 4 | Surface water | 1.148 ± 0.032 | 0.836 ± 0.038 | 0.856 ± 0.021 | 27 ± 4 | 25 ± 3 |
| 5 | Soil extract | 0.886 ± 0.129 | 0.319 ± 0.047 | 0.430 ± 0.064 | 63 ± 10 | 51 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonkanon, C.; Phatthanawiwat, K.; Chuenchom, L.; Lamthornkit, N.; Taweekarn, T.; Wongniramaikul, W.; Choodum, A. Preparation and Characterization of Calcium Cross-Linked Starch Monolithic Cryogels and Their Application as Cost-Effective Green Filters. Polymers 2021, 13, 3975. https://doi.org/10.3390/polym13223975
Boonkanon C, Phatthanawiwat K, Chuenchom L, Lamthornkit N, Taweekarn T, Wongniramaikul W, Choodum A. Preparation and Characterization of Calcium Cross-Linked Starch Monolithic Cryogels and Their Application as Cost-Effective Green Filters. Polymers. 2021; 13(22):3975. https://doi.org/10.3390/polym13223975
Chicago/Turabian StyleBoonkanon, Chanita, Kharittha Phatthanawiwat, Laemthong Chuenchom, Nareumon Lamthornkit, Tarawee Taweekarn, Worawit Wongniramaikul, and Aree Choodum. 2021. "Preparation and Characterization of Calcium Cross-Linked Starch Monolithic Cryogels and Their Application as Cost-Effective Green Filters" Polymers 13, no. 22: 3975. https://doi.org/10.3390/polym13223975
APA StyleBoonkanon, C., Phatthanawiwat, K., Chuenchom, L., Lamthornkit, N., Taweekarn, T., Wongniramaikul, W., & Choodum, A. (2021). Preparation and Characterization of Calcium Cross-Linked Starch Monolithic Cryogels and Their Application as Cost-Effective Green Filters. Polymers, 13(22), 3975. https://doi.org/10.3390/polym13223975