State-of-Art of Standard and Innovative Materials Used in Cranioplasty
Abstract
1. Introduction
2. Short Cranium Anatomy and Cranioplasty Fixation Techniques
3. Discussion on Cranioplasty Materials
3.1. Synthetic Materials
3.2. Bones Substitutes Derived from Biological Sources
3.3. Synthetic Inorganic Bones Substitutes
3.4. Others Synthetic Materials
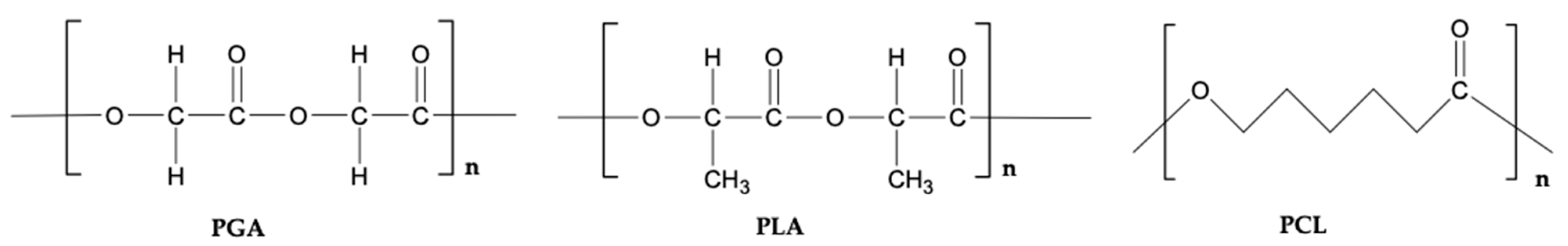
3.5. Synthetic Polymers
3.5.1. Poly(methylmethacrylate) (PMMA)
3.5.2. Polylactic Acid (PLA)
3.5.3. Poly(ε-Caprolactone) (PCL)
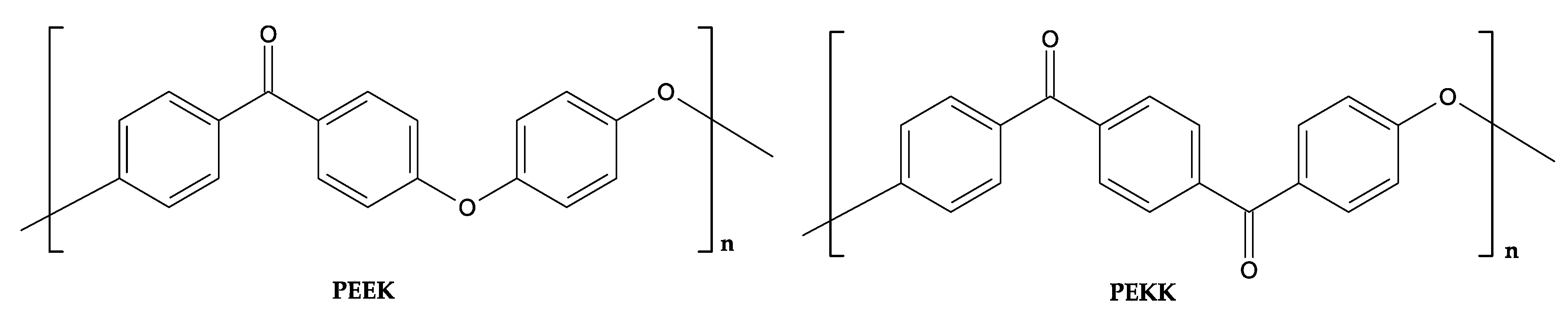
3.5.4. Polyetheretherketone (PEEK)
3.5.5. Polyethylene (PE)
4. Future of Cranioplasty
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, A.M.; Jung, H.; Skirboll, S. Materials used in cranioplasty: A history and analysis. Neurosurg. Focus 2014, 36, E19. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Kucukyuruk, B.; Abuzayed, B.; Aydin, S.; Sanus, G.Z. Cranioplasty: Review of materials and techniques. J. Neurosci. Rural. Pr. 2011, 2, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Feroze, A.H.; Walmsley, G.G.; Choudhri, O.; Lorenz, H.P.; Grant, G.A.; Edwards, M.S.B. Evolution of cranioplasty tech-niques in neurosurgery: Historical review, pediatric considerations, and current trends. J. Neurosurg. 2015, 123, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.A.; Jolley, M.; Ellenbogen, R.G.; Roberts, T.S.; Gruss, J.R.; Loeser, J.D. Failure of autologous bone-assisted cranio-plasty following decompressive craniectomy in children and adolescents. J. Neurosurg. 2004, 100, 163–168. [Google Scholar]
- Alkhaibary, A.; Alharbi, A.; Alnefaie, N.; Almubarak, A.O.; Aloraidi, A.; Khairy, S. Cranioplasty: A Comprehensive Re-view of the History, Materials, Surgical Aspects, and Complications. World Neuros. 2020, 139, 445–452. [Google Scholar] [CrossRef]
- Zanotti, B.; Zingaretti, N.; Verlicchi, A.; Robiony, M.; Alfieri, A.; Parodi, P.C. Cranioplasty: Review of Materials. J. Craniofac. Surg. 2016, 27, 2061–2072. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W.J.; Donovan, J.M.; Pensler, J.M. Cranial Bone Grafting in Children. Plast. Reconstr. Surg. 1995, 95, 1–4. [Google Scholar] [CrossRef]
- Bowers, C.A.; Riva-Cambrin, J.; Hertzler, D.A., 2nd; Walker, M.L. Risk factors and rates of bone flap resorption in pediatric patients after decompressive craniectomy for traumatic brain injury. J. Neurosurg. Pediatr. 2013, 11, 526–532. [Google Scholar] [CrossRef]
- Matsuno, A.; Tanaka, H.; Iwamuro, H.; Takanashi, S.; Miyawaki, S.; Nakashima, M.; Nakaguchi, H.; Nagashima, T. Analyses of the factors influencing bone graft infection after delayed cranioplasty. Acta Neurochir. 2006, 148, 535–540. [Google Scholar] [CrossRef]
- Morselli, C.; Zaed, I.; Tropeano, M.P.; Cataletti, G.; Iaccarino, C.; Rossini, Z.; Servadei, F. Comparison between the different types of heterologous materials used in cranioplasty: A systematic review of the literature. J. Neurosurg. Sci. 2020, 63, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Haufe, J.; Patel, M.K. Product Overview and Market Projection of Emerging Biobased Plastics. Report No: NWS-E-2009-32; University of Utrecht: Utrecht, The Netherlands, 2009; pp. 1–243. [Google Scholar]
- Ebnesajjad, S. Handbook of Biopolymers and Biodegradable Plastics: Properties, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Mülhaupt, R. Green Polymer Chemistry and Bio-based Plastics: Dreams and Reality. Macromol. Chem. Phys. 2012, 214, 159–174. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- European Bioplastics—Report Bioplastics. Available online: https://docs.europeanbioplastics.org/publications/market_data/Report_Bioplastics_Market_Data_2019.pdf (accessed on 3 April 2021).
- Khader, B.A.; Towler, M.R. Materials and techniques used in cranioplasty fixation: A review. Mater. Sci. Eng. C 2016, 66, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Piazza, M.; Grady, M.S. Cranioplasty. Neurosurg. Clin. 2017, 28, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Manjila, S.; Selman, W.R.; Dean, D. The Recent Revolution in the Design and Manufacture of Cranial Implants: Modern Advancements and Future Directions. Neurosurgery 2015, 77, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Spetzger, U.; Vougioukas, V.; Schipper, J. Materials and techniques for osseous skull reconstruction. Minim. Invasive Ther. Allied Technol. 2010, 19, 110–121. [Google Scholar] [CrossRef]
- Antonelli, V.; Maimone, G.; D’Andrea, M.; Tomassini, A.; Bassi, M.; Tosatto, L. “Single-step” resection and cranio-orbital reconstruction for spheno-orbital metastasis with custom made implant. A case report and review of the literature. Int. J. Surg. Case Rep. 2021, 81, 105755. [Google Scholar] [CrossRef]
- Vougioukas, V.I.; Hubbe, U.; Van Velthoven, V.; Freiman, T.M.; Schramm, A.; Spetzger, U. Neuronavigation-assisted Cranial Reconstruction. Neurosurgery 2004, 55, 162–167. [Google Scholar] [CrossRef]
- Tel, A.; Costa, F.; Sembronino, S.; Lazzarotto, A.; Robiony, M. All-in-one surgical guide: A new method for cranial vault re-section and reconstruction. J. Craniomaxillofac. Surg. 2018, 46, 967–973. [Google Scholar] [CrossRef]
- Guerrini, F.; Dallolio, V.; Grimod, G.; Cesana, C.; Vismara, D.; Franzin, A.B. It Is Time to Reduce Free-Hand Manipulation: Case Report of Our Proposal for an Innovative 1-Step Cranioplasty. World Neurosurg. 2017, 107, 1052.e7–1052.e10. [Google Scholar] [CrossRef] [PubMed]
- De Grado, G.F.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 1–18. [Google Scholar] [CrossRef]
- Albanese, A.; E Licata, M.; Polizzi, B.; Campisi, G. Platelet-rich plasma (PRP) in dental and oral surgery: From the wound healing to bone regeneration. Immun. Ageing 2013, 10, 1–23. [Google Scholar] [CrossRef]
- Arenaz-Búa, J.; Luaces-Rey, R.; Sironvalle-Soliva, S.; Otero-Rico, A.; Charro-Huerga, E.; Patino-Seijas, B.; Garcia-Rozado, A.; Ferreras-Granados, J.; Vazquez-Mahia, I.; Lorenzo-Franco, F.; et al. A comparative study of platelet-rich plasma, hydroxyapatite, demineralized bone matrix and autologous bone to promote bone regenera-tion after mandibular impacted third molar extraction. Med. Oral Patol. Oral Cir. Bucal. 2010, 15, 483–489. [Google Scholar] [CrossRef]
- Tressler, M.A.; Richards, J.E.; Sofianos, D.; Comrie, F.K.; Kregor, P.J.; Obremskey, W.T. Bone Morphogenetic Protein-2 Compared to Autologous Iliac Crest Bone Graft in the Treatment of Long Bone Nonunion. Orthopedics 2011, 34, e877–884. [Google Scholar] [CrossRef]
- Kwarcinski, J.; Boughton, P.; Ruys, A.; Doolan, A.; Van Gelder, J. Cranioplasty and Craniofacial Reconstruction: A Review of Implant Material, Manufacturing Method and Infection Risk. Appl. Sci. 2017, 7, 276. [Google Scholar] [CrossRef]
- Khan, S.N.; Tomin, E.; Lane, J.M. Clinical applications of bone graft substitutes. Orthop. Clin. N. Am. 2000, 31, 389–398. [Google Scholar] [CrossRef]
- Afifi, A.M.; Gordon, C.R.; Pryor, L.S.; Sweeney, W.; Papay, F.A.; Zins, J.E. Calcium phosphate cements in skull recon-struction: A meta-analysis. Plast. Reconstr. Surg. 2010, 126, 1300–1309. [Google Scholar] [CrossRef]
- Gunzburg, R.; Szpalski, M.; Passuti, N.; Aebi, M. The Use of Bone Substitutes in Spine Surgery: A State-of-the-Art Review; Springer: Berlin/Heidelberg, Germany, 2002; p. 129. ISBN 13:9783540426875. [Google Scholar]
- Hench, L.L.; Wilson, J. Surface-active biomaterials. Science 1984, 226, 630–636. [Google Scholar] [CrossRef]
- Warren, S.M.; Fong, K.D.; Nacamuli, R.P.; Song, H.M.; Fang, T.D.; Longaker, M.T. Biomaterials for skin and bone re-placement and repair in Plastic Surgery. Operat. Tech. Plastic Reconstr. Surg. 2003, 9, 10–15. [Google Scholar] [CrossRef]
- Eppley, B.L. Alloplastic cranioplasty. Oper. Tech. Plast. Reconstr. Surg. 2002, 9, 16–22. [Google Scholar] [CrossRef]
- Nguyen, B.; Ashraf, O.; Richards, R.; Tra, H.; Huynh, T. Cranioplasty Using Customized 3-Dimensional–Printed Titanium Implants: An International Collaboration Effort to Improve Neurosurgical Care. World Neurosurg. 2021, 149, 174–180. [Google Scholar] [CrossRef]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Enhanced osseointegration of titanium implants with nanostructured surfaces: An experimental study in rabbits. Acta Biomater. 2015, 11, 494–502. [Google Scholar] [CrossRef]
- Gosain, A.K. Biomaterials in facial reconstruction. Oper. Tech. Plast. Reconstr. Surg. 2002, 9, 23–30. [Google Scholar] [CrossRef]
- Eppley, B.L. Use of Resorbable Plate and Screw Fixation in Pediatric Craniofacial Surgery. Operat. Tech. Plastic Reconstr. Surg. 2003, 9, 36–45. [Google Scholar] [CrossRef]
- Gosain, A.K.; Song, L.; Corrao, M.A.; Pintar, F.A. Biomechanical Evaluation of Titanium, Biodegradable Plate and Screw, and Cyanoacrylate Glue Fixation Systems in Craniofacial Surgery. Plast. Reconstr. Surg. 1998, 101, 582–591. [Google Scholar] [CrossRef]
- Charnley, J. Total hip replacement by low-friction arthroplasty. Clin. Orthop. Relat. Res. 1970, 72, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Galicich, J.H.; Hovind, K.H. Stainless Steel Mesh-Acrylic Cranioplasty. J. Neurosurg. 1967, 27, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L. Biomechanical Testing of Alloplastic PMMA Cranioplasty Materials. J. Craniofacial Surg. 2005, 16, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Sanus, G.Z.; Tanriverdi, T.; Ulu, M.O.; Kafadar, A.M.; Tanriover, N.; Ozlen, F. Use of CortossTM as an alternative material in calvarial defects: The first clinical results in cranioplasty. The First Clinical Results in Cranioplasty. J. Craniofacial Surg. 2008, 19, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Asghar, M.; Din, S.U.; Zafar, M.S. Thermoset polymethacrylate-based materials for dental applications. Mater. Biomed. Eng. 2019, 273–308. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Peltoniemi, H.; Waris, E.; Suuronen, R.; Serlo, W.; Kellomäki, M.; Törmälä, P.; Waris, T. Developments in Craniomaxillofacial Surgery: Use of Self-Reinforced Bioabsorbable Osteofixation Devices. Plast. Reconstr. Surg. 2001, 108, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Leenslag, J.W.; Pennings, A.J.; Bos, R.R.; Rozema, F.R.; Boering, G. Resorbable materials of poly(l-lactide). VI. Plates and screws for internal fracture fixation. Biomaterials 1987, 8, 70–73. [Google Scholar] [CrossRef]
- Habal, M. Bioresorbable Skeletal Fixation Systems in Craniofacial Surgery. Biomater. Orthop. 2003, 9, 31–35. [Google Scholar] [CrossRef]
- Roberts, J.C.; Merkle, A.C.; Carneal, C.M.; Voo, L.M.; Johannes, M.S.; Paulson, J.M.; Tankard, S.; Uy, O.M. Development of a Human Cranial Bone Surrogate for Impact Studies. Front. Bioeng. Biotechnol. 2013, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Apriawan, T.; Permana, K.R.; Darlan, D.; Arifianto, M.R.; Fitra, F.; Alfauzi, A.; Bajamal, A.H. Polylactic acid implant for cranioplasty with 3-dimesional printing customization: A case report. Maced. J. Med. Sci. 2020, 8, 151–155. [Google Scholar] [CrossRef]
- English, J.; Perrin, D.; Wiseman, D.; Kost, J.; Domb, A. Polycaprolactone. In Handbook of Biodegradable Polymers; Apple Academic Press: Cambridge, MA, USA, 1998; pp. 63–77. [Google Scholar]
- Vandamme, T.; Legras, R. Physico-mechanical properties of poly(ε-caprolactone) for the construction of rumino-reticulum devices for grazing animals. Biomaterials 1995, 16, 1395–1400. [Google Scholar] [CrossRef]
- Chim, H.; Schantz, J.-T. New Frontiers in Calvarial Reconstruction: Integrating Computer-Assisted Design and Tissue Engineering in Cranioplasty. Plast. Reconstr. Surg. 2005, 116, 1726–1741. [Google Scholar] [CrossRef]
- De Santis, R.; Russo, T.; Rau, J.V.; Papallo, I.; Martorelli, M.; Gloria, A. Design of 3D Additively Manufactured Hybrid Structures for Cranioplasty. Materials 2021, 14, 181. [Google Scholar] [CrossRef]
- Teoh, S.H.; Goh, B.T.; Lim, J. Three-Dimensional Printed Polycaprolactone Scaffolds for Bone Regeneration Success and Future Perspective. Tissue Eng. Part A 2019, 25, 931–935. [Google Scholar] [CrossRef]
- Shi, R.; Xue, J.; Wang, H.; Wang, R.; Gong, M.; Chen, D.; Zhang, L.; Tian, W. Fabrication and evaluation of a homogeneous electrospun PCL–gelatin hybrid membrane as an anti-adhesion barrier for craniectomy. J. Mater. Chem. B 2015, 3, 4063–4073. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, W.; Chen, J.; Yu, J.; Zhang, J.; Chen, J. The application of polyetheretherketone (PEEK) implants in cranioplasty. Brain Res. Bull. 2019, 153, 143–149. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef]
- De Barros, A.; Brauge, D.; Quehan, R.; Cavallier, Z.; Roux, F.E.; Moyse, E. One-step customized peek cranioplasty after 3D printed resection template assisted surgery for a frontal intraosseous meningioma: A case report. Turk. Neurosurg. 2020, 31, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Alqurashi, H.; Khurshid, Z.; Syed, A.U.Y.; Habib, S.R.; Rokaya, D.; Zafar, M.S. Polyetherketoneketone (PEKK): An emerging biomaterial for oral implants and dental prostheses. J. Adv. Res. 2021, 28, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Abuzayed, B.; Tuzgen, S.; Canbaz, B.; Yuksel, O.; Tutunculer, B.; Sanus, G.Z. Reconstruction of Growing Skull Fracture within Situ Galeal Graft Duraplasty and Porous Polyethylene Sheet. J. Craniofacial Surg. 2009, 20, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Kucukyuruk, B.; Biceroglu, H.; Abuzayed, B.; Ulu, M.O.; Sanus, G.Z. Intraosseous meningioma: A rare tumor reconstructed with porous polyethylene. J. Craniofacial Surg. 2010, 21, 936–939. [Google Scholar] [CrossRef]
- Liu, J.K.; Gottfried, O.N.; Cole, C.D.; Dougherty, W.R.; Couldwell, W.T. Porous polyethylene implant for cranioplasty and skull base reconstruction. Neurosurg. Focus 2004, 16, 1–5. [Google Scholar] [CrossRef]
- Janecka, I.P. New reconstructive technologies in skull base surgery: Role of titanium mesh and porous polyethylene. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 396–401. [Google Scholar] [CrossRef]
- Ridwan-Pramana, A.; Wolff, J.; Raziei, A.; Ashton-James, C.E.; Forouzanfar, T. Porous polyethylene implants in facial reconstruction: Outcome and complications. J. Cranio-Maxillofac. Surg. 2015, 43, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-C.; Wei, L.; Xu, J.; Liu, J.-F.; Gui, L. Clinical Outcome of Cranioplasty With High-Density Porous Polyethylene. J. Craniofacial Surg. 2012, 23, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- Thien, A.; King, N.K.; Ang, B.T.; Wang, E.; Ng, I. Comparison of Polyetheretherketone and Titanium Cranioplasty after Decompressive Craniectomy. World Neurosurg. 2015, 83, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M. UHMWPE Biomaterials Handbook: Ultra High Molecular Weight Polyethylene in Total Joint Replacement and Medical Devices; Academic Press: Cambridge, MA, USA, 2009; ISBN 9780123747211. [Google Scholar]
- Karageorgiou, V.; Kaplan, D.L. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.T.W.; Ling, J.M.; Dinesh, S.K. The feasibility of producing patient-specific acrylic cranioplasty implants with a low-cost 3D printer. J. Neurosurg. 2016, 124, 1531–1537. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.; Antonelli, V.; Tomassini, A.; Maimone, G.; D’Andrea, M.; Campobassi, A.; Gessaroli, M.; Tosatto, L. Synchronized “One-Step” Resection and Cranio-Orbital Reconstruction for Spheno-Orbital Lesions with Custom Made Implant. J. Craniofacial Surg. 2021, 81. [Google Scholar] [CrossRef]
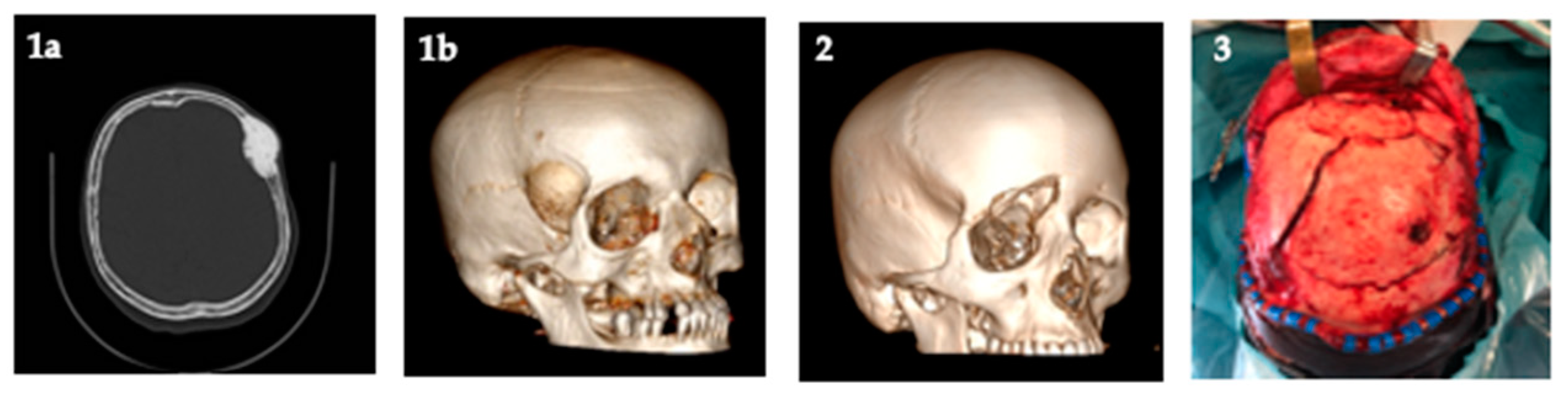

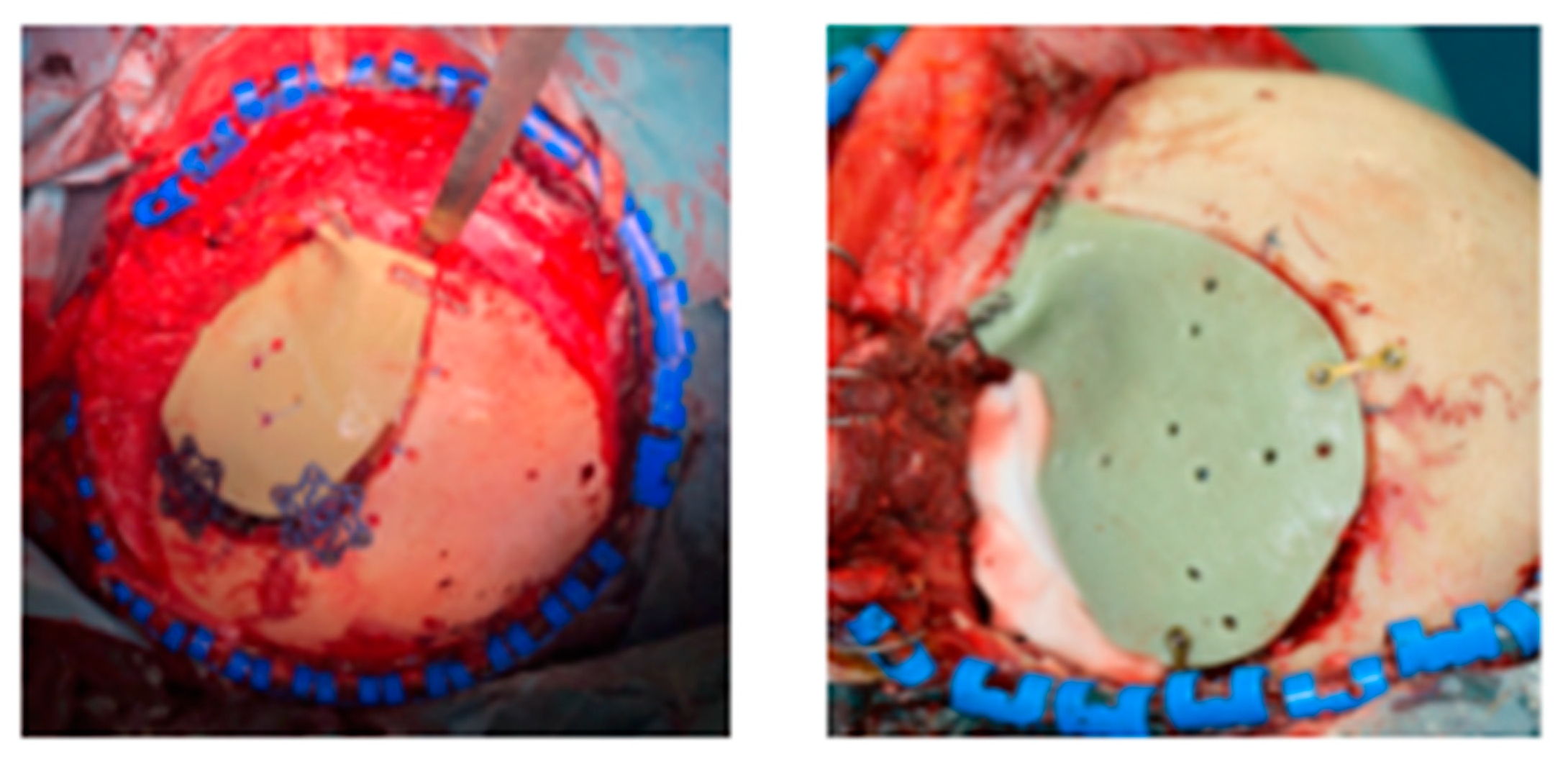
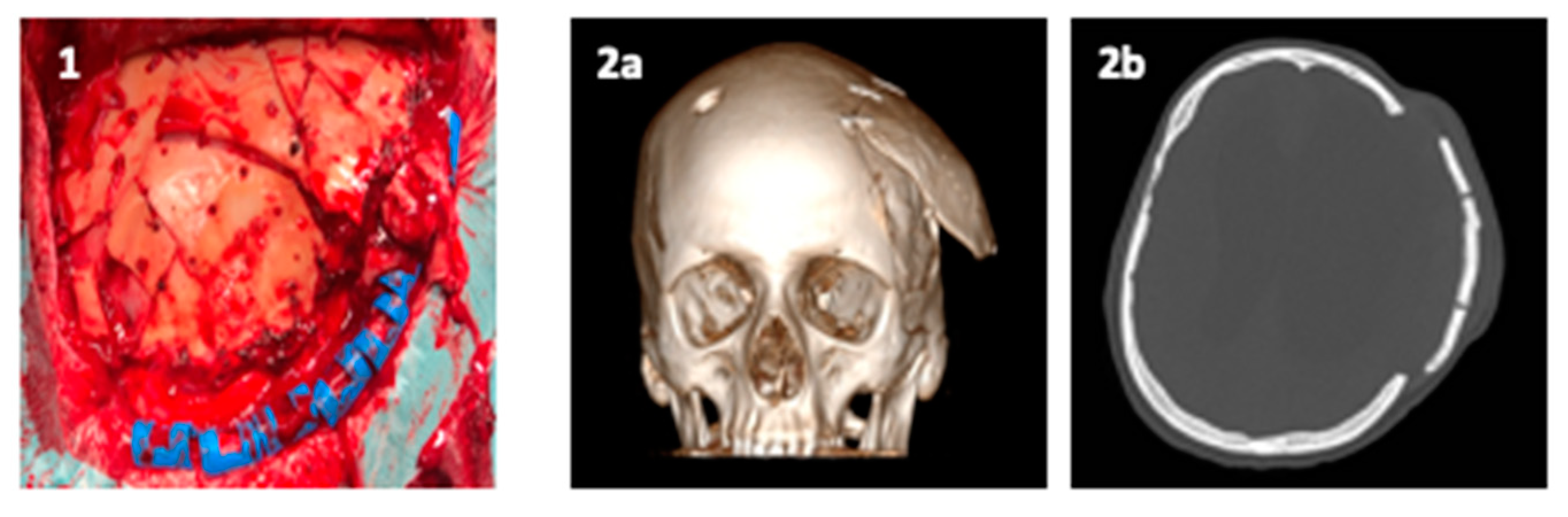

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siracusa, V.; Maimone, G.; Antonelli, V. State-of-Art of Standard and Innovative Materials Used in Cranioplasty. Polymers 2021, 13, 1452. https://doi.org/10.3390/polym13091452
Siracusa V, Maimone G, Antonelli V. State-of-Art of Standard and Innovative Materials Used in Cranioplasty. Polymers. 2021; 13(9):1452. https://doi.org/10.3390/polym13091452
Chicago/Turabian StyleSiracusa, Valentina, Giuseppe Maimone, and Vincenzo Antonelli. 2021. "State-of-Art of Standard and Innovative Materials Used in Cranioplasty" Polymers 13, no. 9: 1452. https://doi.org/10.3390/polym13091452
APA StyleSiracusa, V., Maimone, G., & Antonelli, V. (2021). State-of-Art of Standard and Innovative Materials Used in Cranioplasty. Polymers, 13(9), 1452. https://doi.org/10.3390/polym13091452







