Solution Crystallization of Polycarbonate Surfaces for Hydrophobic State: Water Droplet Dynamics and Life Cycle Assessment towards Self-Cleaning Applications
Abstract
1. Introduction
2. Experimental and Life Cycle Model
2.1. Experimental
2.2. Life Cycle Model
3. Results and Discussion
3.1. Surface Crystallization and Texture Topology
3.2. Hydrophobic Characteristics of Crystallized Surface and Water Droplet Mobility
3.3. Improvement of Optical Transmittance of Crystallized Surfaces
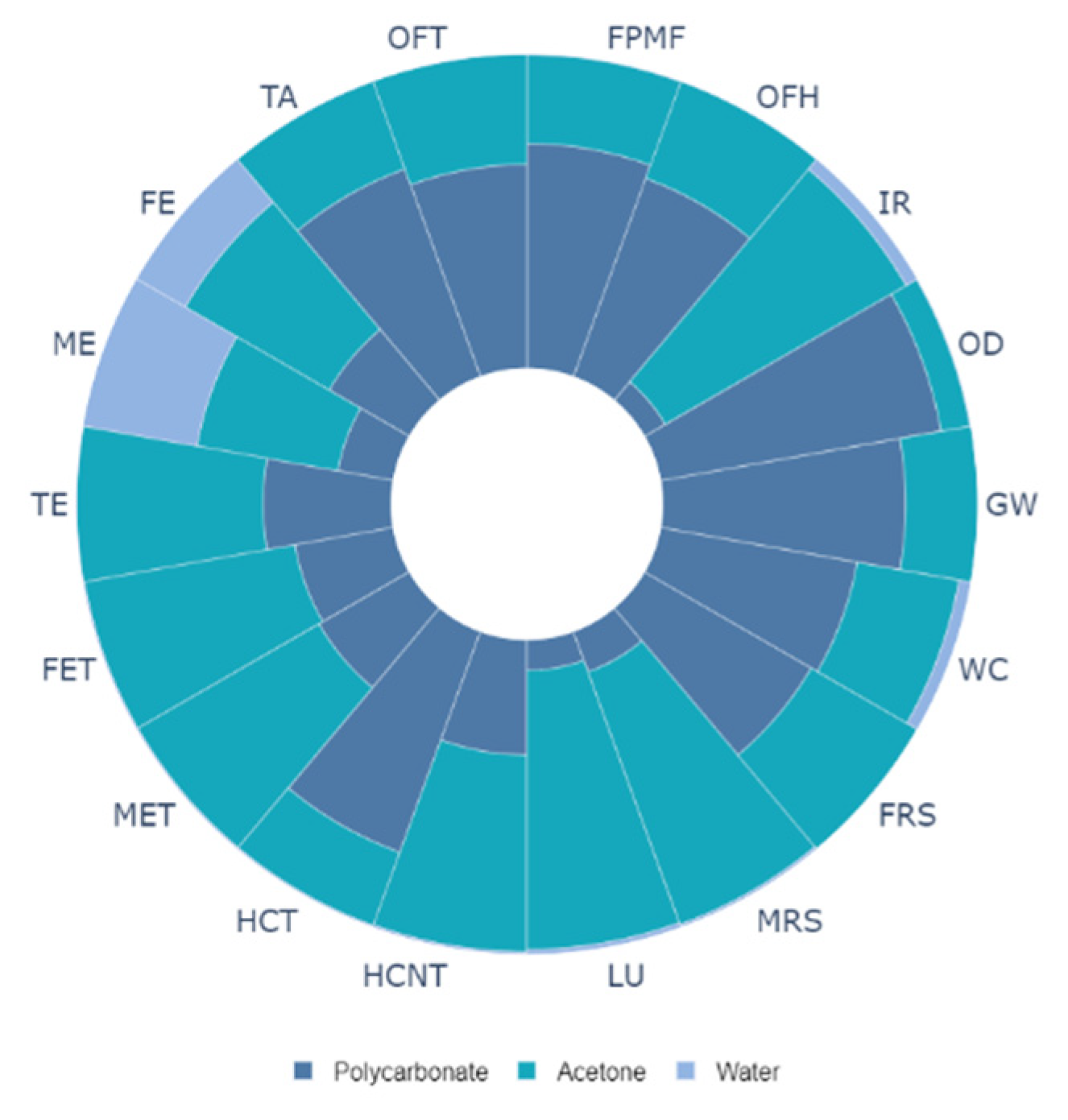
3.4. Life Cycle Analysis for Solution Crystallization of Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Hemoud, A.; Al-Dousari, A.; Al-Shatti, A.; Al-Khayat, A.; Behbehani, W.; Malak, M. Health Impact Assessment Associated with Exposure to PM10 and Dust Storms in Kuwait. Atmosphere 2018, 9, 6. [Google Scholar] [CrossRef]
- Sada, K. Functional polymers in nonpolar solvents induced by dissociation of macromolecular complexes. Polym. J. 2018, 50, 285–299. [Google Scholar] [CrossRef]
- Gumede, T.P.; Luyt, A.S.; Pérez-Camargo, R.A.; Tercjak, A.; Müller, A.J. Morphology, nucleation, and isothermal crystallization kinetics of poly (butylene succinate) mixed with a polycarbonate/MWCNT masterbatch. Polymers 2018, 10, 424. [Google Scholar] [CrossRef]
- Lu, J.; Huang, R. Selective Etching of Pressure-Crystallized Bisphenol-A Polycarbonate by Dimethylacetamide at Room Temperature. In Proceedings of the Advanced Materials Research. Adv. Mater. Res. 2012, 430, 66–69. [Google Scholar] [CrossRef]
- Trantidou, T.; Elani, Y.; Parsons, E.; Ces, O. Hydrophilic surface modification of PDMS for droplet microfluidics using a simple, quick, and robust method via PVA deposition. Microsyst. Nanoeng. 2017, 3, 16091. [Google Scholar] [CrossRef]
- Ogończyk, D.; Jankowski, P.; Garstecki, P. A Method for Simultaneous Polishing and Hydrophobization of Polycarbonate for Microfluidic Applications. Polymers 2020, 12, 2490. [Google Scholar] [CrossRef]
- Yao, W.; Li, O.L.; Kang, Y.-J.; Jeong, M.-Y.; Cho, Y.-R. Impact of pillar configuration on the amphiphobicity of micro-patterned polymer surface. Vacuum 2018, 156, 115–122. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Z.; Ge, T.; Hu, J.; Wang, J.; Yang, L. Self-assembly and in vitro drug release behaviors of amphiphilic copolymers based on functionalized aliphatic liquid crystalline polycarbonate with pH/temperature dual response. J. Mol. Liq. 2020, 316, 113837. [Google Scholar] [CrossRef]
- Zhou, Y.; Dan, Y.; Jiang, L.; Li, G. The effect of crystallization on hydrolytic stability of polycarbonate. Polym. Degrad. Stab. 2013, 98, 1465–1472. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Khaled, M.; Abu-Dheir, N.; Al-Aqeeli, N.; Said, S.A.M.; Ahmed, A.O.M.; Varanasi, K.K.; Toumi, Y.K. Wetting and other physical characteristics of polycarbonate surface textured using laser ablation. Appl. Surf. Sci. 2014, 320, 21–29. [Google Scholar] [CrossRef]
- Alamri, S.; Aguilar-Morales, A.I.; Lasagni, A.F. Controlling the wettability of polycarbonate substrates by producing hierarchical structures using Direct Laser Interference Patterning. Eur. Polym. J. 2018, 99, 27–37. [Google Scholar] [CrossRef]
- Li, P.; Zeng, Y.; Yan, H.; Chen, J. Investigation on stable superhydrophobic polycarbonate based compound induced by KrF excimer laser irradiation. Appl. Surf. Sci. 2018, 457, 1110–1115. [Google Scholar] [CrossRef]
- Rosselot, K.; Allen, D.T. Life-cycle concepts, product stewardship and green engineering. In Green Engineering: Environmentally Conscious Design of Chemical Processes; Prentice-Hall PTR: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Cooper, T. Slower consumption reflections on product life spans and the “throwaway society”. J. Ind. Ecol. 2005, 9, 51–67. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Ali, H.; Al-Aqeeli, N.; Khaled, M.; Abu-Dheir, N.; Varanasi, K.K. Solvent-induced crystallization of a polycarbonate surface and texture copying by polydimethylsiloxane for improved surface hydrophobicity. J. Appl. Polym. Sci. 2016, 133, 43467–43479. [Google Scholar] [CrossRef]
- Heib, F.; Schmitt, M. Statistical Contact Angle Analyses with the High-Precision Drop Shape Analysis (HPDSA) Approach: Basic Principles and Applications. Coatings 2016, 6, 57. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Hassan, G.; Al-Sharafi, A.; Ali, H.; Al-Aqeeli, N.; Al-Sarkhi, A. Water Droplet Dynamics on a Hydrophobic Surface in Relation to the Self-Cleaning of Environmental Dust. Sci. Rep. 2018, 8, 2984. [Google Scholar] [CrossRef]
- ASTM D7027-05 Standard Test Method for Evaluation of Scratch Resistance of Polymeric Coatings and Plastics Using an Instrumented Scratch Machine; ASTM International: West Conshohocken, PA, USA, 2020.
- Simonen, K. Life Cycle Assessment; Routledge: London, UK, 2014; ISBN 1317697367. [Google Scholar]
- SimaPro LCA Software LCA Software for Fact-Based Sustainability. Available online: https://simapro.com/ (accessed on 20 March 2021).
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: A harmonised life cycle impact assessment method at midpoint and endpoint level. Int. J. Life Cycle Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A.; Abe, A.; Bloch, D.R. Polymer Handbook; Wiley: New York, NY, USA, 1999; Volume 89. [Google Scholar]
- Dayal, P.; Guenthner, A.J.; Kyu, T. Morphology development of main-chain liquid crystalline polymer fibers during solvent evaporation. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 429–435. [Google Scholar] [CrossRef]
- Lauritzen, J.I., Jr.; Hoffman, J.D. Theory of formation of polymer crystals with folded chains in dilute solution. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1960, 64, 73. [Google Scholar] [CrossRef]
- Frank, F.C.; Tosi, M. On the theory of polymer crystallization. Proc. R. Soc. London. Ser. A. Math. Phys. Sci. 1961, 263, 323–339. [Google Scholar]
- Fan, Z.; Shu, C.; Yu, Y.; Zaporojtchenko, V.; Faupel, F. Vapor-induced crystallization behavior of bisphenol-A polycarbonate. Polym. Eng. Sci. 2006, 46, 729–734. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C.; Morril, T.C. Spectroscopic Identification of Organic Compounds4; John Wiley and Sons Inc.: New York, NY, USA, 1981. [Google Scholar]
- Nakajima, A. Design of hydrophobic surfaces for liquid droplet control. NPG Asia Mater. 2011, 3, 49–56. [Google Scholar] [CrossRef]
- Koti Reddy, C.; Shailaja, D. Improving hydrophobicity of polyurethane by PTFE incorporation. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Al-Sharafi, A.; Ali, H.; Al-Aqeeli, N. Dynamics of a water droplet on a hydrophobic inclined surface: Influence of droplet size and surface inclination angle on droplet rolling. RSC Adv. 2017, 7, 48806–48818. [Google Scholar] [CrossRef]
- Aussillous, P.; Quéré, D. Shapes of rolling liquid drops. J. Fluid Mech. 2004, 512, 133. [Google Scholar] [CrossRef]
- Kim, D.; Pugno, N.M.; Ryu, S. Wetting theory for small droplets on textured solid surfaces. Sci. Rep. 2016, 6, 37813. [Google Scholar] [CrossRef]
- Ricci, E.; Sangiorgi, R.; Passerone, A. Density and surface tension of dioctylphthalate, silicone oil and their solutions. Surf. Coat. Technol. 1986, 28, 215–223. [Google Scholar] [CrossRef]
- Smith, J.D.; Dhiman, R.; Anand, S.; Reza-Garduno, E.; Cohen, R.E.; McKinley, G.H.; Varanasi, K.K. Droplet mobility on lubricant-impregnated surfaces. Soft Matter 2013, 9, 1772–1780. [Google Scholar] [CrossRef]
- National Institute for Public Health and the Environment LCIA: The ReCiPe Model. Available online: https://www.rivm.nl/en/life-cycle-assessment-lca/recipe (accessed on 20 March 2021).
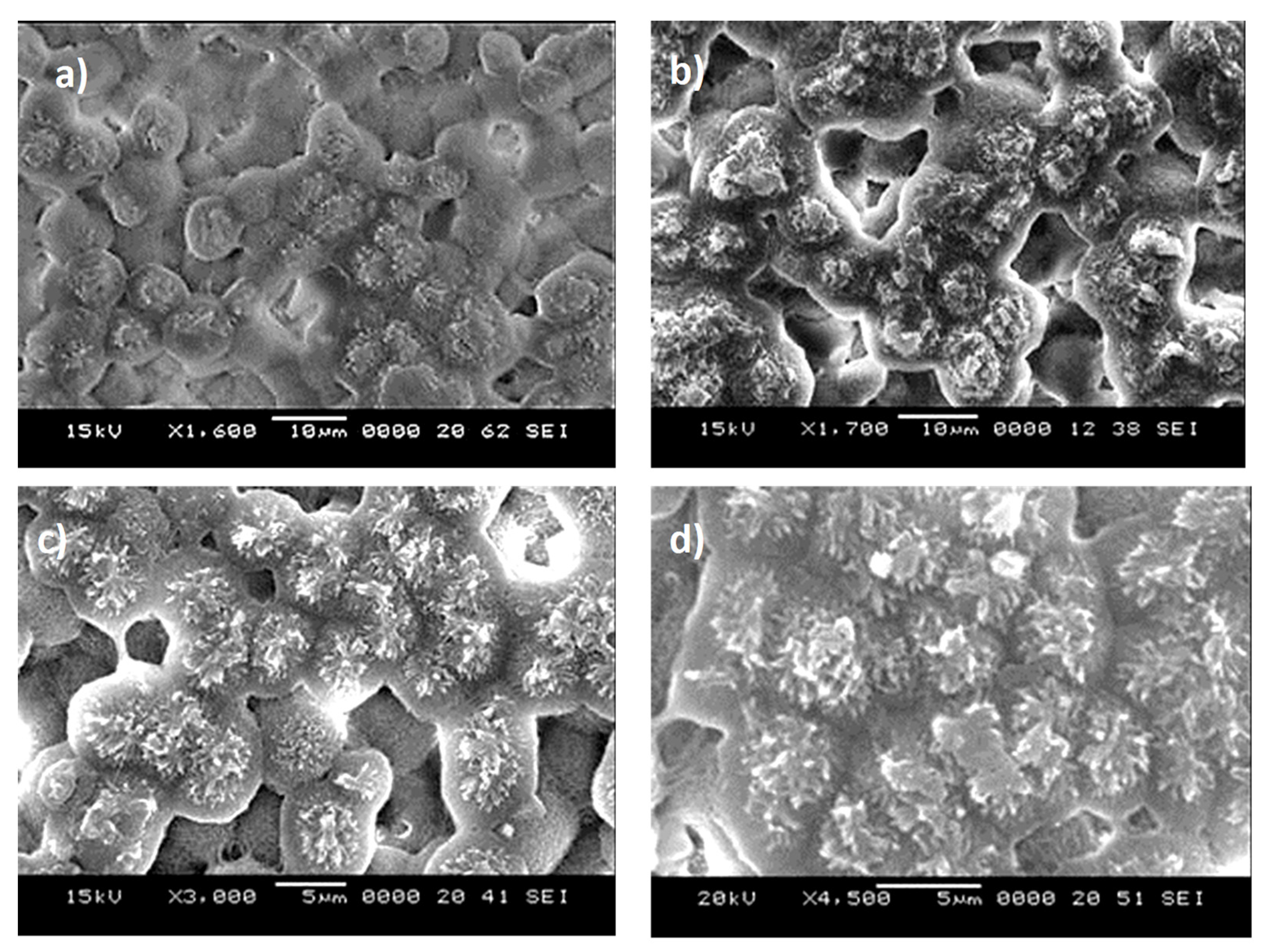
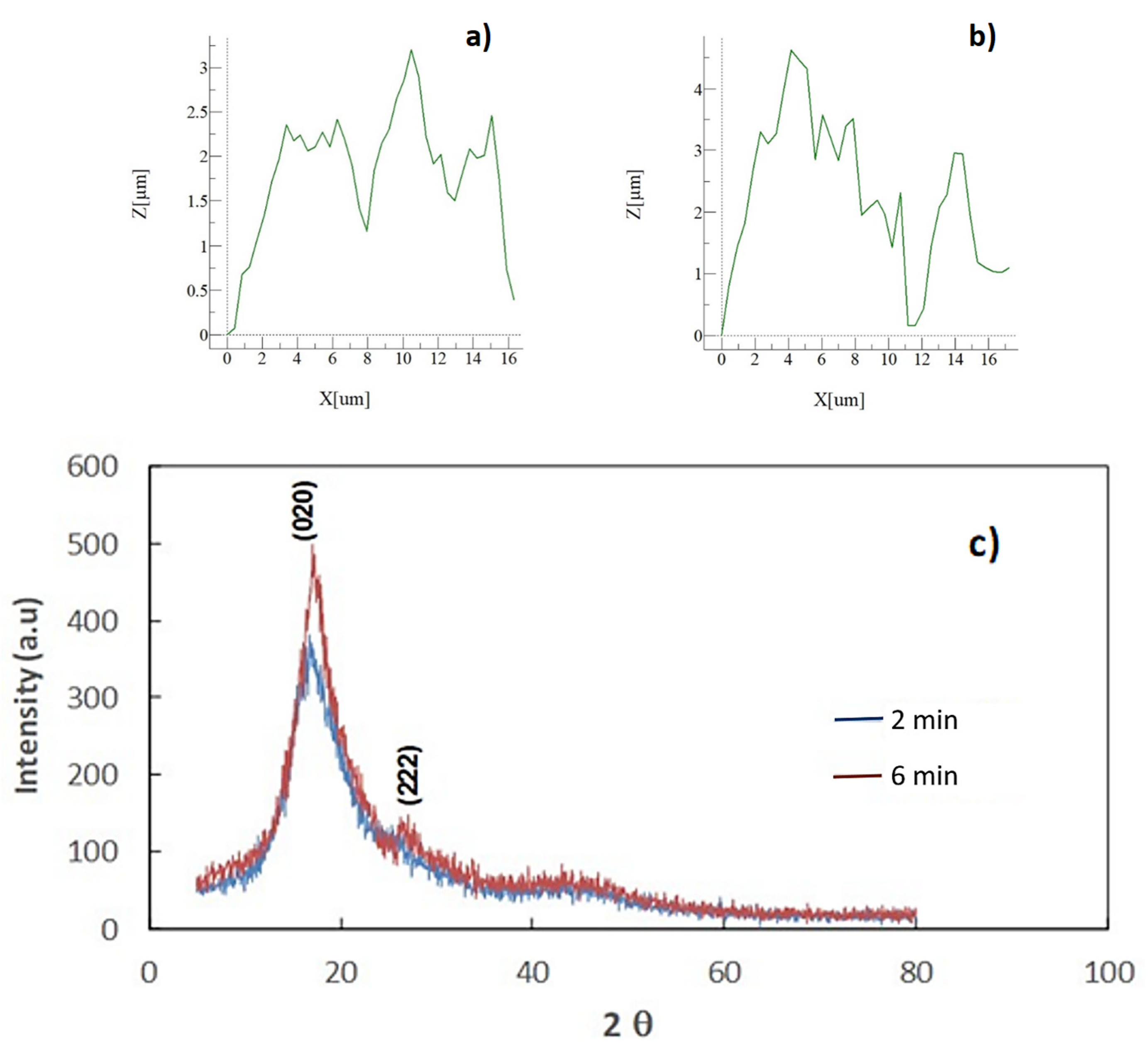
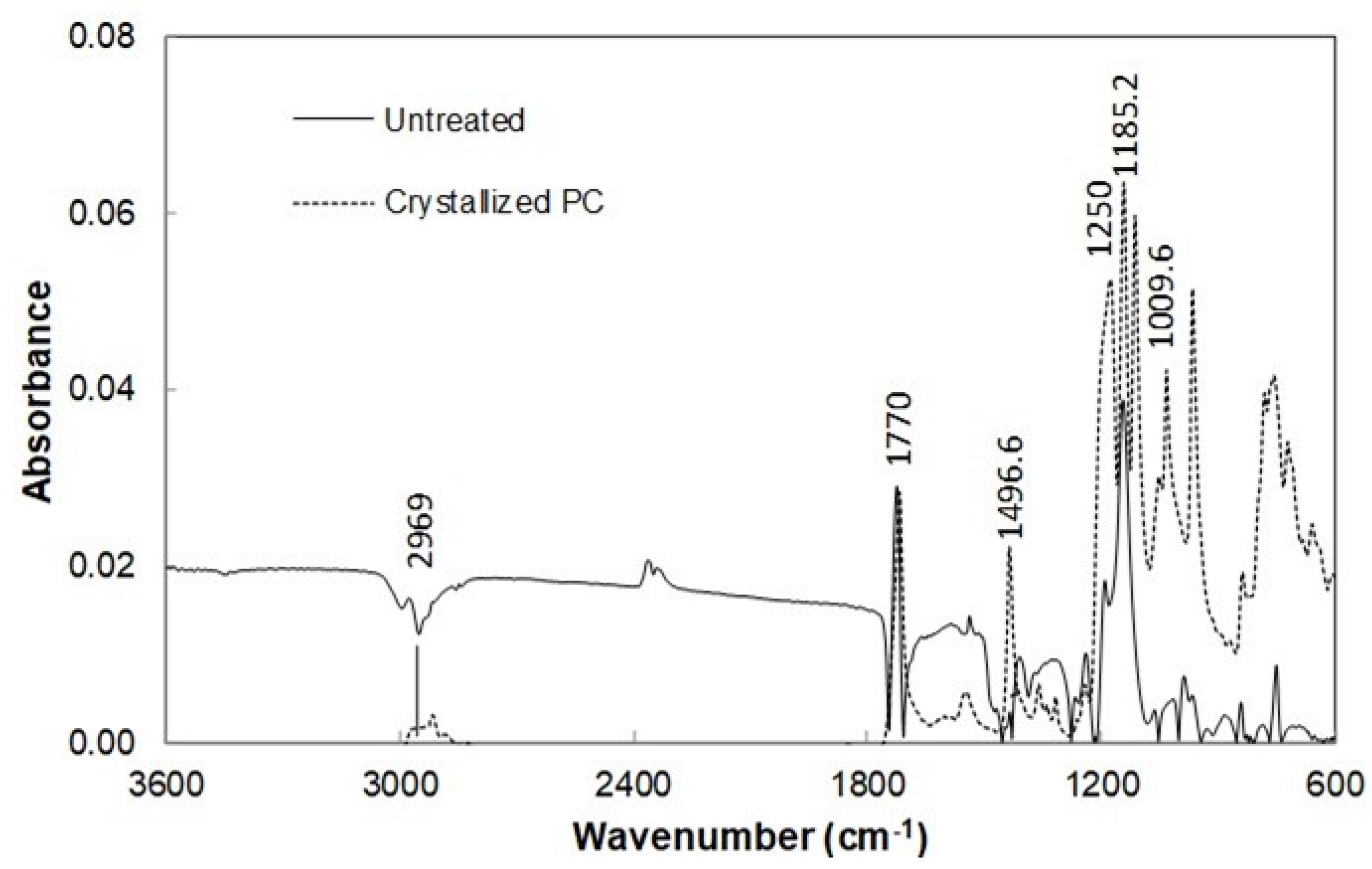

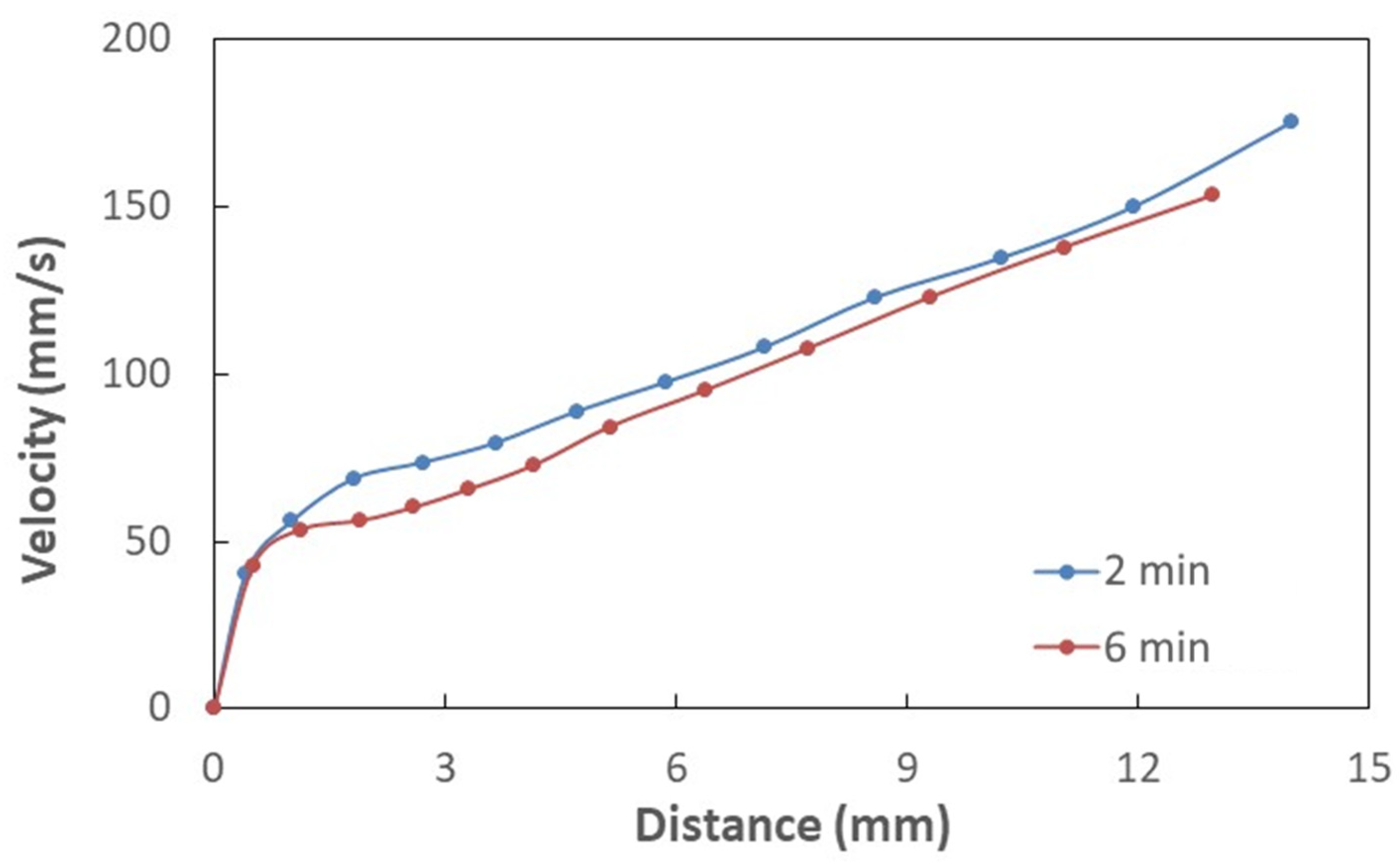


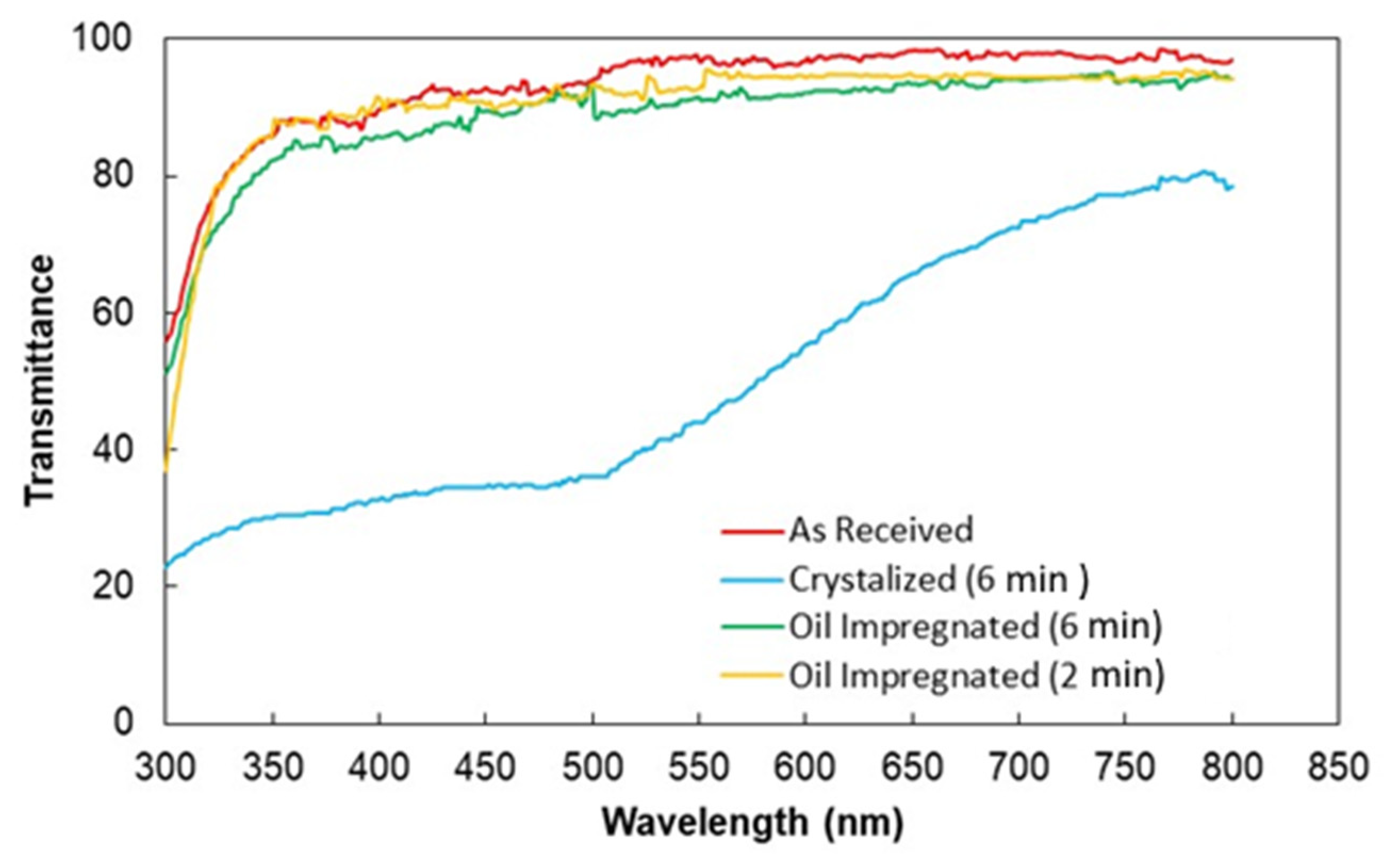
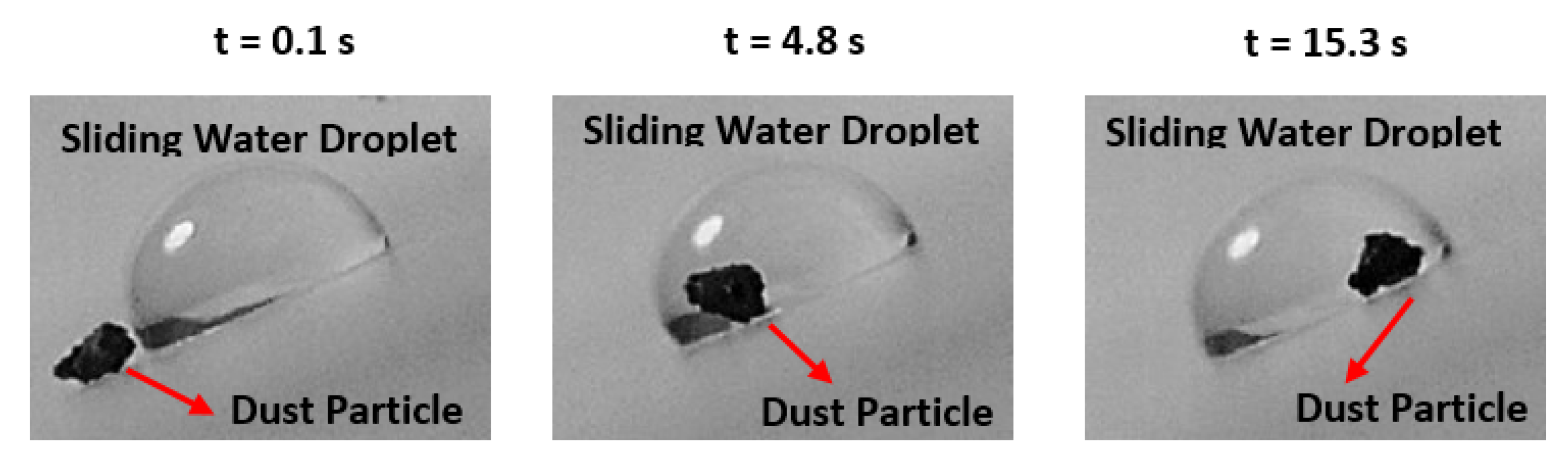
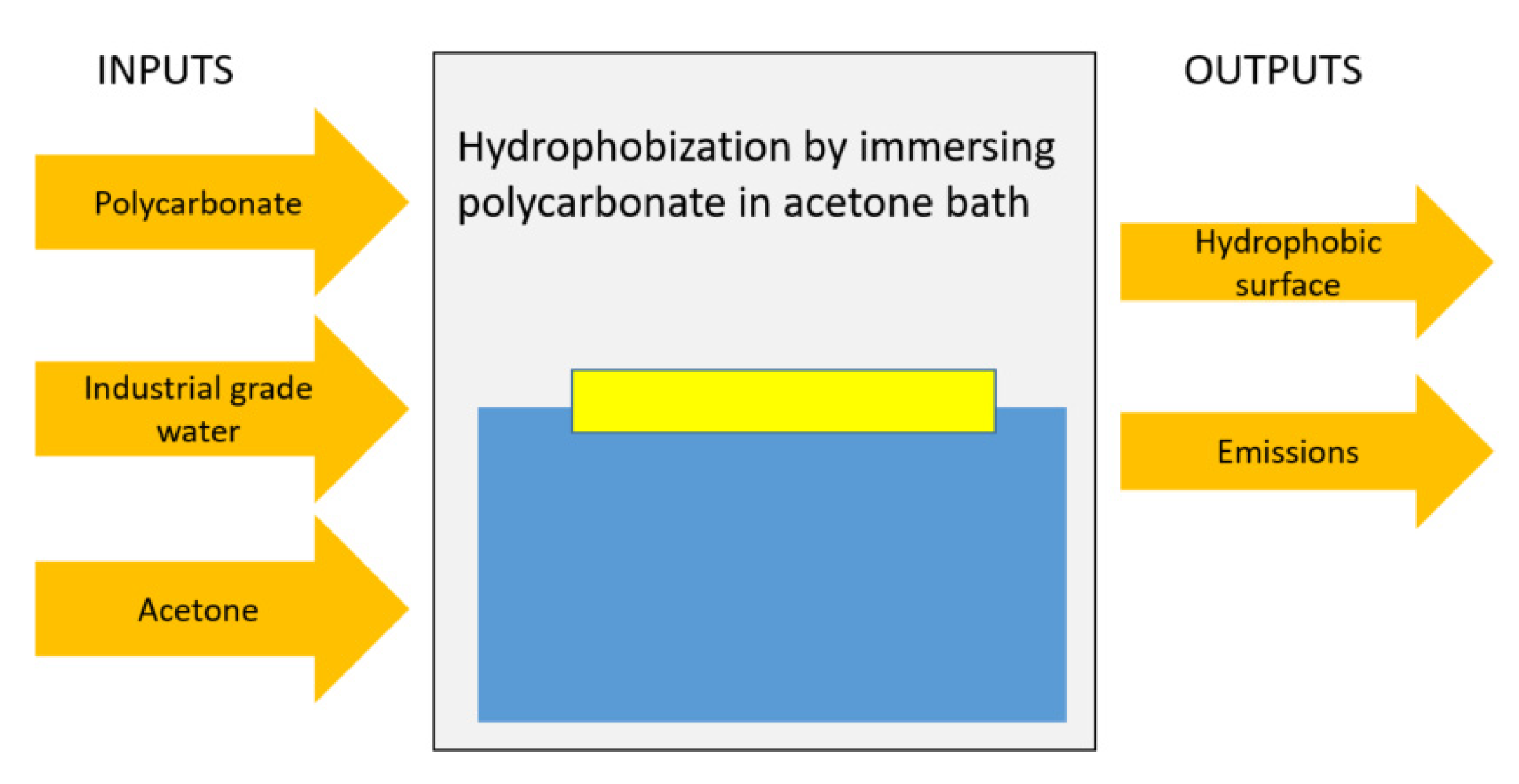

| Angle (Degrees) | Hysteresis (Degrees) | |
|---|---|---|
| As received | 85 ± 3° | 45 ± 3° |
| Crystalized PC (6 min) | 144 ± 3° | 28 ± 3° |
| Crystalized PC (2 min) | 138 ± 3° | 22 ± 3° |
| Impact Category | Symbol | Unit | Impact Value |
|---|---|---|---|
| Global warming | GW | kg CO2 eq | 0.002564 |
| Stratospheric ozone depletion | OD | kg CFC-11 eq | 0.000105 |
| Ionizing radiation | IR | kBq Co-60 eq | 0.000137 |
| Ozone formation, human health | OFH | kg NOx eq | 0.001785 |
| Fine particulate matter formation | FPMF | kg PM2.5 eq | 0.00081 |
| Ozone formation, terrestrial ecosystems | OFT | kg NOx eq | 0.002214 |
| Terrestrial acidification | TA | kg SO2 eq | 0.0013 |
| Freshwater eutrophication | FE | kg P eq | 0.00214 |
| Marine eutrophication | ME | kg N eq | 2.01 × 10−5 |
| Terrestrial ecotoxicity | TE | kg 1,4-DCB | 0.012569 |
| Freshwater ecotoxicity | FET | kg 1,4-DCB | 0.124233 |
| Marine ecotoxicity | MET | kg 1,4-DCB | 0.197215 |
| Human carcinogenic toxicity | HCT | kg 1,4-DCB | 0.155352 |
| Human non-carcinogenic toxicity | HCNT | kg 1,4-DCB | 0.021281 |
| Land use | LU | m2a crop eq | 4.31 × 10−5 |
| Mineral resource scarcity | MRS | kg Cu eq | 9.21 × 10−8 |
| Fossil resource scarcity | FRS | kg oil eq | 0.006773 |
| Water consumption | WC | m3 | 0.000572 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilbas, B.S.; Abubakar, A.A.; Al-Qahtani, H.; Shuja, S.Z.; Shaukat, M.M.; Sahin, A.Z.; Al-Sharafi, A.; Bahatab, S. Solution Crystallization of Polycarbonate Surfaces for Hydrophobic State: Water Droplet Dynamics and Life Cycle Assessment towards Self-Cleaning Applications. Polymers 2021, 13, 1449. https://doi.org/10.3390/polym13091449
Yilbas BS, Abubakar AA, Al-Qahtani H, Shuja SZ, Shaukat MM, Sahin AZ, Al-Sharafi A, Bahatab S. Solution Crystallization of Polycarbonate Surfaces for Hydrophobic State: Water Droplet Dynamics and Life Cycle Assessment towards Self-Cleaning Applications. Polymers. 2021; 13(9):1449. https://doi.org/10.3390/polym13091449
Chicago/Turabian StyleYilbas, Bekir Sami, Abba Abdulhamid Abubakar, Hussain Al-Qahtani, Shahzada Zaman Shuja, Mian Mobeen Shaukat, Ahmet Z. Sahin, Abdullah Al-Sharafi, and Saeed Bahatab. 2021. "Solution Crystallization of Polycarbonate Surfaces for Hydrophobic State: Water Droplet Dynamics and Life Cycle Assessment towards Self-Cleaning Applications" Polymers 13, no. 9: 1449. https://doi.org/10.3390/polym13091449
APA StyleYilbas, B. S., Abubakar, A. A., Al-Qahtani, H., Shuja, S. Z., Shaukat, M. M., Sahin, A. Z., Al-Sharafi, A., & Bahatab, S. (2021). Solution Crystallization of Polycarbonate Surfaces for Hydrophobic State: Water Droplet Dynamics and Life Cycle Assessment towards Self-Cleaning Applications. Polymers, 13(9), 1449. https://doi.org/10.3390/polym13091449






