Application of Furcellaran Nanocomposite Film as Packaging of Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of an Active Film
2.3. Water Content and Physicochemical Properties of Cheese
2.4. Microbiological Quality of Cheese
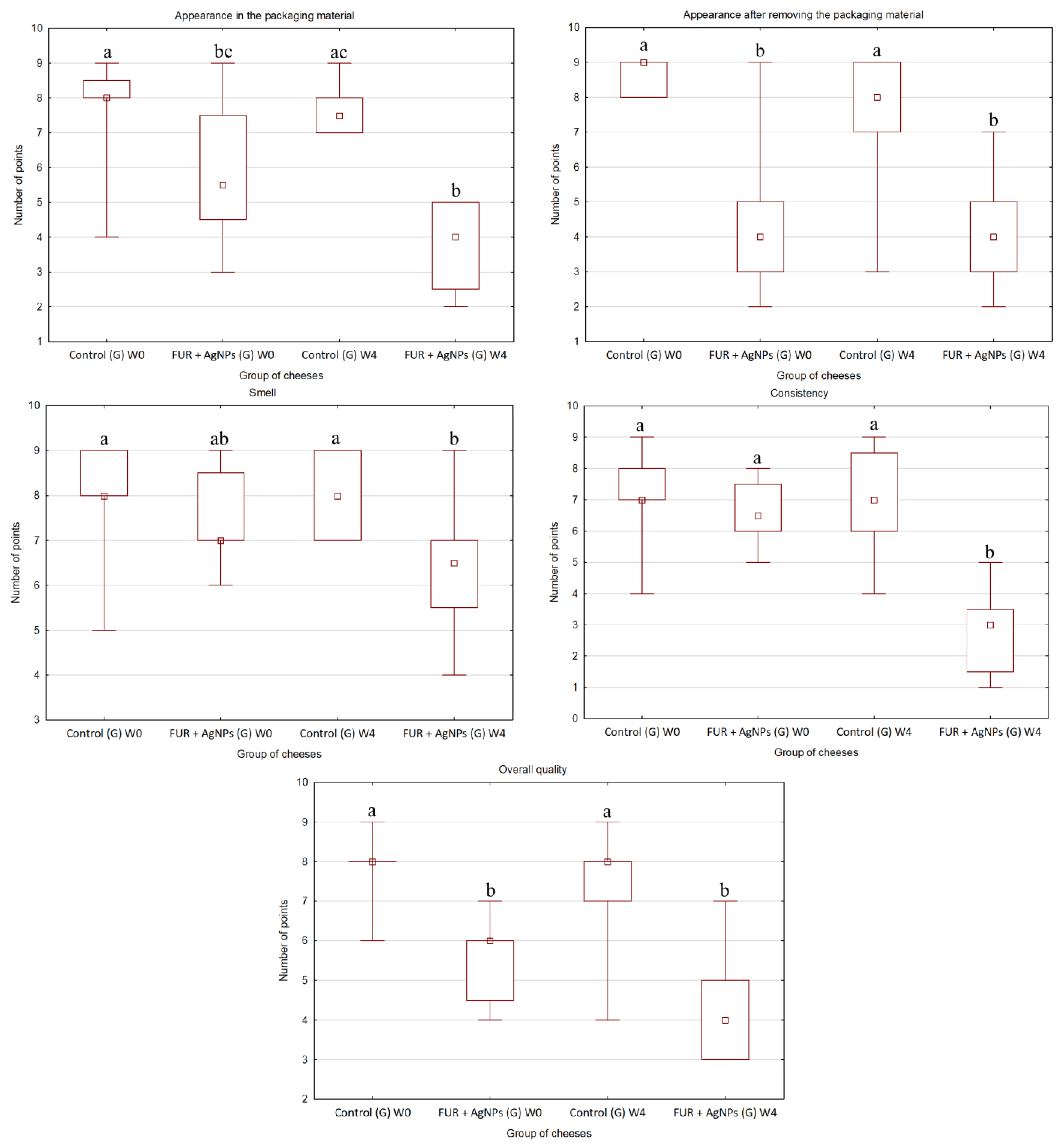
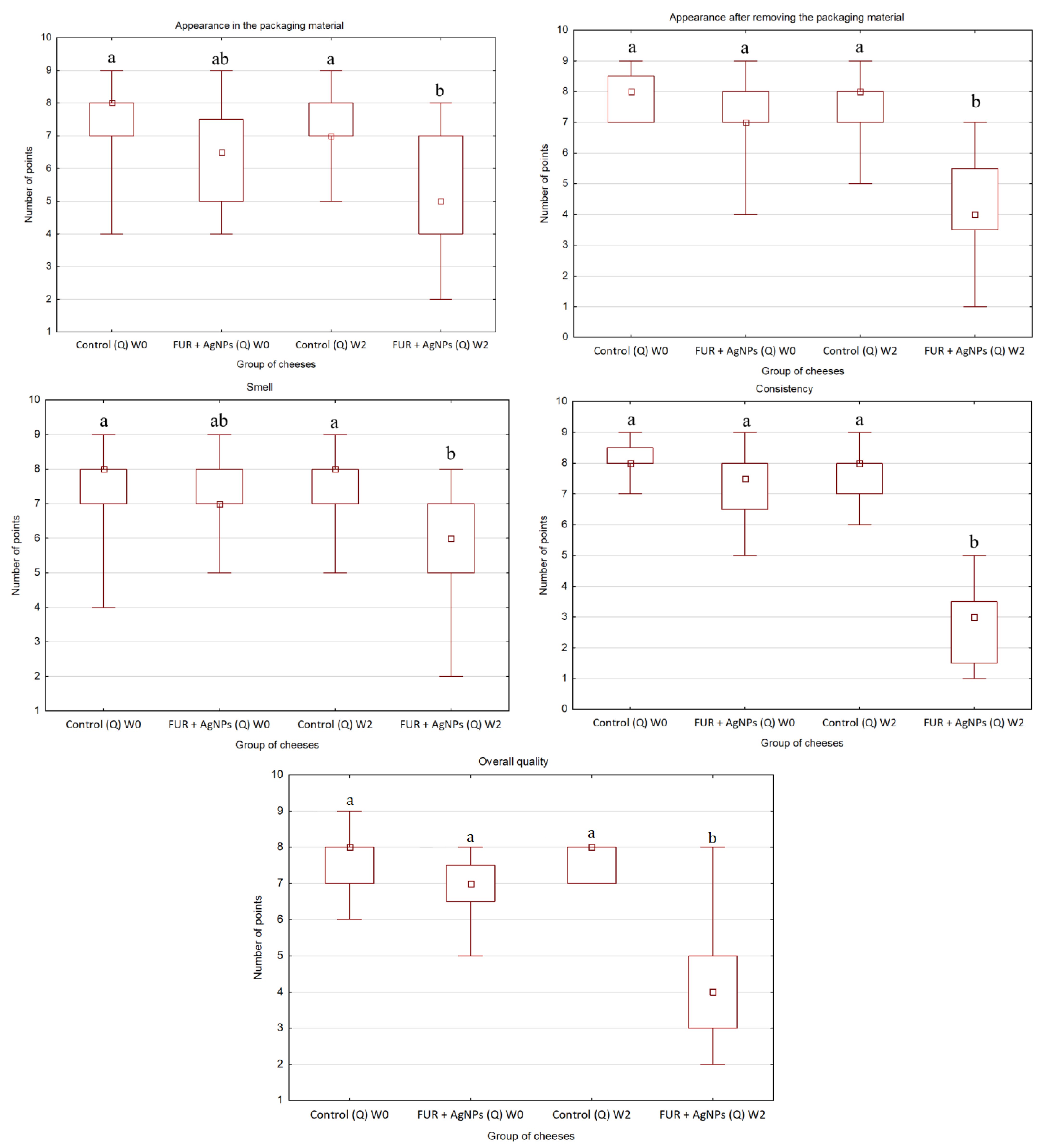
2.5. Organoleptic Quality of Cheese
2.6. Migration of Silver Nanoparticles
2.7. Statistical Analysis
3. Results and Discussion
3.1. Water Content and Physicochemical Properties of Cheese
3.2. Microbiological Quality of Cheese
3.3. Organoleptic Quality of Cheese
3.4. Migration of Silver Nanoparticles
4. Conclusions
- The FUR + AgNPs film applied as packaging of gouda and quark during storage improved the microbiological quality of both cheese varieties;
- The level of silver detected in the samples after storage seemed to not pose a threat to human health;
- All organoleptic characteristics of cheeses wrapped in the active film assessed after storage were found to be less desirable than that of control.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simbine, E.O.; Rodrigues, L.D.C.; Lapa-Guimaraes, J.; Kamimura, E.S.; Corassin, C.H.; Oliveira, C.A.F.D. Application of silver nanoparticles in food packages: A review. Food Sci. Technol. 2019, 39, 793–802. [Google Scholar] [CrossRef]
- Youssef, A.M.; Assem, F.M.; El-Sayed, S.M.; Salama, H.; Abd El-Salam, M.H. Utilization of Edible Films and Coatings as Packaging Materials for Preservation of Cheeses. J. Packag. Technol. Res. 2017, 1, 87–99. [Google Scholar] [CrossRef]
- Ng, S.; Kurisawa, M. Integrating biomaterials and food biopolymers for cultured meat production. Acta Biomater. 2021, 124, 108–129. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Juszczak, L.; Kawecka, A.; Bytesnikova, Z.; Milosavljević, V.; Kucharek, M.; Makarewicz, M.; Adam, V. Development and characterisation of furcellaran-gelatin films containing SeNPs and AgNPs that have antimicrobial activity. Food Hydrocoll. 2018, 83, 9–16. [Google Scholar] [CrossRef]
- Jancikova, S.; Jamróz, E.; Kulawik, P.; Tkaczewska, J.; Dordevic, D. Furcellaran/gelatin hydrolysate/rosemary extract composite films as active and intelligent packaging materials. Int. J. Biol. Macromol. 2019, 131, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Pluta-Kubica, A.; Jamróz, E.; Kawecka, A.; Juszczak, L.; Krzyściak, P. Active edible furcellaran/whey protein films with yerba mate and white tea extracts: Preparation, characterization and its application to fresh soft rennet-curd cheese. Int. J. Biol. Macromol. 2020, 155, 1307–1316. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Juszczak, L.; Kawecka, A.; Bytesnikova, Z.; Milosavljevic, V.; Makarewicz, M. Development of furcellaran-gelatin films with Se-AgNPs as an active packaging system for extension of mini kiwi shelf life. Food Packag. Shelf Life 2019, 21, 100339. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Tkaczewska, J.; Dordevic, D.; Jancikova, S.; Kulawik, P.; Milosavljevic, V.; Dolezelikova, K.; Smerkova, K.; Svec, P.; et al. Nanocomposite furcellaran films-the influence of nanofillers on functional properties of furcellaran films and effect on linseed oil preservation. Polymers 2019, 11, 2046. [Google Scholar] [CrossRef] [PubMed]
- Kulawik, P.; Jamróz, E.; Zając, M.; Guzik, P.; Tkaczewska, J. The effect of furcellaran-gelatin edible coatings with green and pu-erh tea extracts on the microbiological, physicochemical and sensory changes of salmon sushi stored at 4 °C. Food Control. 2019, 100, 83–91. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Tkaczewska, J.; Guzik, P.; Zając, M.; Juszczak, L.; Krzyściak, P.; Turek, K. The effects of active double-layered furcellaran/gelatin hydrolysate film system with Ala-Tyr peptide on fresh Atlantic mackerel stored at −18 °C. Food Chem. 2021, 338, 127867. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Guzik, P.; Duda, I. The verification of intelligent properties of furcellaran films with plant extracts on the stored fresh Atlantic mackerel during storage at 2 °C. Food Hydrocoll. 2019, 97, 105211. [Google Scholar] [CrossRef]
- Ortiz-Duarte, G.; Martínez-Hernández, G.B.; Casillas-Peñuelas, R.; Pérez-Cabrera, L.E. Evaluation of Biopolymer Films Containing Silver–Chitosan Nanocomposites. Food Bioprocess. Technol. 2021. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Nerín, C. Nanoparticle release from nano-silver antimicrobial food containers. Food Chem. Toxicol. 2013, 62, 16–22. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.; Bing, X.; Gao, C.; Wang, T.; Yuan, B. Nanosilver migrated into food-simulating solutions from commercially available food fresh containers. Packag. Technol. Sci. 2011, 24, 291–297. [Google Scholar] [CrossRef]
- Cushen, M.; Kerry, J.; Morris, M.; Cruz-Romero, M.; Cummins, E. Migration and exposure assessment of silver from a PVC nanocomposite. Food Chem. 2013, 139, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, D.; Kanmani, P. Study on physical and mechanical properties of the biopolymer/silver based active nanocomposite films with antimicrobial activity. Carbohydr. Polym. 2019, 224, 115159. [Google Scholar] [CrossRef]
- Mohammadzadeh-Vazifeh, M.; Hosseini, S.M.; Mohammadi, A.; Jahanfar, M.; Maleki, H. Investigation of the antimicrobial properties of nanoclay and chitosan based nanocomposite on the microbial characteristics of Gouda cheese. Iran. J. Microbiol. 2020, 12, 121–126. [Google Scholar] [CrossRef]
- Wemmenhove, E.; van Valenberg, H.J.F.; van Hooijdonk, A.C.M.; Wells-Bennik, M.H.J.; Zwietering, M.H. Factors that inhibit growth of Listeria monocytogenes in nature-ripened Gouda cheese: A major role for undissociated lactic acid. Food Control. 2018, 84, 413–418. [Google Scholar] [CrossRef]
- Jo, Y.; Benoist, D.M.; Ameerally, A.; Drake, M.A. Sensory and chemical properties of Gouda cheese. J. Dairy Sci. 2018, 101, 1967–1989. [Google Scholar] [CrossRef]
- Pachlová, V.; Buňková, L.; Purkrtová, S.; Němečková, I.; Havlíková, Š.; Purevdorj, K.; Buňka, F. Contaminating microorganisms in quark-type cheese and their capability of biogenic amine production. Int. J. Dairy Technol. 2018, 71, 1018–1022. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science, 2nd ed.; Springer: Berlin, Germany, 2017; Volume 16, ISBN 9781489976819. [Google Scholar] [CrossRef]
- Dmytrów, I.; Szczepanik, G.; Kryza, K.; Mituniewicz-Małek, A.; Lisiecki, S. Impact of polylactic acid packaging on the organoleptic and physicochemical properties of tvarog during storage. Int. J. Dairy Technol. 2011, 64, 569–577. [Google Scholar] [CrossRef]
- Chromik, C.; Partschefeld, C.; Jaros, D.; Henle, T.; Rohm, H. Adjustment of vat milk treatment to optimize whey protein transfer into semi-hard cheese: A case study. J. Food Eng. 2010, 100, 496–503. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Codex Standard for Gouda; CODEX STAN 266-1966; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; pp. 1–6. [Google Scholar]
- Jasińska, M.; Harabin, K.; Dmytrów, I. Effect of packaging and season of milk production on selected quality characteristics of organic acid curd cheese during storage. Acta Sci. Pol. Technol. Aliment. 2014, 13, 231–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jamróz, E.; Khachatryan, G.; Kopel, P.; Juszczak, L.; Kawecka, A.; Krzyściak, P.; Kucharek, M.; Bębenek, Z.; Zimowska, M. Furcellaran nanocomposite films: The effect of nanofillers on the structural, thermal, mechanical and antimicrobial properties of biopolymer films. Carbohydr. Polym. 2020, 240, 116244. [Google Scholar] [CrossRef] [PubMed]
- Pluta-Kubica, A.; Jamróz, E.; Juszczak, L.; Krzyściak, P.; Zimowska, M. Characterization of Furcellaran-Whey Protein Isolate Films with Green Tea or Pu-erh Extracts and Their Application as Packaging of an Acid-Curd Cheese. Food Bioprocess. Technol. 2021, 14, 78–92. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Dairy Products; AOAC International: Arlington, VA, USA, 2007; Volume 33. [Google Scholar]
- Berti, S.; Ollé Resa, C.P.; Basanta, F.; Gerschenson, L.N.; Jagus, R.J. Edible coatings on Gouda cheese as a barrier against external contamination during ripening. Food Biosci. 2019, 31, 100447. [Google Scholar] [CrossRef]
- PN-EN ISO 6887-5:2010. In Microbiology of Food and Animal Feeding Stuffs—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 5: Specific Rules for the Preparation of Milk and Milk Products; International Organization for Standardization: Geneva, Switzerland, 2010.
- Ong, L.; Shah, N.P. Probiotic Cheddar cheese: Influence of ripening temperatures on survival of probiotic microorganisms, cheese composition and organic acid profiles. LWT Food Sci. Technol. 2009, 42, 1260–1268. [Google Scholar] [CrossRef]
- PN-EN ISO 4833-1:2013-12. In Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 degrees C by the Pour Plate Technique; International Organization for Standardization: Geneva, Switzerland, 2013.
- PN-ISO 21527-1:2009. In Microbiology of Food and Animal Feeding stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95; International Organization for Standardization: Geneva, Switzerland, 2009.
- PN-ISO 4832:2007. In Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coliforms—Colony-count Technique; International Organization for Standardization: Geneva, Switzerland, 2009.
- Baryłko-Pikielna, N.; Matuszewska, I. Sensoryczne Badania Żywności. Podstawy-Metody-Zastosowania; Wydawnictwo Naukowe PTTŻ: Kraków, Poland, 2014; ISBN 978-83-935421-3-0. [Google Scholar]
- PN-EN 14084:2004. In Foodstuffs-Determination of Trace Elements-Determination of Lead, Cadmium, Sinc, Copper and Iron by Atomic Absorption Spectrometry (AAS) after Microwave Digestion; International Organization for Standardization: Geneva, Switzerland, 2004.
- PN-EN 13804:2013-06. In Foodstuffs. Determination of Elements and Their Chemical Species. General Considerations and Specific Requirements; International Organization for Standardization: Geneva, Switzerland, 2013.
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and Incorporation of Functional Ingredients in Edible Films and Coatings. Food Bioprocess. Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Youssef, A.M.; Assem, F.M.; Abdel-Aziz, M.E.; Elaaser, M.; Ibrahim, O.A.; Mahmoud, M.; Abd El-Salam, M.H. Development of bionanocomposite materials and its use in coating of Ras cheese. Food Chem. 2019, 270, 467–475. [Google Scholar] [CrossRef]
- Panrong, T.; Karbowiak, T.; Harnkarnsujarit, N. Thermoplastic starch and green tea blends with LLDPE films for active packaging of meat and oil-based products. Food Packag. Shelf Life 2019, 21, 100331. [Google Scholar] [CrossRef]
- Pinto, M.S.; de Carvalho, A.F.; Pires, A.C.D.S.; Campos Souza, A.A.; Fonseca da Silva, P.H.; Sobral, D.; de Paula, J.C.J.; de Lima Santos, A. The effects of nisin on Staphylococcus aureus count and the physicochemical properties of Traditional Minas Serro cheese. Int. Dairy J. 2011, 21, 90–96. [Google Scholar] [CrossRef]
- Amjadi, S.; Emaminia, S.; Nazari, M.; Davudian, S.H.; Roufegarinejad, L.; Hamishehkar, H. Application of Reinforced ZnO Nanoparticle-Incorporated Gelatin Bionanocomposite Film with Chitosan Nanofiber for Packaging of Chicken Fillet and Cheese as Food Models. Food Bioprocess. Technol. 2019, 12, 1205–1219. [Google Scholar] [CrossRef]
- Panfil-Kuncewicz, H.; Lis, A.; Majewska, M. Wpływ opakowań aktywnych na trwałość mikrobiologiczną i cechy sensoryczne serów twarogowych. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2014, 21, 190–203. [Google Scholar] [CrossRef]
- Salazar, J.K.; Gonsalves, L.J.; Natarajan, V.; Shazer, A.; Reineke, K.; Mhetras, T.; Sule, C.; Carstens, C.K.; Schill, K.M.; Tortorello, M. Lou Population dynamics of listeria monocytogenes, Escherichia coli O157:H7, and native microflora during manufacture and aging of gouda cheese made with unpasteurized milk. J. Food Prot. 2020, 83, 266–276. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable hybrid nanocomposites of chitosan/gelatin and silver nanoparticles for active food packaging applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Lan, W.; Li, S.; Shama, S.; Zhao, Y.; Sameen, D.E.; He, L. Investigation of Ultrasonic Treatment on Physicochemical, Structural and Morphological Properties of Sodium Alginate/AgNPs/Apple Polyphenol Films and Its Preservation Effect on Strawberry. Polymers 2020, 12, 2096. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Rani, M.S.; Venkataswamy, P.; Mohan Reddy, B.J.; Vithal, M. Biosynthesis of CMC-Guar gum-Ag0 nanocomposites for inactivation of food pathogenic microbes and its effect on the shelf life of strawberries. Carbohydr. Polym. 2020, 236, 116053. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Zhang, H.; Yuan, M.; Qin, Y. Evaluation of PLA nanocomposite films on physicochemical and microbiological properties of refrigerated cottage cheese. J. Food Process. Preserv. 2018, 42, 1–9. [Google Scholar] [CrossRef]
- Metak, A.M.; Nabhani, F.; Connolly, S.N. Migration of engineered nanoparticles from packaging into food products. LWT Food Sci. Technol. 2015, 64, 781–787. [Google Scholar] [CrossRef]
- Introna, B.; De Benedetto, G.; Genga, A.; Pennetta, A.; Rella, S.; Siciliano, T.; Malitesta, C.; Conte, A.; Del Nobile, M.A. Analytical characterization of silver-nanoparticle antimicrobial coatings for fiordilatte cheese. In Proceedings of the 2015 1st Workshop on Nanotechnology in Instrumentation and Measurement, Lecce, Italy, 24–25 July 2015; pp. 216–219. [Google Scholar] [CrossRef]
- Commission Regulation (EU). No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 2011, L12, 1–89. [Google Scholar]
| Parameters | Type of Film | Storage Weeks | |
|---|---|---|---|
| 0 | 4 | ||
| Water content (%) | Control | 42.0 b ± 0.2 | 41.2 b ± 1.0 |
| Active | 32.6 a ± 0.1 | ||
| Water activity | Control | 0.971 b ± 0.001 | 0.966 ab ± 0.002 |
| Active | 0.935 a ± 0.003 | ||
| pH | Control | 5.64 b ± 0.01 | 5.63 ab ± 0.00 |
| Active | 5.58 a ± 0.01 | ||
| Lactococcus count (log cfu/g) | Control | 9.4 ± 0.3 | 9.5 ± 0.3 |
| Active | 9.7 ± 0.1 | ||
| TBC (log cfu/g) | Control | 9.8 ± 0.4 | 10.1 ± 0.1 |
| Active | 9.9 ± 0.2 | ||
| Yeast count (log cfu/g) | Control | 0.6 a ± 0.5 | 5.2 c ± 0.3 |
| Active | 2.6 b ± 0.3 | ||
| Mold count (log cfu/g) | Control | 0.1 a ± 0.1 | 2.5 b ± 0.2 |
| Active | ND | ||
| Parameters | Type of Film | Storage Weeks | |
|---|---|---|---|
| 0 | 4 | ||
| Water content [%] | Control | 74.3 b ± 1.3 | 75.0 b ± 0.5 |
| Active | 44.4 a ± 4.5 | ||
| Water activity | Control | 0.990 b ± 0.002 | 0.990 b ± 0.006 |
| Active | 0.963 a ± 0.016 | ||
| pH | Control | 4.61 ± 0.02 | 4.67 ± 0.11 |
| Active | 4.66 ± 0.03 | ||
| Lactococcus count (log cfu/g) | Control | 8.3 b ± 0.1 | 5.8 a ± 0.6 |
| Active | 6.3 a ± 0.7 | ||
| TBC (log cfu/g) | Control | 8.2 ± 0.1 | 6.2 ± 0.9 |
| Active | 6.0 ± 0.3 | ||
| Yeast count (log cfu/g) | Control | ND | 3.7 ± 1.2 |
| Active | ND | ||
| Mold count (log cfu/g) | Control | ND | 0.6 ± 0.5 |
| Active | 1.2 ± 1.0 | ||
| Gouda | ||
|---|---|---|
| Appearance at the beginning of storage | Control | Active |
| Appearance after four weeks of storage | Control | Active |
| Quark | ||
| Appearance at the beginning of storage | Control | Active |
| Appearance after two weeks of storage | Control | Active |
| Type of Cheese | Type of Sample | Content of Silver (mg/kg) |
|---|---|---|
| Gouda | Before storage | <LOQ |
| After storage (rind) | 3.897 ± 1.750 | |
| After storage (interior) | <LOQ | |
| Quark | Before storage | <LOQ |
| After storage (rind) | 0.717 b ± 0.690 | |
| After storage (interior) | 0.036 a ± 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pluta-Kubica, A.; Jamróz, E.; Khachatryan, G.; Florkiewicz, A.; Kopel, P. Application of Furcellaran Nanocomposite Film as Packaging of Cheese. Polymers 2021, 13, 1428. https://doi.org/10.3390/polym13091428
Pluta-Kubica A, Jamróz E, Khachatryan G, Florkiewicz A, Kopel P. Application of Furcellaran Nanocomposite Film as Packaging of Cheese. Polymers. 2021; 13(9):1428. https://doi.org/10.3390/polym13091428
Chicago/Turabian StylePluta-Kubica, Agnieszka, Ewelina Jamróz, Gohar Khachatryan, Adam Florkiewicz, and Pavel Kopel. 2021. "Application of Furcellaran Nanocomposite Film as Packaging of Cheese" Polymers 13, no. 9: 1428. https://doi.org/10.3390/polym13091428
APA StylePluta-Kubica, A., Jamróz, E., Khachatryan, G., Florkiewicz, A., & Kopel, P. (2021). Application of Furcellaran Nanocomposite Film as Packaging of Cheese. Polymers, 13(9), 1428. https://doi.org/10.3390/polym13091428









