Fabrication of Carbon Nanomaterials Using Laser Scribing on Copper Nanoparticles-Embedded Polyacrylonitrile Films and Their Application in a Gas Sensor
Abstract
1. Introduction
2. Experimental
2.1. Materials
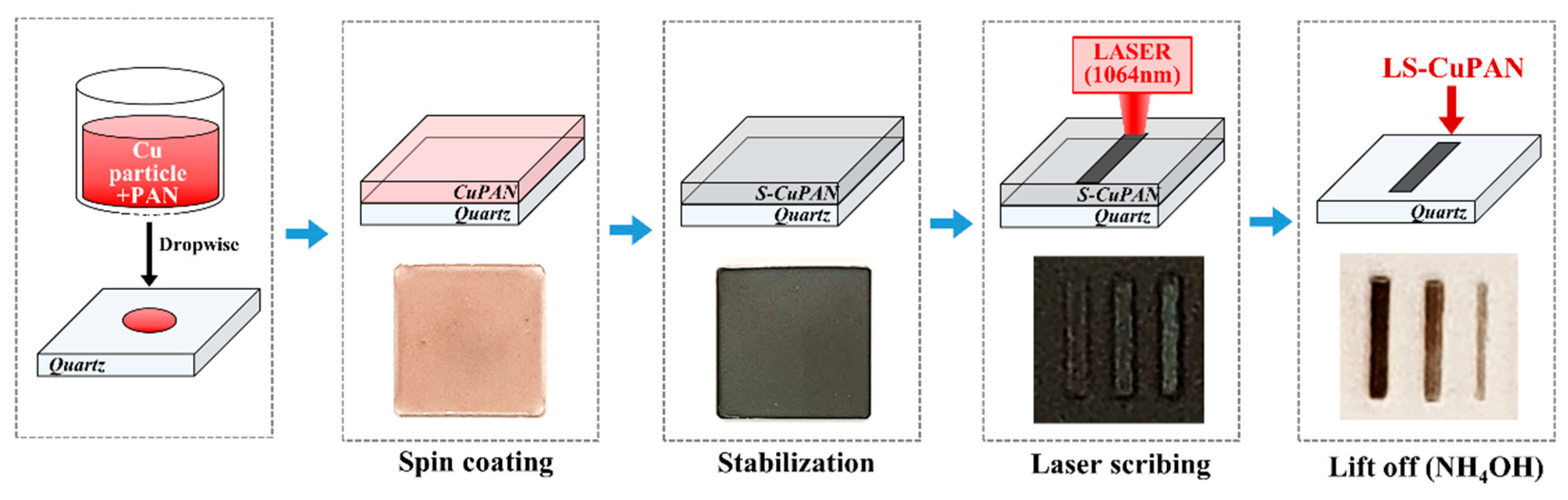
2.2. Stabilization of Copper-Embedded PAN Thin Film
2.3. Laser Scribing on S-CuPAN and Lift-Off Untreated Area
2.4. Gas Sensing
2.5. Characterization
3. Results and Discussion
3.1. Effect of Stabilization of CuPAN
3.2. Laser Scribing Conditions of LS-CuPAN
3.2.1. Spin-Coating Conditions of CuPAN Thin Film
3.2.2. Weight Ratio of Cu Particle to PAN
3.2.3. Optimization of the Laser Scribing Process (Power, Repeat Time)
3.3. Chemical and Morphological Analysis of LS-CuPAN
3.3.1. X-ray Photoelectron Spectroscopy (XPS)
3.3.2. Scanning Electron Microscopy (SEM)
3.4. Gas Sensing Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gopinath, K.P.; Vo, D.V.N.; Prakash, D.G.; Joseph, A.A.; Viswanathan, S.; Arun, J. Environmental applications of carbon-based materials: A review. Environ. Chem. Lett. 2021, 19, 557–582. [Google Scholar] [CrossRef]
- Zhang, B.T.; Zheng, X.; Li, H.F.; Lin, J.M. Application of carbon-based nanomaterials in sample preparation: A review. Anal. Chim. Acta 2013, 784, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lü, K.; Zhao, G.; Wang, X. A brief review of graphene-based material synthesis and its application in environmental pollution management. Chin. Sci. Bull. 2012, 57, 1223–1234. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, L.B.; Ruan, S.; Li, X.; Yu, S.; Li, X.; Jiang, Y.; Yao, Z.; Ye, S.; Wang, C. Recent development in nanocarbon materials for gas sensor applications. Sens. Actuators B Chem. 2018, 274, 235–267. [Google Scholar] [CrossRef]
- Yoon, H.J.; Jun, D.H.; Yang, J.H.; Zhou, Z.; Yang, S.S.; Cheng, M.M.C. Carbon dioxide gas sensor using a graphene sheet. Sens. Actuators B Chem. 2011, 157, 310–313. [Google Scholar] [CrossRef]
- Aslam, S.; Bokhari, T.H.; Anwar, T.; Khan, U.; Nairan, A.; Khan, K. Graphene oxide coated graphene foam based chemical sensor. Mater. Lett. 2019, 235, 66–70. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Hyeon, T. Recent Progress in the Synthesis of Porous Carbon Materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Muñoz, R.; Gómez-Aleixandre, C. Review of CVD Synthesis of Graphene. Chem. Vap. Depos. 2013, 19, 297–322. [Google Scholar] [CrossRef]
- Choi, D.S.; Kim, K.S.; Kim, H.; Kim, Y.; Kim, T.; Rhy, S.; Yang, C.-M.; Yoon, D.H.; Yang, W.S. Effect of Cooling Condition on Chemical Vapor Deposition Synthesis of Graphene on Copper Catalyst. ACS Appl. Mater. Interfaces 2014, 6, 19574–19578. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Lee, D.Y.; Nam, J.; Kim, K.S. Pattern Synthesis of Designed Graphene by using a LASER Scribing Process. New Phys. Sae Mulli 2019, 69, 590–595. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser scribing of high-performance and flexible graphene-based electrochemical capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef]
- Ruan, X.; Wang, R.; Luo, J.; Yao, Y.; Liu, T. Experimental and modeling study of CO2 laser writing induced polyimide carbonization process. Mater. Des. 2018, 160, 1168–1177. [Google Scholar] [CrossRef]
- Dunham, M.G.; Edie, D.D. Model of stabilization for pan-based carbon fiber precursor bundles. Carbon N. Y. 1992, 30, 435–450. [Google Scholar] [CrossRef]
- Frank, E.; Ingildeev, D.; Buchmeiser, M.R. High-performance PAN-based carbon fibers and their performance requirements. In Structure and Properties of High-Performance Fibers; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 7–30. ISBN 9780081005514. [Google Scholar]
- Ko, T.-H. Influence of continuous stabilization on the physical properties and microstructure of PAN-based carbon fibers. J. Appl. Polym. Sci. 1991, 42, 1949–1957. [Google Scholar] [CrossRef]
- Kidambi, P.R.; Bayer, B.C.; Blume, R.; Wang, Z.J.; Baehtz, C.; Weatherup, R.S.; Willinger, M.G.; Schloegl, R.; Hofmann, S. Observing graphene grow: Catalyst-graphene interactions during scalable graphene growth on polycrystalline copper. Nano Lett. 2013, 13, 4769–4778. [Google Scholar] [CrossRef] [PubMed]
- Bhaviripudi, S.; Jia, X.; Dresselhaus, M.S.; Kong, J. Role of kinetic factors in chemical vapor deposition synthesis of uniform large area graphene using copper catalyst. Nano Lett. 2010, 10, 4128–4133. [Google Scholar] [CrossRef] [PubMed]
- Doremus, R.; Kao, S.-C.; Garcia, R. Optical absorption of small copper particles and the optical properties of copper. Appl. Opt. 1992, 31, 5773. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Guo, L.; Hao, Y.-W.; Li, P.-L.; Song, J.-F.; Yang, R.-Z.; Fu, X.-Y.; Xie, S.-Y.; Zhao, J.; Zhang, Y.-L. Improved NO2 Gas Sensing Properties of Graphene Oxide Reduced by Two-beam-laser Interference. Sci. Rep. 2018, 8, 4918. [Google Scholar] [CrossRef]
- Fei, H.; Wu, G.; Cheng, W.-Y.; Yan, W.; Xu, H.; Zhang, D.; Zhao, Y.; Lv, Y.; Chen, Y.; Zhang, L.; et al. Enhanced NO2 Sensing at Room Temperature with Graphene via Monodisperse Polystyrene Bead Decoration. ACS Omega 2019, 4, 3812–3819. [Google Scholar] [CrossRef]
- Ben Aziza, Z.; Zhang, Q.; Baillargeat, D. Graphene/mica based ammonia gas sensors. Appl. Phys. Lett. 2014, 105, 254102. [Google Scholar] [CrossRef]
- Seekaew, Y.; Pon-On, W.; Wongchoosuk, C. Ultrahigh Selective Room-Temperature Ammonia Gas Sensor Based on Tin–Titanium Dioxide/reduced Graphene/Carbon Nanotube Nanocomposites by the Solvothermal Method. ACS Omega 2019, 4, 16916–16924. [Google Scholar] [CrossRef]
- Su, Z.; Tan, L.; Yang, R.; Zhang, Y.; Tao, J.; Zhang, N.; Wen, F. Cu-modified carbon spheres/reduced graphene oxide as a high sensitivity of gas sensor for NO2 detection at room temperature. Chem. Phys. Lett. 2018, 695, 153–157. [Google Scholar] [CrossRef]
- Wei, D.; Mitchell, J.I.; Tansarawiput, C.; Nam, W.; Qi, M.; Ye, P.D.; Xu, X. Laser direct synthesis of graphene on quartz. Carbon N. Y. 2013, 53, 374–379. [Google Scholar] [CrossRef]
- Mahmoud, W.E.; Al-Hazmi, F.S.; Al-Ghamdi, A.A.; Shokr, F.S.; Beall, G.W.; Bronstein, L.M. Structure and spectroscopic analysis of the graphene monolayer film directly grown on the quartz substrate via the HF-CVD technique. Superlattices Microstruct. 2016, 96, 174–178. [Google Scholar] [CrossRef]
- Al-Salman, H.S.; Abdullah, M.J. Hydrogen gas sensing based on ZnO nanostructure prepared by RF-sputtering on quartz and PET substrates. Sens. Actuators B Chem. 2013, 181, 259–266. [Google Scholar] [CrossRef]
- Danel, J.S.; Delapierre, G. Quartz: A material for microdevices. J. Micromech. Microeng. 1991, 1, 187–198. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Hofmann, M. Raman spectroscopy as a probe of graphene and carbon nanotubes. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2008, 366, 231–236. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.L.; Couzi, M. Raman spectroscopy as a tool for the analysis of carbon-based materials (highly oriented pyrolitic graphite, multilayer graphene and multiwall carbon nanotubes) and of some of their elastomeric composites. Vib. Spectrosc. 2014, 74, 57–63. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, J.; Zhang, H.; Wang, Y.; Gao, X.; Wang, L.; Zhu, Z. One-pot synthesis of 3D hierarchical SnO2 nanostructures and their application for gas sensor. Sens. Actuators B Chem. 2015, 207, 83–89. [Google Scholar] [CrossRef]
- Wu, J.; Tao, K.; Guo, Y.; Li, Z.; Wang, X.; Luo, Z.; Feng, S.; Du, C.; Chen, D.; Miao, J.; et al. A 3D Chemically Modified Graphene Hydrogel for Fast, Highly Sensitive, and Selective Gas Sensor. Adv. Sci. 2017, 4, 1600319. [Google Scholar] [CrossRef]
- Mittal, M.; Kumar, A. Carbon nanotube (CNT) gas sensors for emissions from fossil fuel burning. Sens. Actuators B Chem. 2014, 203, 349–362. [Google Scholar] [CrossRef]







| Parameter | Wavelength (nm) | Laser Power (W) | Scan Speed (mm/s) | Beam Diameter (μm) | Frequency (kHz) |
|---|---|---|---|---|---|
| Data | 1064 ± 4 | 1–20 | 0–1500 | 6–9 | 30–60 |
| Stabilization |
Cu/PAN
Ratio (wt%) |
Spin
Coating (rpm) | Thickness (μm) |
Absorbance
(a.u.) |
Laser
Power (W) |
Repeat Count
(times) | ID/IG | I2D/IG |
FWHM
of G + D′ (cm−1) |
|---|---|---|---|---|---|---|---|---|---|
| X | 10 | 1000 | 31 | 0.13 | 4 | - | n/a | n/a | n/a |
| O | 10 | 1000 | 23 | 0.77 | 4 | - | 1.45 | 0.26 | 111.5 |
| O | 0 | 1000 | 20 | 0.60 | 4 | 1 | 1.13 | 0.16 | 100.1 |
| O | 5 | 1000 | 22 | 0.74 | 4 | 1 | 1.08 | 0.33 | 82.6 |
| O | 10 | 1000 | 23 | 0.77 | 4 | 1 | 0.94 | 0.51 | 64.2 |
| O | 10 | 1000 | 23 | 0.77 | 4 | 1 | 0.94 | 0.51 | 64.2 |
| O | 10 | 1500 | 19 | 0.37 | 4 | 1 | 1.02 | 0.43 | 79.2 |
| O | 10 | 2000 | 12 | 0.18 | 4 | 1 | 1.43 | n/a | 107.7 |
| O | 10 | 1000 | 23 | 0.77 | 2 | 1 | 1.37 | n/a | 114.5 |
| O | 10 | 1000 | 23 | 0.77 | 3 | 1 | 1.02 | 0.25 | 87.3 |
| O | 10 | 1000 | 23 | 0.77 | 4 | 1 | 0.94 | 0.51 | 64.2 |
| O | 10 | 1000 | 23 | 0.77 | 5 | 1 | 1.09 | 0.23 | 94.8 |
| O | 10 | 1000 | 23 | 0.77 | 6 | 1 | 1.75 | n/a | 109.3 |
| O | 10 | 1000 | 23 | 0.77 | 4 | 1 | 0.94 | 0.51 | 64.2 |
| O | 10 | 1000 | 23 | 0.77 | 4 | 3 | 1.08 | 0.25 | 92.7 |
| O | 10 | 1000 | 23 | 0.77 | 4 | 5 | 1.64 | n/a | 118.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, Y.-i.; Kim, M.-J.; Lee, D.-Y.; Nam, J.; Jang, A.-R.; Lee, J.-O.; Kim, K.-S. Fabrication of Carbon Nanomaterials Using Laser Scribing on Copper Nanoparticles-Embedded Polyacrylonitrile Films and Their Application in a Gas Sensor. Polymers 2021, 13, 1423. https://doi.org/10.3390/polym13091423
Ko Y-i, Kim M-J, Lee D-Y, Nam J, Jang A-R, Lee J-O, Kim K-S. Fabrication of Carbon Nanomaterials Using Laser Scribing on Copper Nanoparticles-Embedded Polyacrylonitrile Films and Their Application in a Gas Sensor. Polymers. 2021; 13(9):1423. https://doi.org/10.3390/polym13091423
Chicago/Turabian StyleKo, Yong-il, Min-Jae Kim, Dong-Yun Lee, Jungtae Nam, A-Rang Jang, Jeong-O Lee, and Keun-Soo Kim. 2021. "Fabrication of Carbon Nanomaterials Using Laser Scribing on Copper Nanoparticles-Embedded Polyacrylonitrile Films and Their Application in a Gas Sensor" Polymers 13, no. 9: 1423. https://doi.org/10.3390/polym13091423
APA StyleKo, Y.-i., Kim, M.-J., Lee, D.-Y., Nam, J., Jang, A.-R., Lee, J.-O., & Kim, K.-S. (2021). Fabrication of Carbon Nanomaterials Using Laser Scribing on Copper Nanoparticles-Embedded Polyacrylonitrile Films and Their Application in a Gas Sensor. Polymers, 13(9), 1423. https://doi.org/10.3390/polym13091423







