Photocatalytic Activity and Filtration Performance of Hybrid TiO2-Cellulose Acetate Nanofibers for Air Filter Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of TiO2 Nanoparticles
2.3. Fabrication of TiO2-CA Nanofiber Webs
2.4. Characteristics of Synthesized TiO2 Nanoparticles and TiO2-CA Nanofibers
2.5. Contact Angle Measurement and Morphology of TiO2-CA Nanofiber Webs
2.6. Photocatalytic Activity of TiO2-CA Nanofiber Webs for NO Removal
2.7. Filtration Efficiency of TiO2-CA Nanofiber Webs
3. Results and Discussion
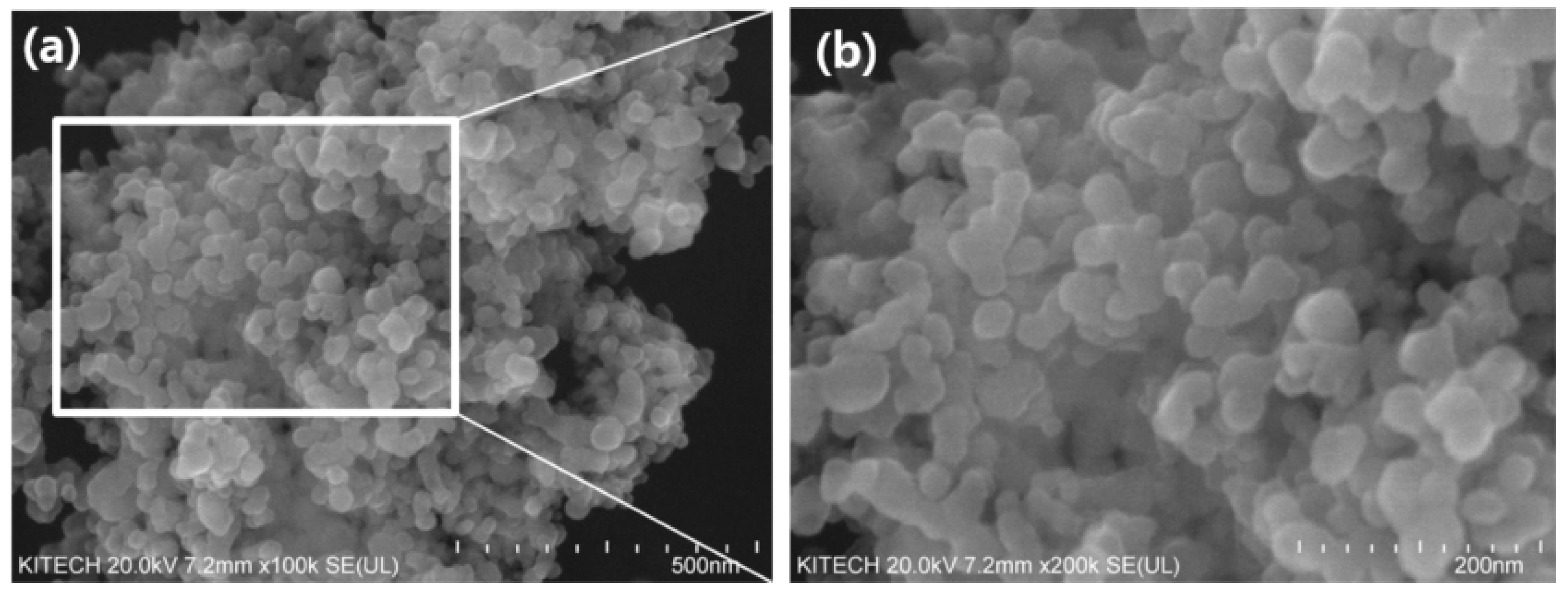
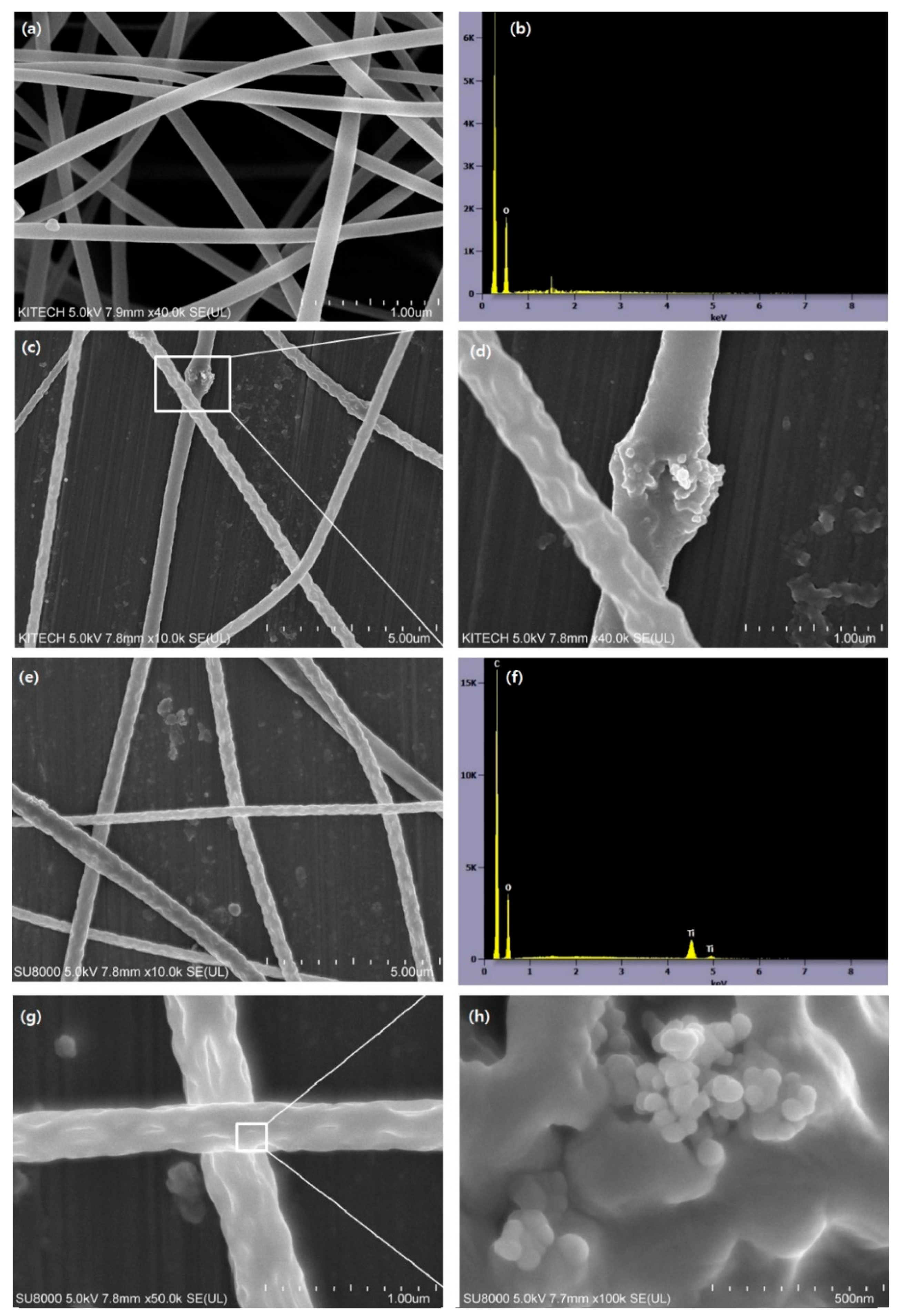
3.1. The Morphology of TiO2 and TiO2-CA Nanofibers
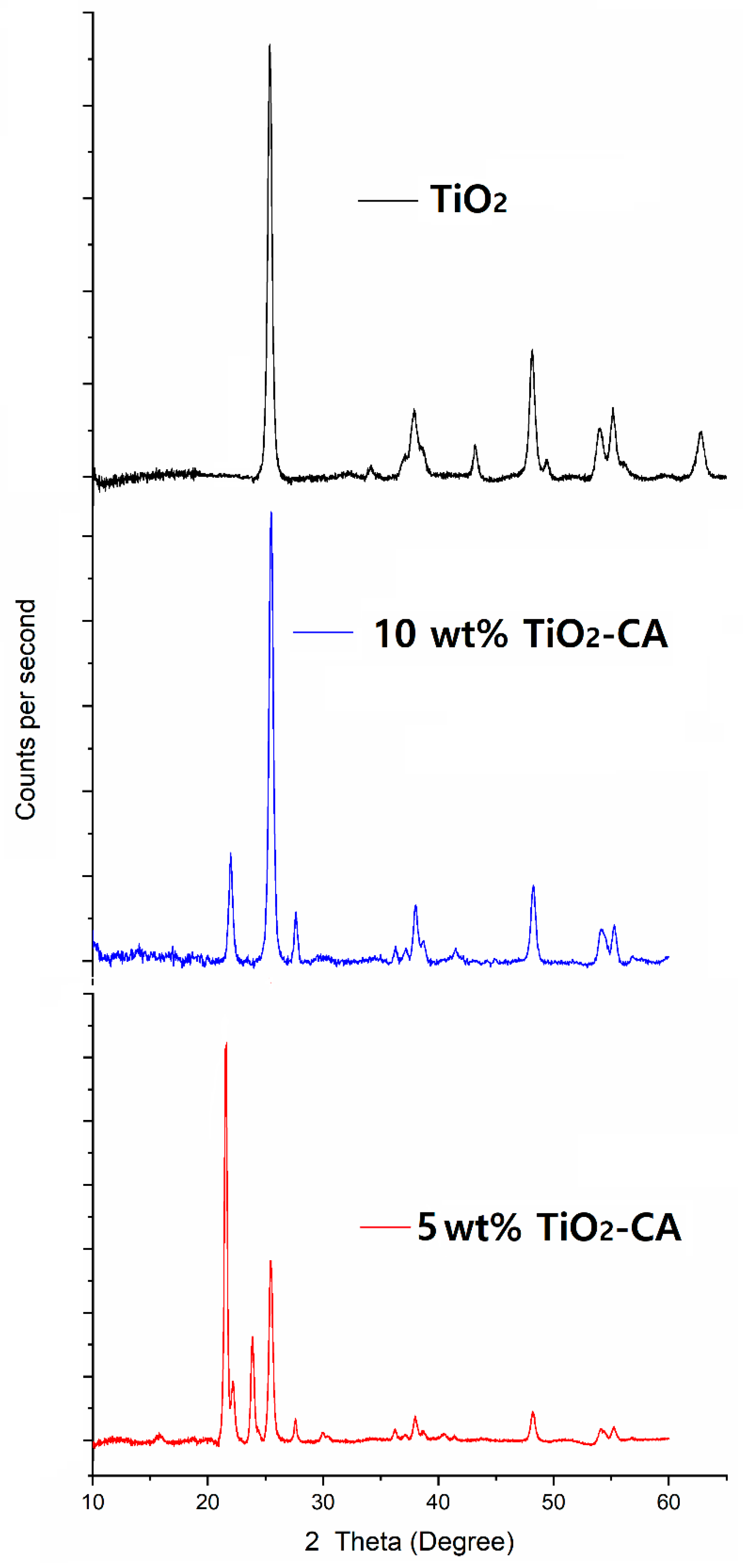
3.2. XRD Analysis of TiO2 Nanoparticles and TiO2-CA Nanofibers
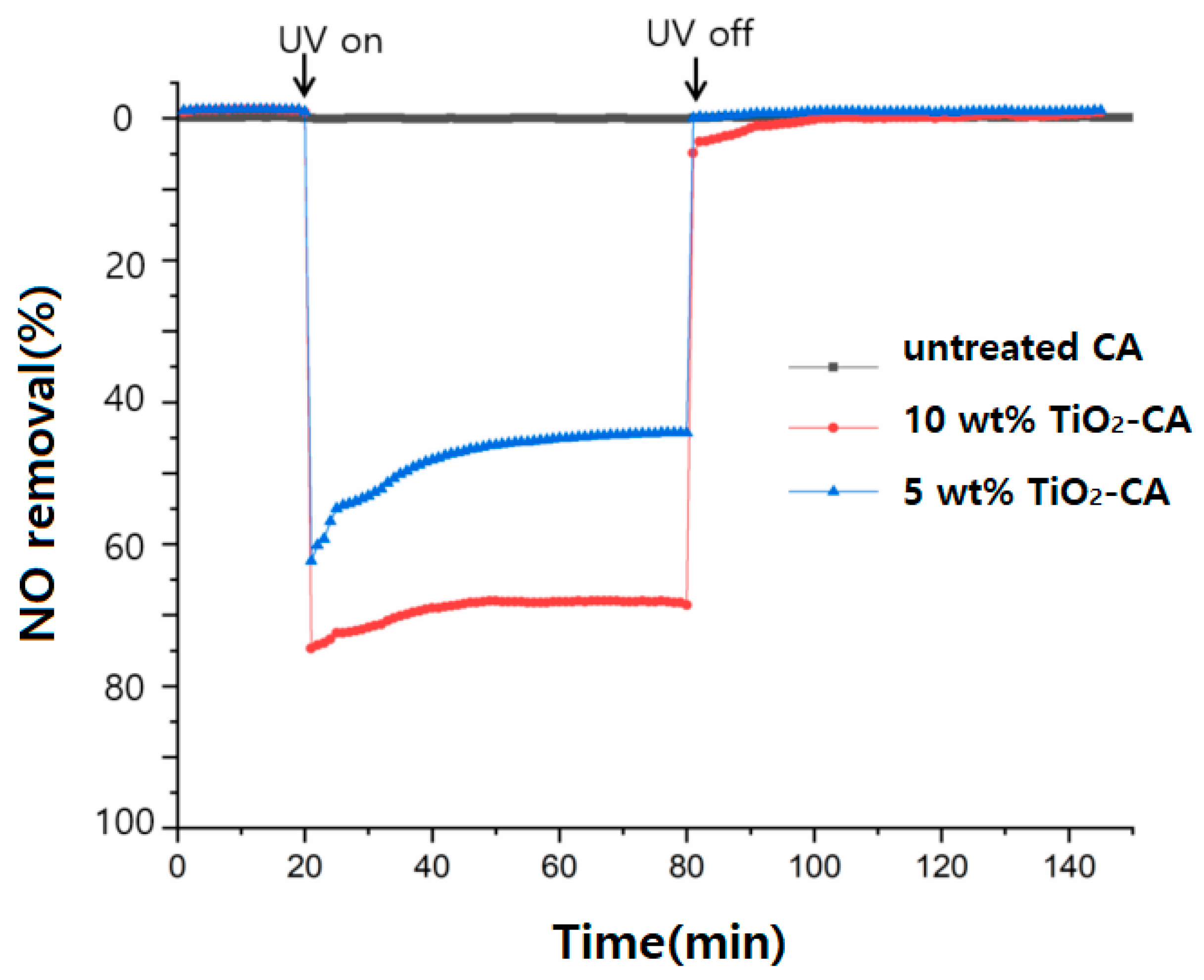
3.3. The Photocatalytic Effect of NO Removal
3.4. The Filtration Efficiency of TiO2-CA Nanofiber Webs
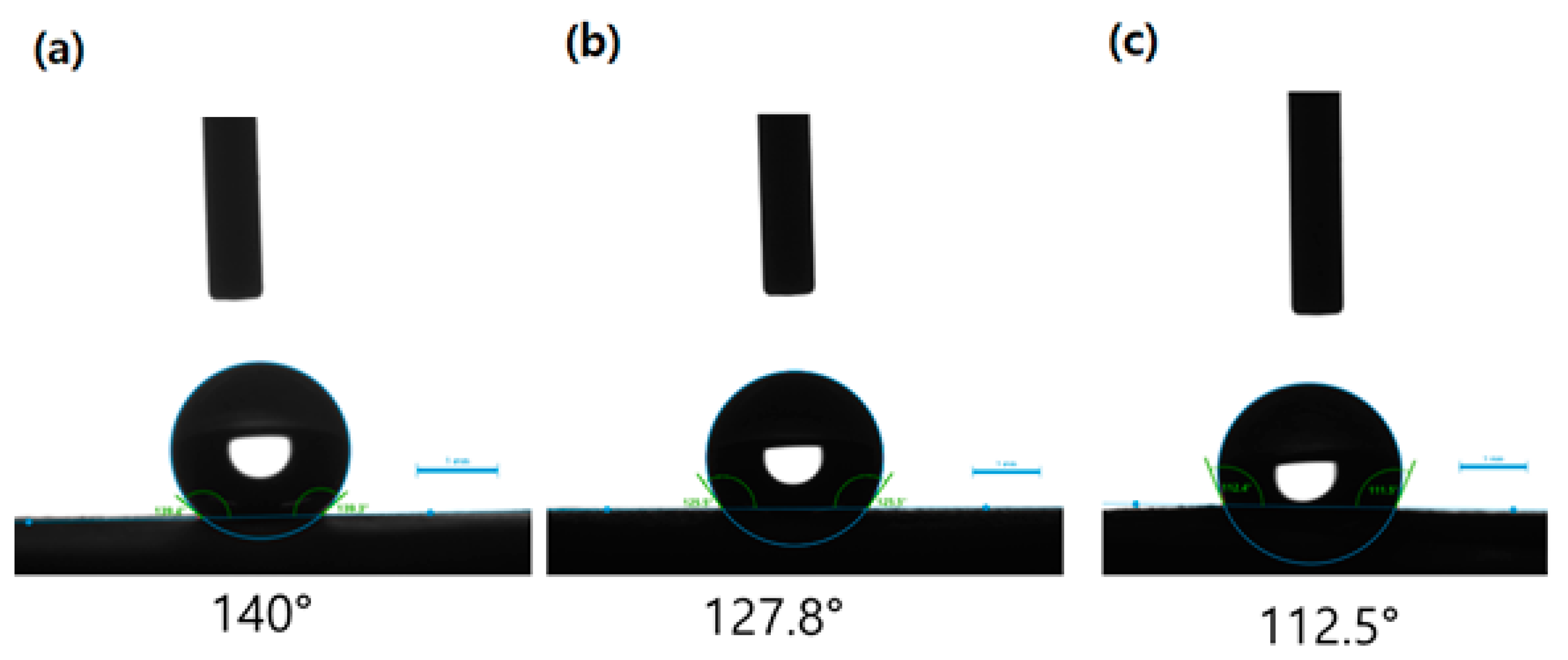
3.5. Contact Angle Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Xiang, J.; Weschler, C.J.; Wang, Q.; Zhang, L.; Mo, J.; Ma, R.; Zhang, J.; Zhang, Y. Reducing Indoor Levels of “Outdoor PM2.5” in Urban China: Impact on Mortalities. Environ. Sci. Technol. 2019, 53, 3119–3127. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Bai, H.; Liu, Y.; Liu, Y.; Zhang, Q. Efficient As(III) removal directly as basic iron arsenite by in-situ generated Fe(III) hydroxide from ferrous sulfate on the surface of CaCO3. Appl. Surf. Sci. 2019, 493, 569–576. [Google Scholar] [CrossRef]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun Nanofibers Membranes for Effective Air Filtration. Macromol. Mater. Eng. 2017, 302, 1600353. [Google Scholar] [CrossRef]
- Jiang, F.; Kittle, J.D.; Tan, X.; Esker, A.R.; Roman, M. Effects of Sulfate Groups on the Adsorption and Activity of Cellulases on Cellulose Substrates. Langmuir 2013, 29, 3280–3291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Cao, Q.; Liu, B.; Guo, H.; Wang, X.; Han, Y.; Sun, G.; Li, Y.; Zhou, J. A novel cellulose acetate/poly (ionic liquid) composite air filter. Cellulose 2020, 27, 3889–3902. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, K.; Zhang, Y.; Wu, J.; Lin, X.; Liu, C.; Hua, J. Study on electro-spin performance of different types of cellulose by activation in the solvent of LiCl/DMAc. J. For. Eng. 2020, 5, 108–113. [Google Scholar]
- Su, C.; Ran, X.; Hu, J.; Shao, C. Photocatalytic Process of Simultaneous Desulfurization and Denitrification of Flue Gas by TiO2–Polyacrylonitrile Nanofibers. Environ. Sci. Technol. 2013, 47, 11562–11568. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Krogman, K.C.; Ma, M.; Hill, R.M.; Hammond, P.T.; Rutledge, G.C. Highly Reactive Multilayer-Assembled TiO2 Coating on Electrospun Polymer Nanofibers. Adv. Mater. 2009, 21, 1252–1256. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Lu, Y.; Huang, J. A hierarchical Ag2O-nanoparticle/TiO2-nanotube composite derived from natural cellulose substance with enhanced photocatalytic performance. Cellulose 2019, 26, 6683–6700. [Google Scholar] [CrossRef]
- Fallah, Z.; Nasr Isfahani, H.; Tajbakhsh, M.; Tashakkorian, H.; Amouei, A. TiO2-grafted cellulose via click reaction: An efficient heavy metal ions bioadsorbent from aqueous solutions. Cellulose 2018, 25, 639–660. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Park, J.K. Bacterial cellulose-titanium dioxide nanocomposites: Nanostructural characteristics, antibacterial mechanism, and biocompatibility. Cellulose 2015, 22, 565–579. [Google Scholar] [CrossRef]
- Chen, K.-N.; Sari, F.N.I.; Ting, J.-M. Multifunctional TiO2/polyacrylonitrile nanofibers for high efficiency PM2.5 capture, UV filter, and anti-bacteria activity. Appl. Surf. Sci. 2019, 493, 157–164. [Google Scholar] [CrossRef]
- Dong, P.; Huang, Z.; Nie, X.; Cheng, X.; Jin, Z.; Zhang, X. Plasma enhanced decoration of nc-TiO2 on electrospun PVDF fibers for photocatalytic application. Mater. Res. Bull. 2019, 111, 102–112. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Q.; Liu, T.; Yang, D.; Liu, Y.; Li, J. Fabrication, characterization and photocatalytic properties of millimeter-long TiO2 fiber with nanostructures using cellulose fiber as a template. J. Alloy. Compd. 2013, 577, 569–574. [Google Scholar] [CrossRef]
- Razzaz, A.; Ghorban, S.; Hosayni, L.; Irani, M.; Aliabadi, M. Chitosan nanofibers functionalized by TiO2 nanoparticles for the removal of heavy metal ions. J. Taiwan Inst. Chem. Eng. 2016, 58, 333–343. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Fabrication of Titania Nanofibers by Electrospinning. Nano Lett. 2003, 3, 555–560. [Google Scholar] [CrossRef]
- Zhai, C.; Jana, S.C. Tuning Porous Networks in Polyimide Aerogels for Airborne Nanoparticle Filtration. ACS Appl. Mater. Interfaces 2017, 9, 30074–30082. [Google Scholar] [CrossRef]
- Lee, E.-J.; An, A.K.; He, T.; Woo, Y.C.; Shon, H.K. Electrospun nanofiber membranes incorporating fluorosilane-coated TiO2 nanocomposite for direct contact membrane distillation. J. Membr. Sci. 2016, 520, 145–154. [Google Scholar] [CrossRef]
- Ding, B.; Lin, J.; Wang, X.; Yu, J.; Yang, J.; Cai, Y. Investigation of silica nanoparticle distribution in nanoporous polystyrene fibers. Soft Matter. 2011, 7, 8376–8383. [Google Scholar] [CrossRef]
- Ananpattarachai, J.; Kajitvichyanukul, P.; Seraphin, S. Visible light absorption ability and photocatalytic oxidation activity of various interstitial N-doped TiO2 prepared from different nitrogen dopants. J. Hazard. Mater. 2009, 168, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, Y.; Yang, D.; Sun, Q.; Liu, Y.; Zhao, H. Lignocellulose Aerogel from Wood-Ionic Liquid Solution (1-Allyl-3-methylimidazolium Chloride) under Freezing and Thawing Conditions. Biomacromolecules 2011, 12, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Martakov, I.S.; Torlopov, M.A.; Mikhaylov, V.I.; Krivoshapkina, E.F.; Silant’ev, V.E.; Krivoshapkin, P.V. Interaction of cellulose nanocrystals with titanium dioxide and peculiarities of hybrid structures formation. J. Sol Gel Sci. Technol. 2018, 88, 13–21. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, R.; Fane, A.G. Fabrication of Bioinspired Composite Nanofiber Membranes with Robust Superhydrophobicity for Direct Contact Membrane Distillation. Environ. Sci. Technol. 2014, 48, 6335–6341. [Google Scholar] [CrossRef]
- Guan, K. Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf. Coat. Technol. 2005, 191, 155–160. [Google Scholar] [CrossRef]
- Liu, J.; Ye, L.; Wooh, S.; Kappl, M.; Steffen, W.; Butt, H.-J. Optimizing Hydrophobicity and Photocatalytic Activity of PDMS-Coated Titanium Dioxide. ACS Appl. Mater. Interfaces 2019, 11, 27422–27425. [Google Scholar] [CrossRef]
- Chen, L.; Sheng, X.; Wang, D.; Liu, J.; Sun, R.; Jiang, L.; Feng, X. High-Performance Triphase Bio-Photoelectrochemical Assay System Based on Superhydrophobic Substrate-Supported TiO2 Nanowire Arrays. Adv. Funct. Mater. 2018, 28, 1801483. [Google Scholar] [CrossRef]
- Sheng, X.; Liu, Z.; Zeng, R.; Chen, L.; Feng, X.; Jiang, L. Enhanced Photocatalytic Reaction at Air-Liquid-Solid Joint Interfaces. J. Am. Chem. Soc. 2017, 139, 12402. [Google Scholar] [CrossRef]

| Materials | 5 wt% TiO2-CA Nanofibers | 10 wt% TiO2-CA Nanofibers | Untreated CA Nanofibers | |||
|---|---|---|---|---|---|---|
| Element | Weight % | Atom % | Weight % | Atom % | Weight % | Atom % |
| C | 47.1 | 56.2 | 42.7 | 53.4 | 49.5 | 56.6 |
| O | 46.7 | 41.9 | 45.7 | 42.9 | 50.5 | 43.4 |
| Ti | 6.3 | 1.9 | 11.6 | 3.6 | - | - |
| Sample | Compound | 2θ (°) |
|---|---|---|
| TiO2 Nanoparticles | Anatase | 25.25°, 37.80°, 38.50°, 48.05°, 53.9°, 55.05°, 62.65° |
| Rutile | 27°, 36°, 55° | |
| TiO2-CA nanofibers | Cellulose | 22.6° |
| Anatase | 25.25°, 37.80°, 38.50°, 48.05°, 53.9°, 55.05° | |
| Rutile | 27°, 36°, 55° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.; Kim, J.; Kim, J. Photocatalytic Activity and Filtration Performance of Hybrid TiO2-Cellulose Acetate Nanofibers for Air Filter Applications. Polymers 2021, 13, 1331. https://doi.org/10.3390/polym13081331
Kwon M, Kim J, Kim J. Photocatalytic Activity and Filtration Performance of Hybrid TiO2-Cellulose Acetate Nanofibers for Air Filter Applications. Polymers. 2021; 13(8):1331. https://doi.org/10.3390/polym13081331
Chicago/Turabian StyleKwon, Miyeon, Juhea Kim, and Juran Kim. 2021. "Photocatalytic Activity and Filtration Performance of Hybrid TiO2-Cellulose Acetate Nanofibers for Air Filter Applications" Polymers 13, no. 8: 1331. https://doi.org/10.3390/polym13081331
APA StyleKwon, M., Kim, J., & Kim, J. (2021). Photocatalytic Activity and Filtration Performance of Hybrid TiO2-Cellulose Acetate Nanofibers for Air Filter Applications. Polymers, 13(8), 1331. https://doi.org/10.3390/polym13081331







