“All Polyimide” Mixed Matrix Membranes for High Performance Gas Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Membranes Fabrication
2.2.1. Synthesis of Tris(4-aminophenyl)amine (TAPA)
2.2.2. Synthesis of 1,3,5-Tris(4-nitrophenyl)benzene (TNPB)
2.2.3. Synthesis of 1,3,5-Tris(4-aminophenyl)benzene (TAPB)
2.2.4. Synthesis of 2,4,6-Tris(4-aminophenyl)-1,3,5-triazine (TAPT)
2.2.5. Synthesis of Microporous Polyimides
2.2.6. Fabrication of Pristine Membrane (6FDA-Durene)
2.2.7. Fabrication of MMMs
2.3. Material Characterization Methods
2.4. Gas Transport Characterization Methods
3. Results and Discussion
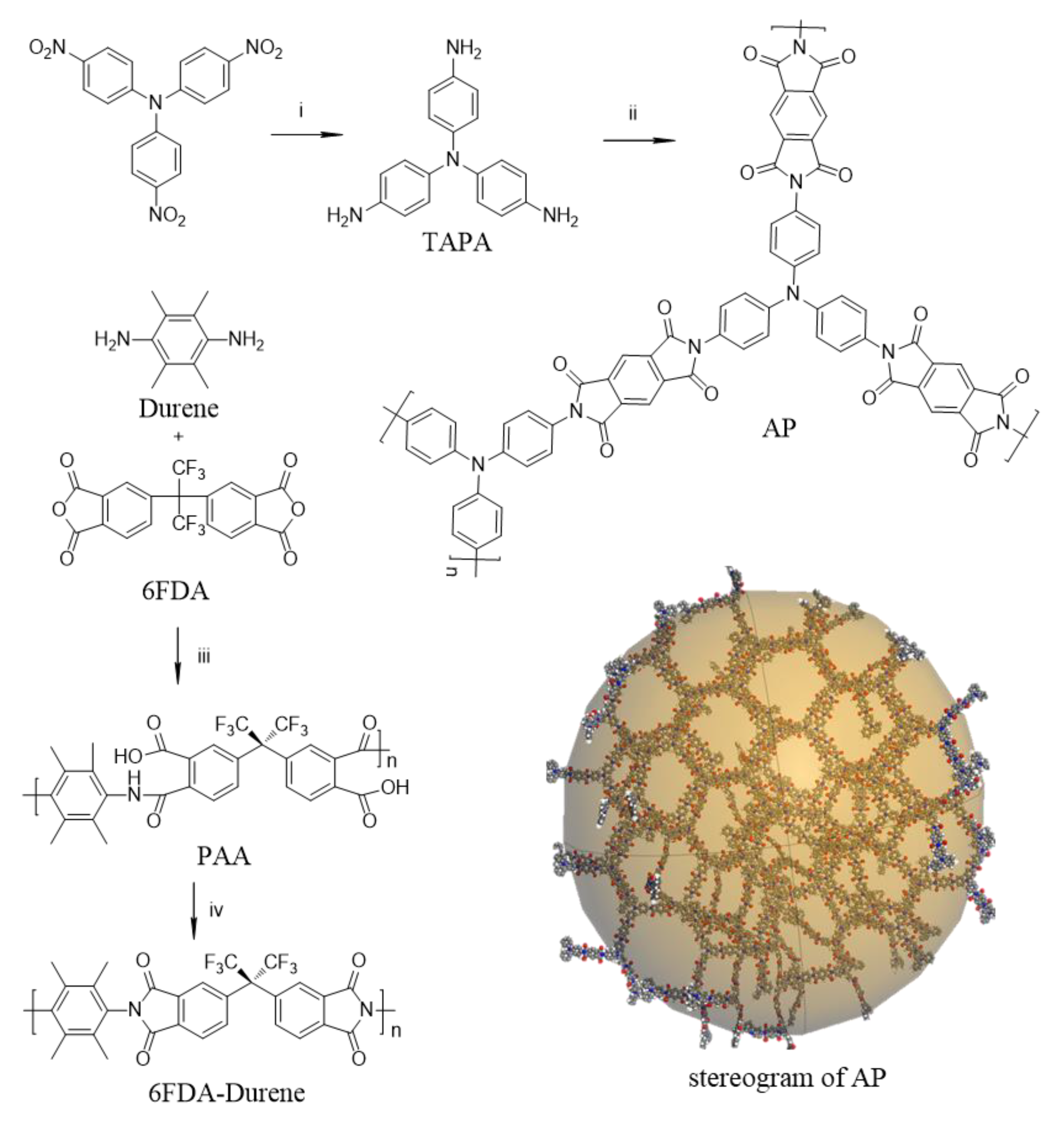
3.1. Synthetic Procedure and Structures of Monomers, Microporous PIs and 6FDA-Durene
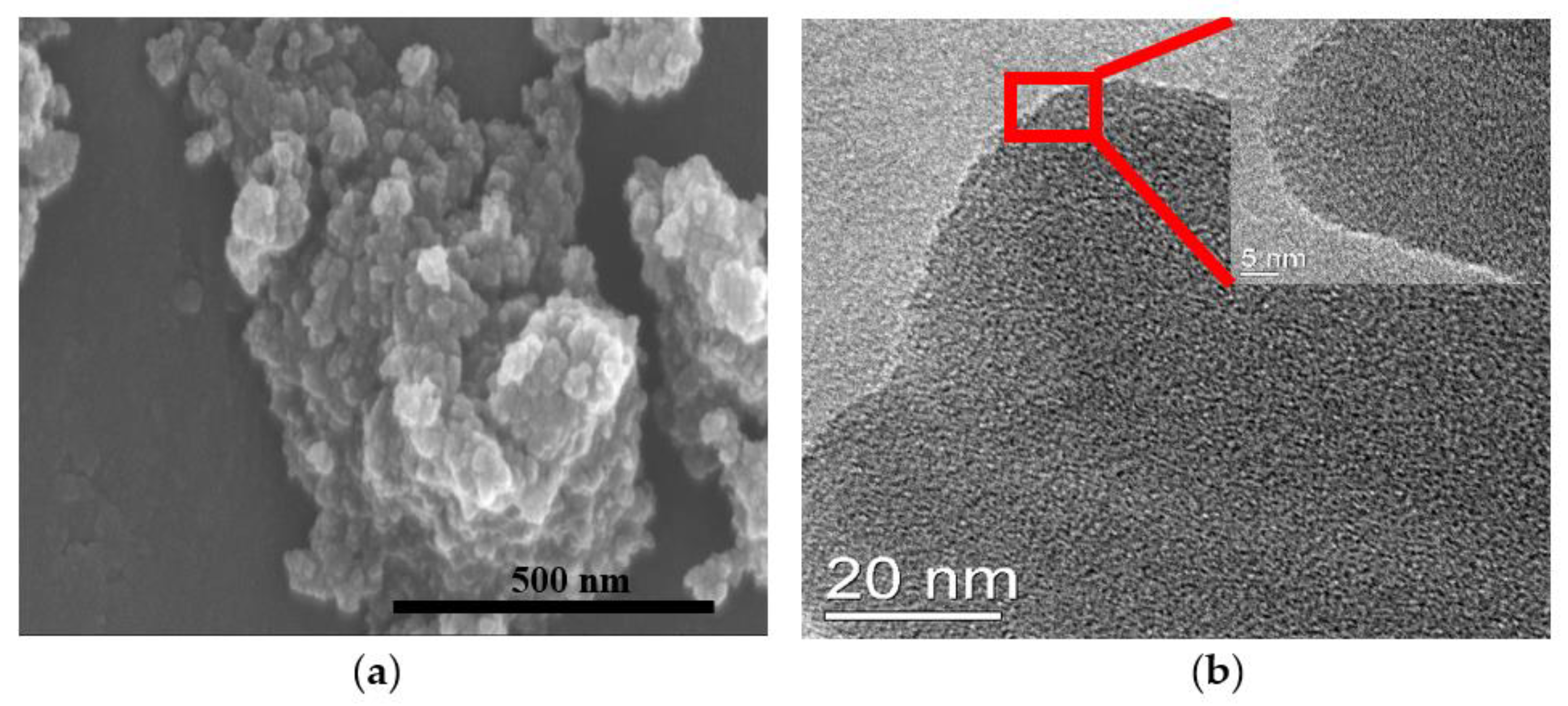
3.2. Morphology of the Microporous PIs
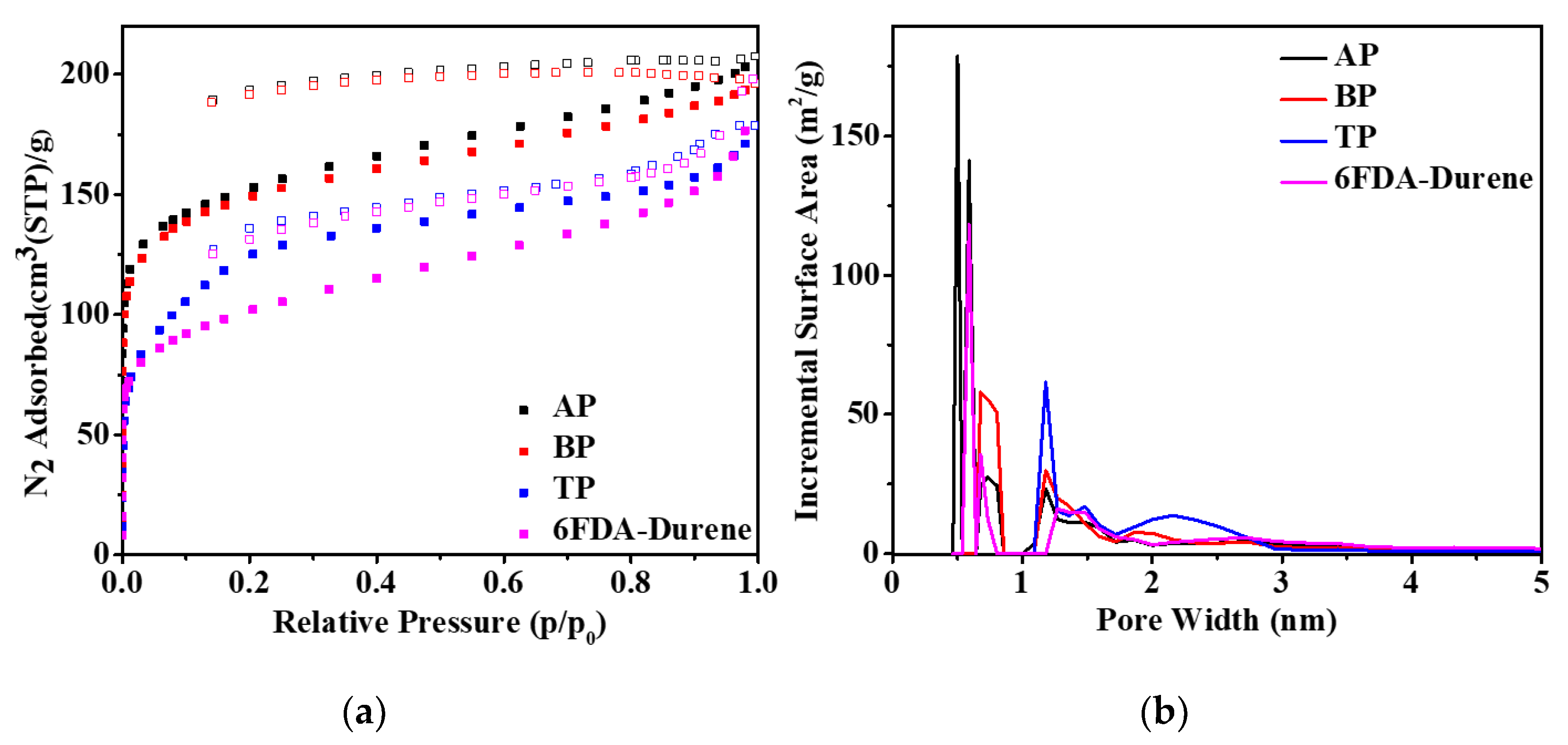
3.3. Porosity Structures of the Microporous PIs and 6FDA-Durene
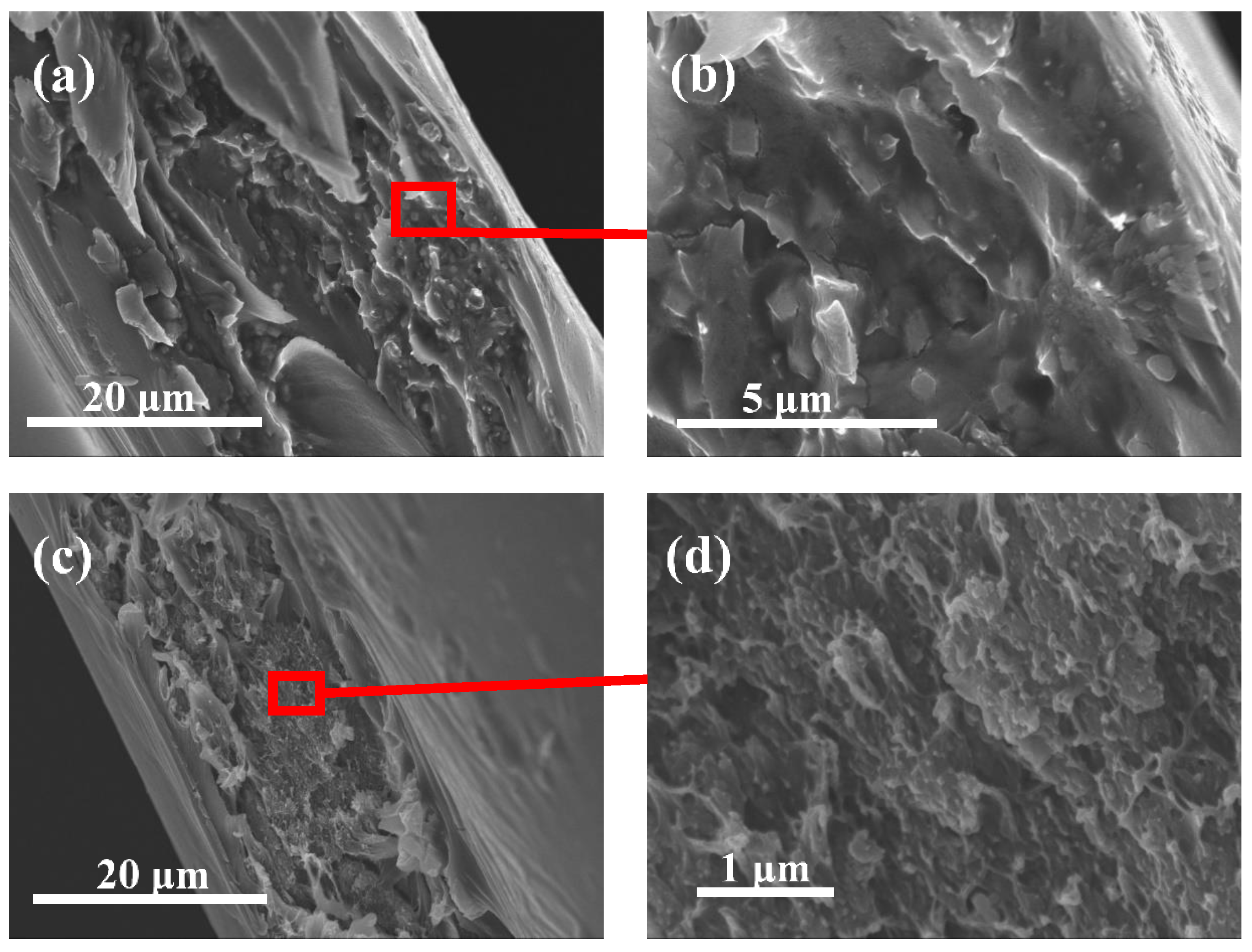
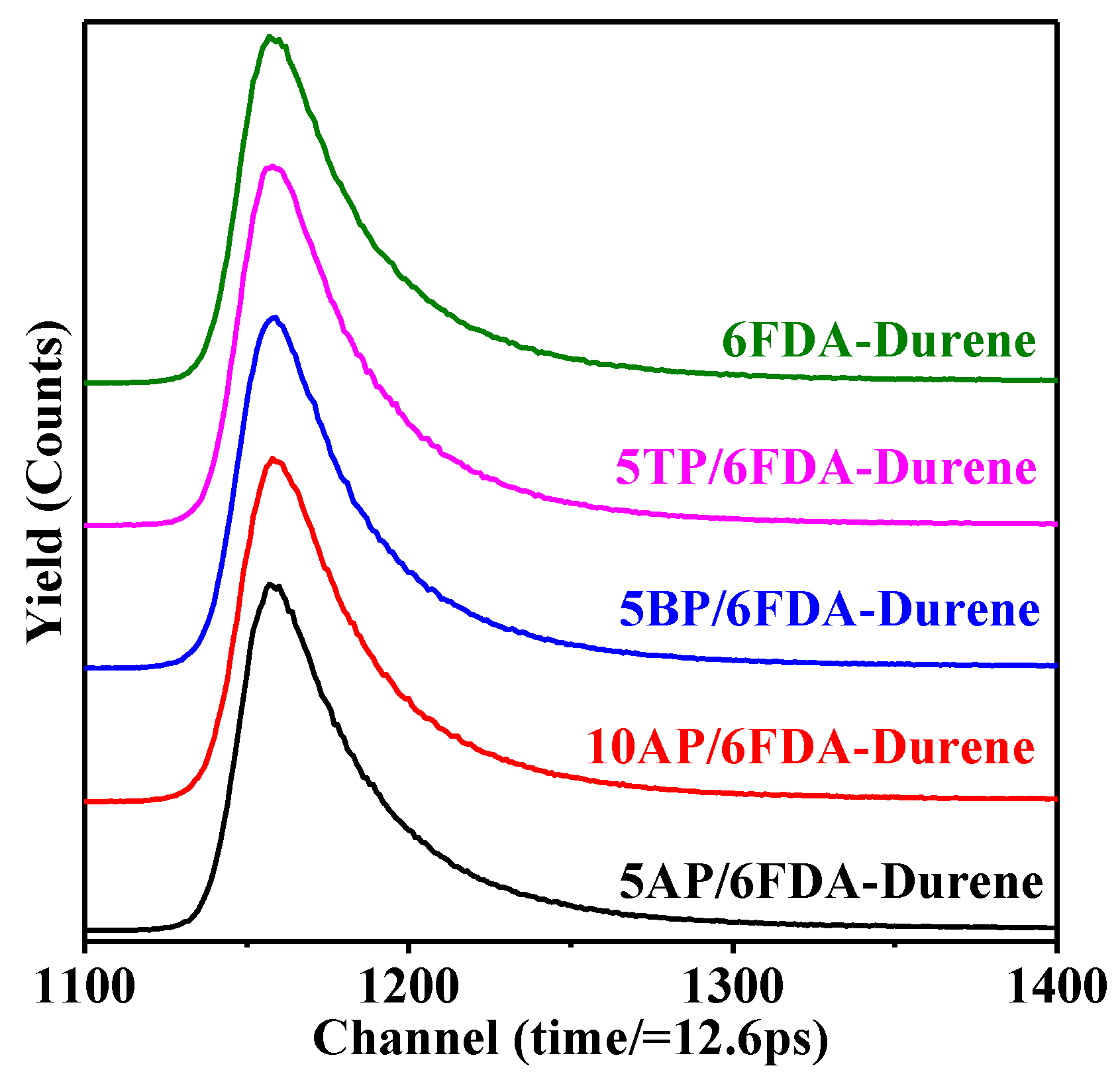
3.4. Interfacial Compatibility Studies of the “All Polyimide” MMMs
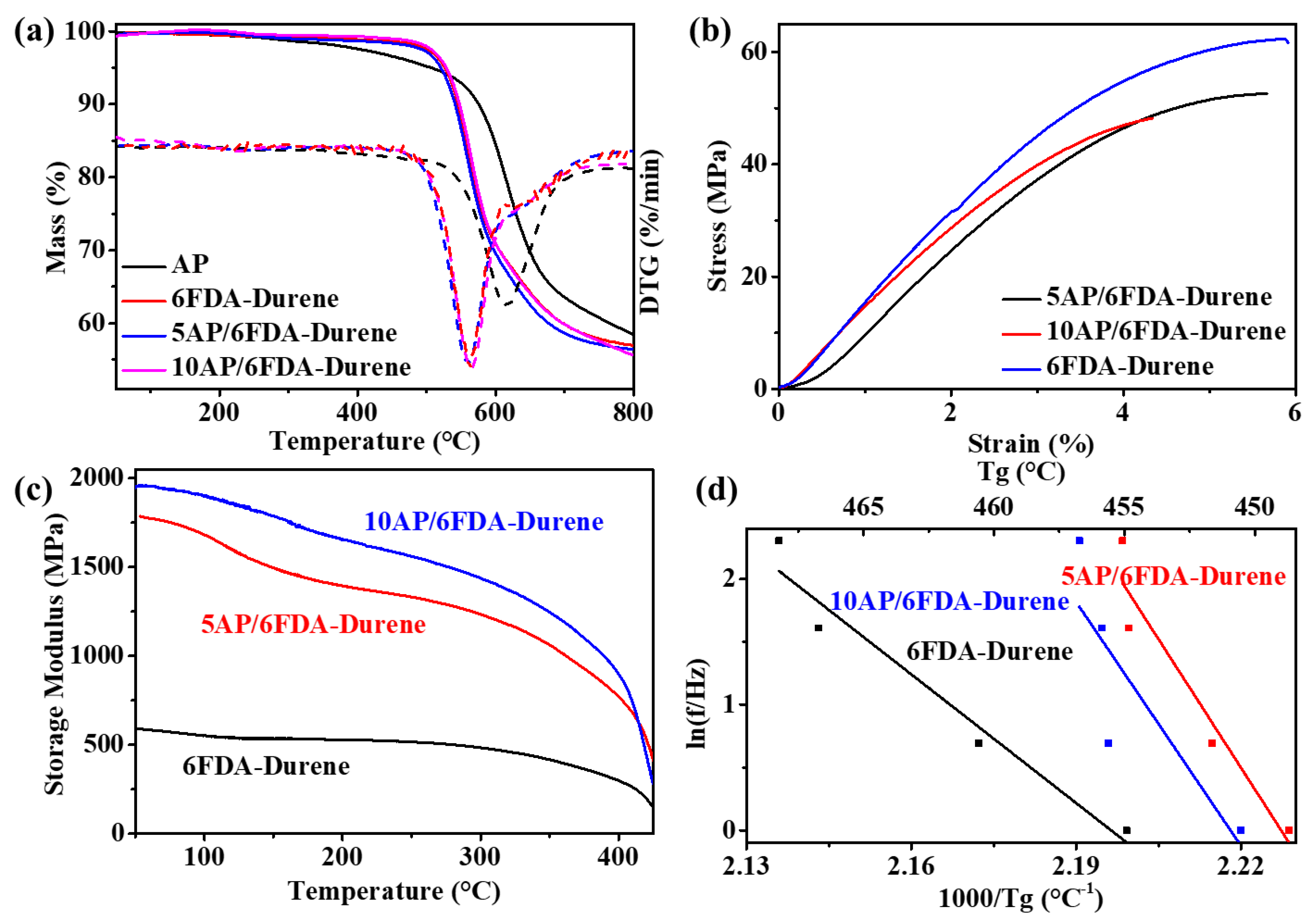
3.5. Thermal and Mechanical Performances of Microporous PIs and Films
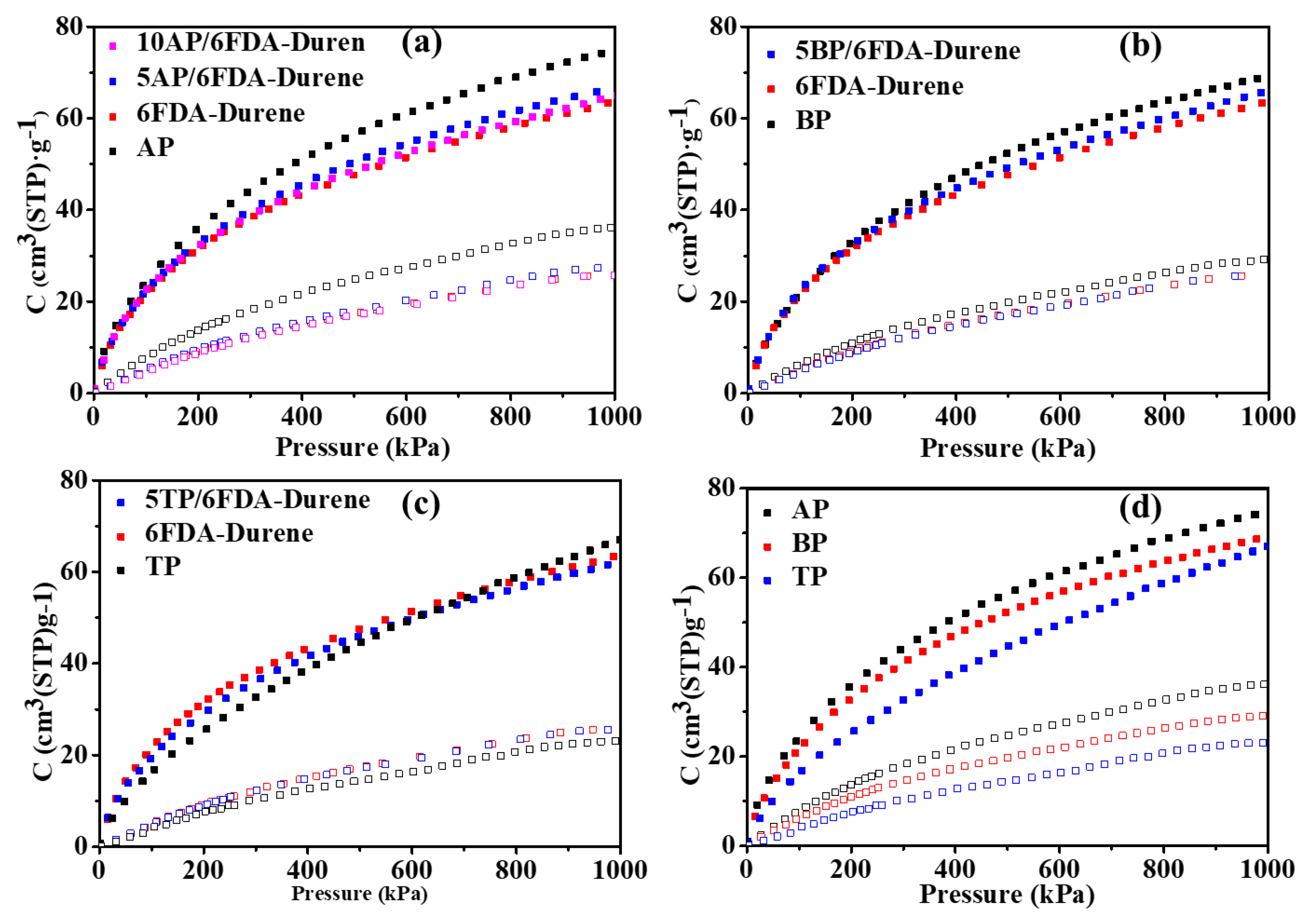
3.6. Gas Transport Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Jeon, E.; Moon, S.Y.; Bae, J.S.; Park, J.W. In situ Generation of Reticulate Micropores through Covalent Network/Polymer Nanocomposite Membranes for Reverse-Selective Separation of Carbon Dioxide. Angew. Chem. Int. Ed. Engl. 2016, 55, 1318–1323. [Google Scholar] [CrossRef]
- Topuz, F.; Abdulhamid, M.A.; Holtzl, T.; Szekely, G. Nanofiber Engineering of Microporous Polyimides Through Electrospinning: Influence of Electrospinning Parameters and Salt Addition. Mater. Des. 2021, 198, 109280. [Google Scholar] [CrossRef]
- Ansaloni, L.; Louradour, E.; Radmanesh, F.; van Veen, H.; Pilz, M.; Simon, C.; Benes, N.E.; Peters, T.A. Upscaling PolyPOSS-Imide Membranes for High Temperature H2 Upgrading. J. Membrane Sci. 2021, 620, 118875. [Google Scholar] [CrossRef]
- Cseri, L.; Topuz, F.; Abdulhamid, M.A.; Alammar, A.; Budd, P.M.; Szekely, G. Electrospun Adsorptive Nanofibrous Membranes from Ion Exchange Polymers to Snare Textile Dyes from Wastewater. Adv. Mater. Tech. 2021. [Google Scholar] [CrossRef]
- Hayek, A.; Alsamah, A.; Alaslai, N.; Maab, H.; Qasem, E.A.; Alhajry, R.H.; Alyami, N.M. Unprecedented Sour Mixed-Gas Permeation Properties of Fluorinated Polyazole-Based Membranes. ACS Appl. Polym. Mater. 2020, 2, 2199–2210. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Robeson, L.M. The Upper Bound Revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, Permeance and Delectivity: A Preferred Way of Reporting Pervaporation Performance Data. J. Membrane Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Carta, M.; Croad, M.; Malpass-Evans, R.; Jansen, J.C.; Bernardo, P.; Clarizia, G.; Friess, K.; Lanc, M.; McKeown, N.B. Triptycene Induced Enhancement of Membrane Gas Selectivity for Microporous Trogers Base Polymers. Adv. Mater. 2014, 26, 3526–3531. [Google Scholar] [CrossRef]
- Park, H.B.; Jung, C.H.; Lee, Y.M.; Hill, A.J.; Pas, S.J.; Mudie, S.T.; Van Wagner, E.; Freeman, B.D.; Cookson, D.J. Polymers with Cavities Tuned for Fast Selective Transport of Small Molecules and Ions. Science 2007, 318, 254–258. [Google Scholar] [CrossRef]
- Chung, T.S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed Matrix Membranes (MMMs) Comprising Organic Polymers with Dispersed Inorganic Fillers for Gas Separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed Matrix Membranes Using Carbon Molecular Sieves—I. Preparation and Experimental Results. J. Membr. Sci. 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed Matrix Membranes Using Carbon Molecular Dieves—II. Modeling Permeation Behavior. J. Membr. Sci. 2003, 211, 335–348. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zhang, X.; Li, J.; Wang, J.; Li, N. Polyvinylamine/Amorphous Metakaolin Mixed-Matrix Composite Membranes with Facilitated Transport Carriers for Highly Efficient CO2/N2 Separation. J. Membr. Sci. 2020, 599, 117828. [Google Scholar] [CrossRef]
- Bachman, J.E.; Smith, Z.P.; Li, T.; Xu, T.; Long, J.R. Enhanced Ethylene Separation and Plasticization Resistance in Polymer Membranes Incorporating Metal-Organic Framework Nanocrystals. Nat. Mater. 2016, 15, 845–849. [Google Scholar] [CrossRef]
- Ahmadi, M.; Tas, E.; Kilic, A.; Kumbaraci, V.; Talinli, N.; Ahunbay, M.G.; Tantekin-Ersolmaz, S.B. Highly CO2 Selective Microporous Metal-Imidazolate Framework-Based Mixed Matrix Membranes. ACS Appl. Mater. Interfaces 2017, 9, 35936–35946. [Google Scholar] [CrossRef]
- Hartini Suhaimi, N.; Fong Yeong, Y.; Jusoh, N.; Farid Mohd Asri, M. Amine-Functionalized Metal Organic Framework (MOF)/6FDA-Durene Composite Membranes for CO2 Removal from CH4. Mater. Today Proc. 2019, 19, 1730–1737. [Google Scholar] [CrossRef]
- Lin, R.; Ge, L.; Hou, L.; Strounina, E.; Rudolph, V.; Zhu, Z. Mixed Matrix Membranes with Strengthened MOFs/Polymer Interfacial Interaction and Improved Membrane Performance. ACS Appl. Mater. Interfaces 2014, 6, 5609–5618. [Google Scholar] [CrossRef]
- Lin, R.; Ge, L.; Diao, H.; Rudolph, V.; Zhu, Z. Ionic Liquids as the MOFs/Polymer Interfacial Binder for Efficient Membrane Separation. ACS Appl. Mater. Interfaces 2016, 8, 32041–32049. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Wu, A.X.; Chi, W.S.; Asinger, P.A.; Lin, S.; Hypsher, A.; Smith, Z.P. Mixed-Matrix Membranes Formed from Imide-Functionalized UiO-66-NH2 for Improved Interfacial Compatibility. ACS Appl. Mater. Interfaces 2019, 11, 31257–31269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.; Jin, J. Microporous Polyimides with Rationally Designed Chain Structure Achieving High Performance for Gas Separation. Macromolecules 2014, 47, 7477–7483. [Google Scholar] [CrossRef]
- Rangel Rangel, E.; Maya, E.M.; Sánchez, F.; de Abajo, J.; de la Campa, J.G. Gas Separation Properties of Mixed-Matrix Membranes Containing Porous Polyimides Fillers. J. Membr. Sci. 2013, 447, 403–412. [Google Scholar] [CrossRef]
- Li, G.Y.; Wang, Z.G. Microporous Polyimides with Uniform Pores for Adsorption and Separation of CO2 Gas and Organic Vapors. Macromolecules 2013, 46, 3058–3066. [Google Scholar] [CrossRef]
- Liebl, M.R.; Senker, J. Microporous Functionalized Triazine-Based Polyimides with High CO2 Capture Capacity. Chem. Mater. 2013, 25, 970–980. [Google Scholar] [CrossRef]
- Fang, Q.; Zhuang, Z.; Gu, S.; Kaspar, R.B.; Zheng, J.; Wang, J.; Qiu, S.; Yan, Y. Designed Synthesis of Large-Pore Crystalline Polyimide Covalent Organic Frameworks. Nat. Commun. 2014, 5, 4503. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Park, J.Y.; Paul, D.R. Correlation and Prediction of Gas Permeability in Glassy Polymer Membrane Materials via a Modified Free Volume Based Group Contribution Method. J. Membr. Sci. 1997, 125, 23–39. [Google Scholar] [CrossRef]
- Li, P.; Paul, D.R.; Chung, T.-S. High Performance Membranes Based on Ionic Liquid Polymers for CO2 Separation from the Flue Gas. Green Chem. 2012, 14, 1052–1063. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, Q.; Zhu, L.; Lin, H.; Kazanowska, B.A.; Doherty, C.M.; Hill, A.J.; Gao, P.; Guo, R. Highly Selective and Permeable Microporous Polymer Membranes for Hydrogen Purification and CO2 Removal from Natural Gas. Chem. Mater. 2018, 30, 5322–5332. [Google Scholar] [CrossRef]
- Omole, I.C.; Adams, R.T.; Miller, S.J.; Koros, W.J. Effects of CO2 on a High Performance Hollow-Fiber Membrane for Natural Gas Purification. Ind. Eng. Chem. Res. 2010, 49, 4887–4896. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Liu, G.; Qiu, W.; Bhuwania, N.; Chinn, D.; Koros, W.J. Surprising Plasticization Benefits in Natural Gas Upgrading Using Polyimide Membranes. J. Membr. Sci. 2020, 593, 117430. [Google Scholar] [CrossRef]
- Wu, S.; Gu, S.; Zhang, A.; Yu, G.; Wang, Z.; Jian, J.; Pan, C. A Rational Construction of Microporous Imide-Bridged Covalent–Organic Polytriazines for High-Enthalpy Dmall Gas Absorption. J. Mater. Chem. A 2015, 3, 878–885. [Google Scholar] [CrossRef]
- Thompson, K.A.; Mathias, R.; Kim, D.; Kim, J.; Rangnekar, N.; Johnson, J.R.; Hoy, S.J.; Bechis, I.; Tarzia, A.; Jelfs, K.E.; et al. N-Aryl-linked spirocyclic polymers for membrane separations of complex hydrocarbon mixtures. Science 2020, 369, 310–315. [Google Scholar] [CrossRef]
- Ma, X.; Salinas, O.; Litwiller, E.; Pinnau, I. Novel Spirobifluorene- and Dibromospirobifluorene-Based Polyimides of Intrinsic Microporosity for Gas Separation Applications. Macromolecules 2013, 46, 9618–9624. [Google Scholar] [CrossRef]
- Ma, X.; Swaidan, R.J.; Wang, Y.; Hsiung, C.-e.; Han, Y.; Pinnau, I. Highly Compatible Hydroxyl-Functionalized Microporous Polyimide-ZIF-8 Mixed Matrix Membranes for Energy Efficient Propylene/Propane Separation. ACS Appl. Nano. Mater. 2018, 1, 3541–3547. [Google Scholar] [CrossRef]
- Moore, T.T.; Koros, W.J. Non-Ideal Effects in Organic-Inorganic Materials for Gas Separation Membranes. J. Membr. Sci. 2005, 739, 87–98. [Google Scholar] [CrossRef]
- Zhuang, Y.; Seong, J.G.; Do, Y.S.; Jo, H.J.; Cui, Z.; Lee, J.; Lee, Y.M.; Guiver, M.D. Intrinsically Microporous Soluble Polyimides Incorporating Trögers Base for Membrane Gas Separation. Macromolecules 2014, 47, 3254–3262. [Google Scholar] [CrossRef]
- Chi, W.S.; Sundell, B.J.; Zhang, K.; Harrigan, D.J.; Hayden, S.C.; Smith, Z.P. Mixed-Matrix Membranes Formed from Multi-Dimensional Metal-Organic Frameworks for Enhanced Gas Transport and Plasticization Resistance. ChemSusChem 2019, 12, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chang, J.Y. Preparation of a Compressible and Hierarchically Porous Polyimide Sponge via the Sol-Gel Process of an Aliphatic Tetracarboxylic Dianhydride and an Aromatic Triamine. Chem. Commun. 2016, 52, 10419–10422. [Google Scholar] [CrossRef]
- Ito, A.; Yasuda, T.; Yoshioka, T.; Yoshida, A.; Li, X.; Hashimoto, K.; Nagai, K.; Shibayama, M.; Watanabe, M. Sulfonated Polyimide/Ionic Liquid Composite Membranes for CO2 Separation: Transport Properties in Relation to Their Nanostructures. Macromolecules 2018, 51, 7112–7120. [Google Scholar] [CrossRef]
- Mashhadikhan, S.; Moghadassi, A.; Ebadi Amooghin, A.; Sanaeepur, H. Interlocking a Synthesized Polymer and Bifunctional Filler Containing the Same Polymers Monomer for Conformable Hybrid Membrane Systems. J. Mater. Chem. A 2020, 8, 3942–3955. [Google Scholar] [CrossRef]
- Hasebe, S.; Aoyama, S.; Tanaka, M.; Kawakami, H. CO2 Separation of Polymer Membranes Containing Silica Nanoparticles with Gas Permeable Nano-Space. J. Membr. Sci. 2017, 536, 148–155. [Google Scholar] [CrossRef]
- Japip, S.; Xiao, Y.; Chung, T.-S. Particle-Size Effects on Gas Transport Properties of 6FDA-Durene/ZIF-71 Mixed Matrix Membranes. Ind. Eng. Chem. Res. 2016, 55, 9507–9517. [Google Scholar] [CrossRef]
- Lin, R.; Ge, L.; Liu, S.; Rudolph, V.; Zhu, Z. Mixed-Matrix Membranes with Metal–Organic Framework-Decorated CNT Fillers for Efficient CO2 Separation. ACS Appl. Mater. Interfaces 2015, 7, 14750–14757. [Google Scholar] [CrossRef] [PubMed]
- Japip, S.; Liao, K.S.; Chung, T.S. Molecularly Tuned Free Volume of Vapor Cross-Linked 6FDA-Durene/ZIF-71 MMMs for H2/CO2 Separation at 150 °C. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, K.; Ho Li, J.P.; Huang, J.; Yuan, B.; Zhang, C.; Yu, Y.; Yang, Y.; Lee, Y.; Li, T. Engineering Plasticization Resistant Gas Separation Membranes Using Metal–Organic Nanocapsules. Chem. Sci. 2020, 11, 4687–4694. [Google Scholar] [CrossRef]
- Bachman, J.E.; Long, J.R. Plasticization-Resistant Ni2(dobdc)/Polyimide Composite Membranes for the Removal of CO2 from Natural Gas. Ener. Environ. Sci. 2016, 9, 2031–2036. [Google Scholar] [CrossRef]
- Japip, S.; Liao, K.-S.; Xiao, Y.; Chung, T.-S. Enhancement of Molecular-Sieving Properties by Constructing Surface Nano-Metric Layer via Vapor Cross-Linking. J. Membr. Sci. 2016, 497, 248–258. [Google Scholar] [CrossRef]
- Vu, M.-T.; Lin, R.; Diao, H.; Zhu, Z.; Bhatia, S.K.; Smart, S. Effect of Ionic Liquids (ILs) on MOFs/Polymer Interfacial Enhancement in Mixed Matrix Membranes. J. Membr. Sci. 2019, 587. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. Enhanced Gas Separation Performance Using Mixed Matrix Membranes Containing Zeolite T and 6FDA-Durene Polyimide. J. Membr. Sci. 2017, 525, 175–186. [Google Scholar] [CrossRef]
- Maserati, L.; Meckler, S.M.; Bachman, J.E.; Long, J.R.; Helms, B.A. Diamine-Appended Mg2(dobpdc) Nanorods as Phase-Change Fillers in Mixed-Matrix Membranes for Efficient CO2/N2 Separations. Nano. Lett. 2017, 17, 6828–6832. [Google Scholar] [CrossRef]
- Aguilar-Lugo, C.; Suárez-García, F.; Hernández, A.; Miguel, J.A.; Lozano, Á.E.; de la Campa, J.G.; Álvarez, C. New Materials for Gas Separation Applications: Mixed Matrix Membranes Made from Linear Polyimides and Porous Polymer Networks Having Lactam Groups. Ind. Eng. Chem. Res. 2019, 58, 9585–9595. [Google Scholar] [CrossRef]
- Yang, Y.; Goh, K.; Wang, R.; Bae, T.H. High-Performance Nanocomposite Membranes Realized by Efficient Molecular Sieving with CuBDC Nanosheets. Chem. Commun. 2017, 53, 4254–4257. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Goh, K.; Weerachanchai, P.; Bae, T.-H. 3D Covalent Organic Framework for Morphologically Induced High-Performance Membranes with Strong Resistance Toward Physical Aging. J. Membr. Sci. 2019, 574, 235–242. [Google Scholar] [CrossRef]
- Liu, G.; Labreche, Y.; Chernikova, V.; Shekhah, O.; Zhang, C.; Belmabkhout, Y.; Eddaoudi, M.; Koros, W.J. Zeolite-Like MOF Nanocrystals Incorporated 6FDA-Polyimide Mixed-Matrix Membranes for CO2/CH4 Separation. J. Membr. Sci. 2018, 565, 186–193. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Seoane, B.; Keskin, D.; Duim, N.; Rodenas, T.; Shahid, S.; Sorribas, S.; Le Guillouzer, C.; Clet, G.; Tellez, C.; et al. Metal Organic Framework Crystals in Mixed-Matrix Membranes: Impact of the Filler Morphology on the Gas Separation Performance. Adv. Funct. Mater. 2016, 26, 3154–3163. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Koros, W.J. Performance of 6FDA–6FpDA Polyimide for Propylene/Propane Separations. J. Membr. Sci. 2010, 365, 399–408. [Google Scholar] [CrossRef]
- Liao, K.-S.; Lai, J.-Y.; Chung, T.-S. Metal Ion Modified PIM-1 and Its Application for Propylene/Propane Separation. J. Membr.Sci. 2016, 515, 36–44. [Google Scholar] [CrossRef]
- Peng, D.; Wang, S.; Tian, Z.; Wu, X.; Wu, Y.; Wu, H.; Xin, Q.; Chen, J.; Cao, X.; Jiang, Z. Facilitated Transport Membranes by Incorporating Graphene Nanosheets with High Zinc IonLoading for Enhanced CO2 Separation. J. Membr. Sci. 2017, 522, 351–362. [Google Scholar] [CrossRef]
- Li, T.Y.; Liu, J.J.; Zhao, S.S.; Chen, Z.Q.; Huang, H.H.; Guo, R.L.; Chen, Y.M. Microporous Polyimides Containing Bulky Tetra-o-Isopropyl and Naphthalene Groups for Gas Separation Membranes. J. Membr. Sci. 2019, 585, 282–288. [Google Scholar] [CrossRef]
- Popp, N.; Homburg, T.; Stock, N.; Senker, J. Porous Imine-Based Networks with Protonated Imine Linkages for Carbon Dioxide Separation from Mixtures with Nitrogen and Methane. J. Mater. Chem. A 2015, 3, 18492–18504. [Google Scholar] [CrossRef]
- Zhao, S.; Liao, J.; Li, D.; Wang, X.; Li, N. Blending of Compatible Polymer of Intrinsic Microporosity (PIM-1) with Trögers Base Polymer for Gas Separation Membranes. J. Membr. Sci. 2018, 566, 77–86. [Google Scholar] [CrossRef]
- Aframehr, W.M.; Molki, B.; Bagheri, R.; Heidarian, P.; Davodi, S.M. Characterization and Enhancement of the Gas Separation Properties of Mixed Matrix Membranes: Polyimide with Nickel Oxide Nanoparticles. Chem. Eng. Res. Des. 2020, 153, 789–805. [Google Scholar] [CrossRef]



| SBET (m2·g−1) | Particle Size (μm) a | D 0.9 (μm) b | FFV (%) c | |
|---|---|---|---|---|
| AP | 520.58 ± 27.89 | 3.14 ± 0.06 | 5.62 | 18.00 |
| BP | 510.52 ± 30.50 | 2.98 ± 0.08 | 7.23 | 11.56 |
| TP | 453.46 ± 16.47 | 2.62 ± 0.03 | 6.50 | NA |
| 6FDA-Durene | 353.87 ± 6.28 | NA | NA | 14.93 |
| d-Spacing (Å) a |
Young’s Modulus
(GPa) |
Elongation at Break
(%) |
Tensile Strength
(MPa) |
Density
(g·cm−3) b |
Density
(g·cm−3) | |
|---|---|---|---|---|---|---|
| AP | 4.09 | NA | NA | NA | NA | 1.29 c |
| BP | 4.07 | NA | NA | NA | NA | 1.36 c |
| TP | 4.12 | NA | NA | NA | NA | 1.41 c |
| 6FDA-Durene | 6.57 | 1.00 ± 0.26 | 5.92 ± 1.53 | 53.89 ± 1.77 | NA | 1.33 d |
| 5AP/FDA-Durene | 6.34 | 1.59 ± 0.01 | 4.35 ± 1.11 | 46.89 ± 2.43 | 1.32 | 1.31 d |
| 10AP/FDA-Durene | 6.30 | 1.66 ± 0.02 | 3.29 ± 0.59 | 48.95 ± 5.75 | 1.32 | 1.31 d |
| 5BP/FDA-Durene | 6.69 | 1.52 ± 0.01 | 5.05 ± 0.78 | 49.50 ± 2.06 | 1.33 | 1.32 d |
| 5TP/FDA-Durene | 6.72 | 1.47 ± 0.22 | 3.94 ± 0.57 | 45.53 ± 6.69 | 1.33 | 1.34 d |
| E’ (GPa) a | Eα (kJ·mol−1) | |
|---|---|---|
| 6FDA-Durene | 0.60 ± 0.01 | 284 |
| 5AP/FDA-Durene | 1.79 ± 0.11 | 537 |
| 10AP/FDA-Durene | 1.95 ± 0.09 | 566 |
| H2 | N2 | O2 | CH4 | CO2 | H2/CH2 | O2/N2 | CO2/N2 | CO2/CH4 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 6FDA-Durene | P a | 549.14 | 34.95 | 108.11 | 23.20 | 500.17 | 23.67 | 3.09 | 14.31 | 21.56 |
| D b | 43.35 | 23.41 | 28.63 | 10.11 | 21.38 | 4.28 | 1.22 | 0.91 | 2.12 | |
| S c | 12.67 | 1.49 | 3.78 | 2.30 | 23.39 | 5.52 | 2.53 | 15.67 | 10.19 | |
| 5AP/FDA-Durene | P | 1277.25 | 85.58 | 293.91 | 71.45 | 1291.13 | 17.88 | 3.43 | 15.09 | 18.07 |
| D | 73.68 | 54.74 | 60.60 | 26.41 | 47.03 | 2.79 | 1.11 | 0.85 | 1.78 | |
| S | 17.33 | 1.56 | 4.85 | 2.71 | 27.45 | 6.41 | 3.10 | 17.56 | 10.14 | |
| 10AP/FDA-Durene | P | 1015.20 | 72.13 | 228.14 | 53.32 | 959.16 | 19.04 | 3.16 | 13.30 | 17.99 |
| D | 125.48 | 76.24 | 97.95 | 27.38 | 58.65 | 4.58 | 1.28 | 0.77 | 2.14 | |
| S | 8.09 | 0.95 | 2.33 | 1.95 | 16.35 | 4.15 | 2.46 | 17.29 | 8.40 | |
| 5BP/FDA-Durene | P | 1164.25 | 77.15 | 253.00 | 60.89 | 1047.43 | 19.12 | 3.28 | 13.58 | 17.20 |
| D | 116.44 | 59.86 | 86.31 | 28.13 | 56.74 | 4.14 | 1.44 | 0.95 | 2.02 | |
| S | 10.00 | 1.29 | 2.93 | 2.16 | 18.46 | 4.62 | 2.27 | 14.32 | 8.53 | |
| 5TP/FDA-Durene | P | 861.58 | 49.20 | 173.83 | 37.89 | 753.07 | 22.74 | 3.53 | 15.31 | 19.88 |
| D | 100.71 | 35.29 | 63.57 | 16.53 | 35.58 | 6.09 | 1.80 | 1.01 | 2.15 | |
| S | 8.55 | 1.39 | 2.73 | 2.29 | 21.17 | 3.73 | 1.96 | 15.18 | 9.24 |
| kDc | be | R2 | I (%) | τ3 (ns) | V3 (Å3) | FFV f (%) | FFV g (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6FDA-Durene | 13.80 | 36.25 | 0.025 | 44.02 | 0.0077 | 99.96 | 10.7 | 3.4 | 244.6 | 4.7 | 14.93 |
| 5AP/FDA-Durene | 14.60 | 88.44 | 0.025 | 48.69 | 0.0069 | 99.93 | 11.1 | 3.4 | 245.7 | 4.9 | 15.78 |
| 10AP/FDA-Durene | 13.93 | 68.87 | 0.026 | 43.88 | 0.0082 | 99.97 | 11.4 | 3.3 | 234.7 | 4.8 | 15.99 |
| 5BP/FDA-Durene | 14.26 | 73.48 | 0.026 | 45.65 | 0.0076 | 99.98 | 10.6 | 3.4 | 246.9 | 4.7 | 15.21 |
| 5TP/FDA-Durene | 13.81 | 54.54 | 0.025 | 43.04 | 0.0067 | 99.92 | 6.7 | 3.0 | 202.4 | 2.4 | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zheng, Z.; Zhang, Z.; Li, N.; Liu, S.; Chi, Z.; Xu, J.; Zhang, Y. “All Polyimide” Mixed Matrix Membranes for High Performance Gas Separation. Polymers 2021, 13, 1329. https://doi.org/10.3390/polym13081329
Li M, Zheng Z, Zhang Z, Li N, Liu S, Chi Z, Xu J, Zhang Y. “All Polyimide” Mixed Matrix Membranes for High Performance Gas Separation. Polymers. 2021; 13(8):1329. https://doi.org/10.3390/polym13081329
Chicago/Turabian StyleLi, Maijun, Zhibo Zheng, Zhiguang Zhang, Nanwen Li, Siwei Liu, Zhenguo Chi, Jiarui Xu, and Yi Zhang. 2021. "“All Polyimide” Mixed Matrix Membranes for High Performance Gas Separation" Polymers 13, no. 8: 1329. https://doi.org/10.3390/polym13081329
APA StyleLi, M., Zheng, Z., Zhang, Z., Li, N., Liu, S., Chi, Z., Xu, J., & Zhang, Y. (2021). “All Polyimide” Mixed Matrix Membranes for High Performance Gas Separation. Polymers, 13(8), 1329. https://doi.org/10.3390/polym13081329





