Optical pH Sensor Based on Immobilization Anthocyanin from Dioscorea alata L. onto Polyelectrolyte Complex Pectin–Chitosan Membrane for a Determination Method of Salivary pH
Abstract
1. Introduction
2. Chemicals and Apparatus
3. Experimental
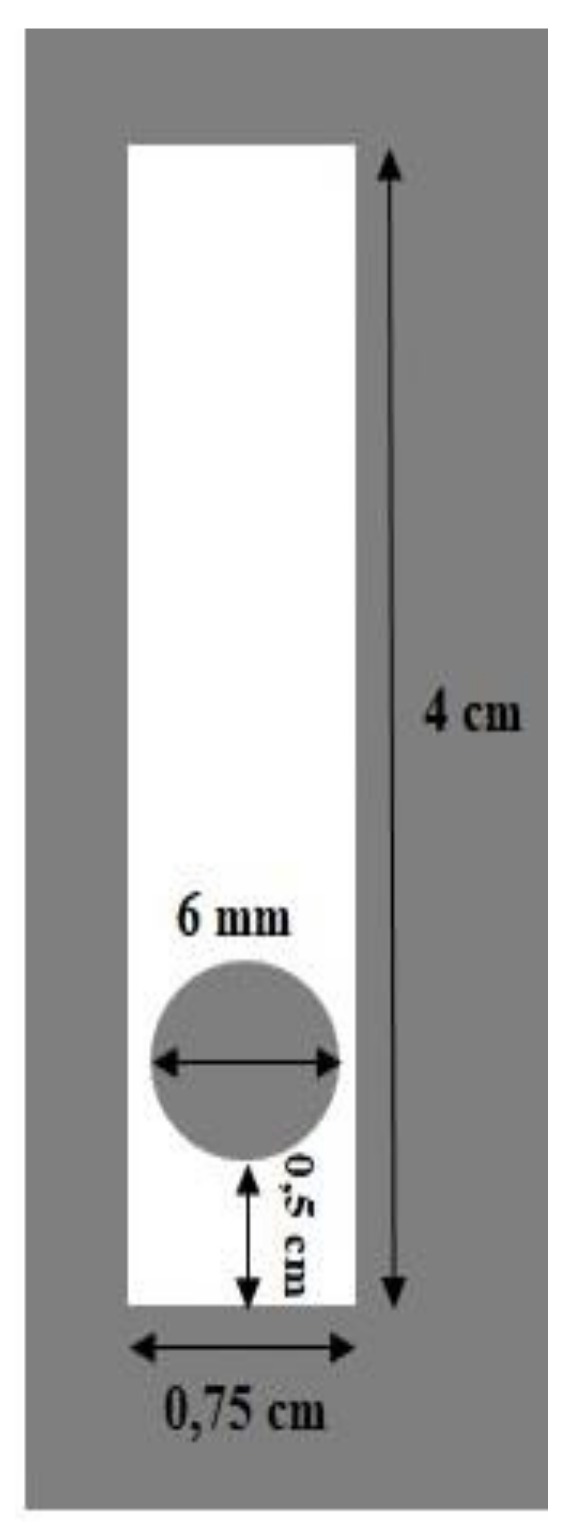
3.1. Preparation of Anthocyanin/Pectin–Chitosan
3.2. Membrane Characterization
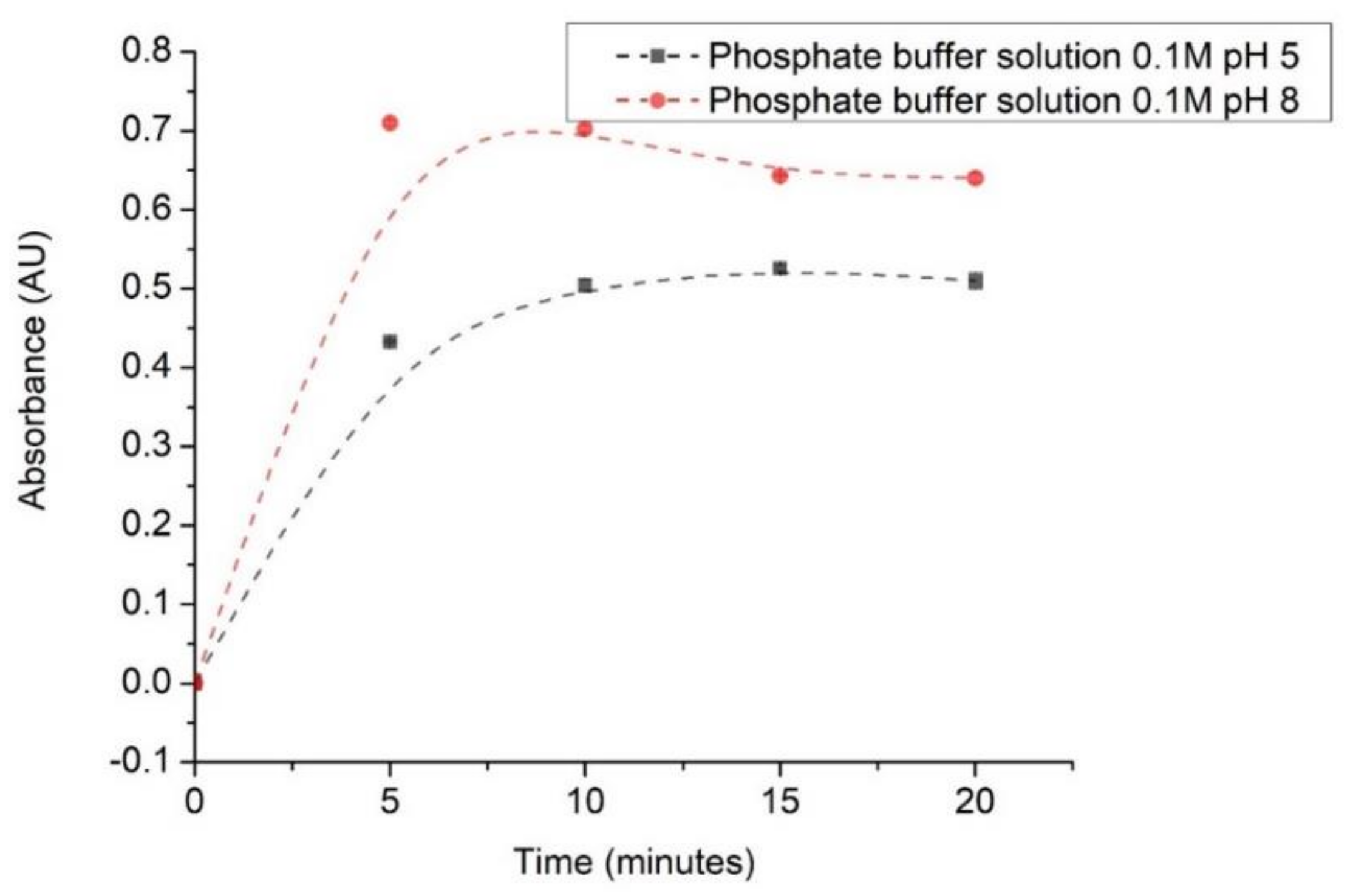
3.3. Sensitivity Optical pH Sensor Determination in Citrate and Phosphate Buffer Solutions
3.4. Determination of Sensitivity of Optical pH Sensors at Various Phosphate Buffer Concentrations
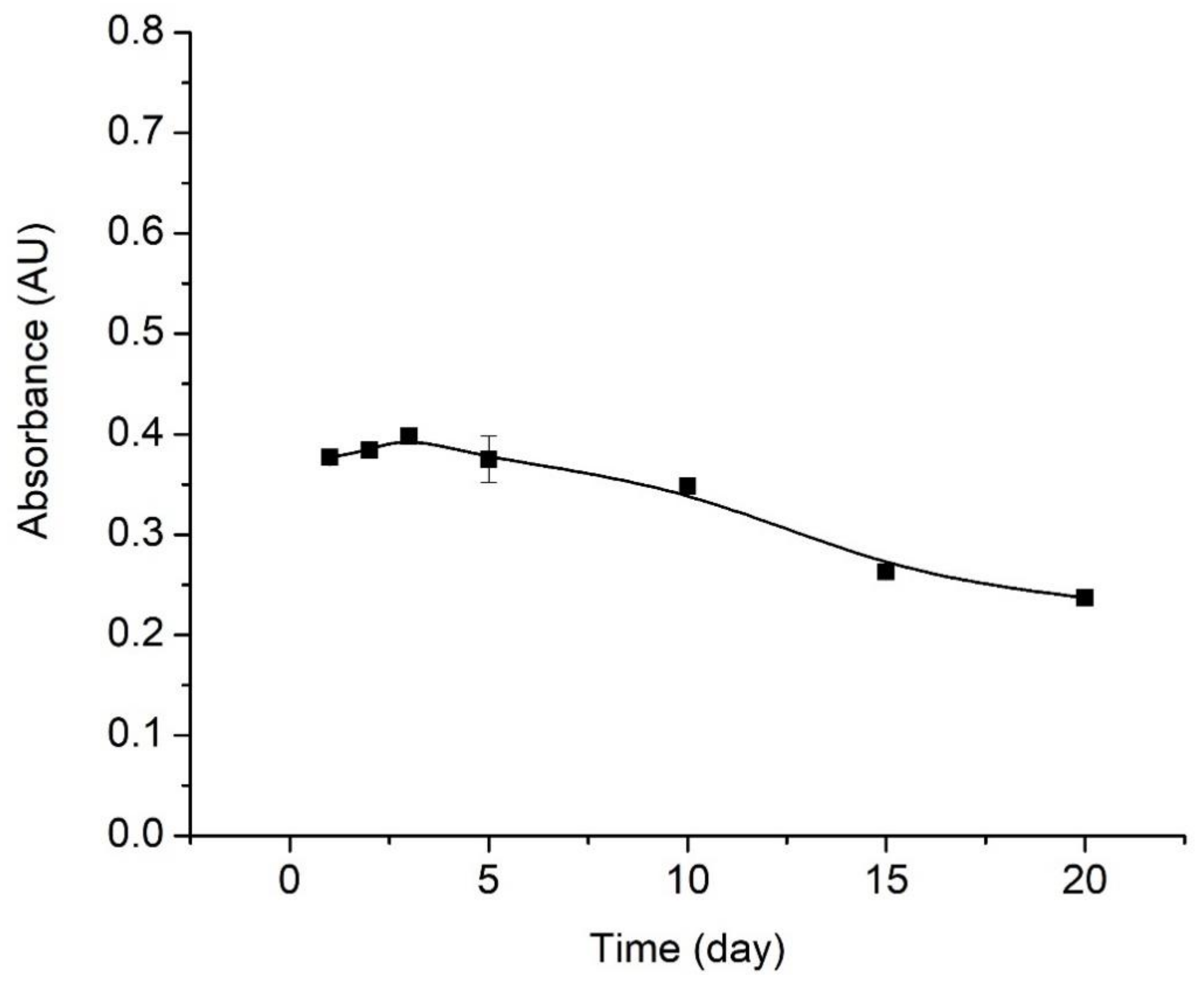
3.5. Reproducibility, Response Time, and Lifetime Studies on the Optical pH Sensor
3.6. Real Sample Measurement
4. Results and Discussion
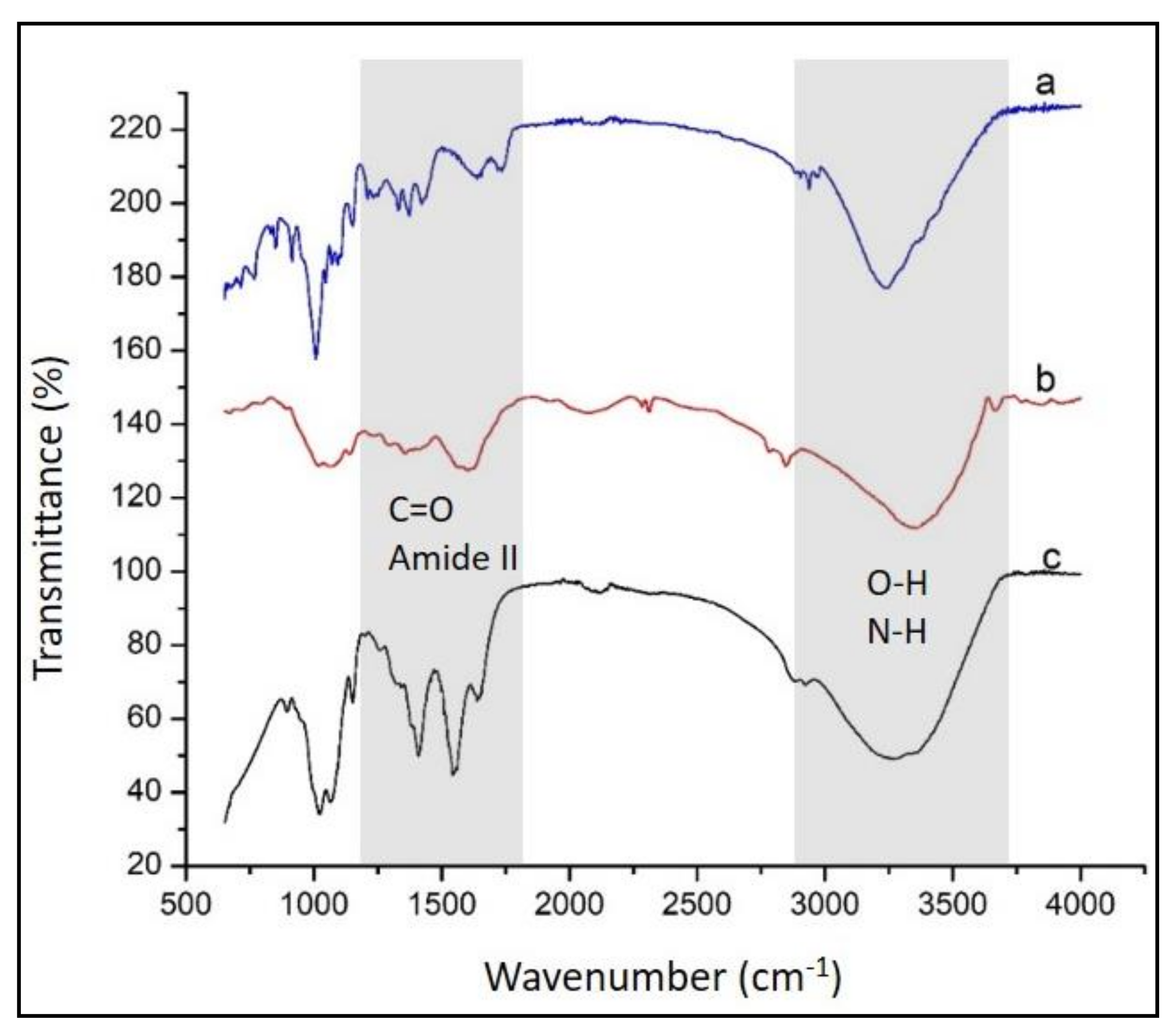
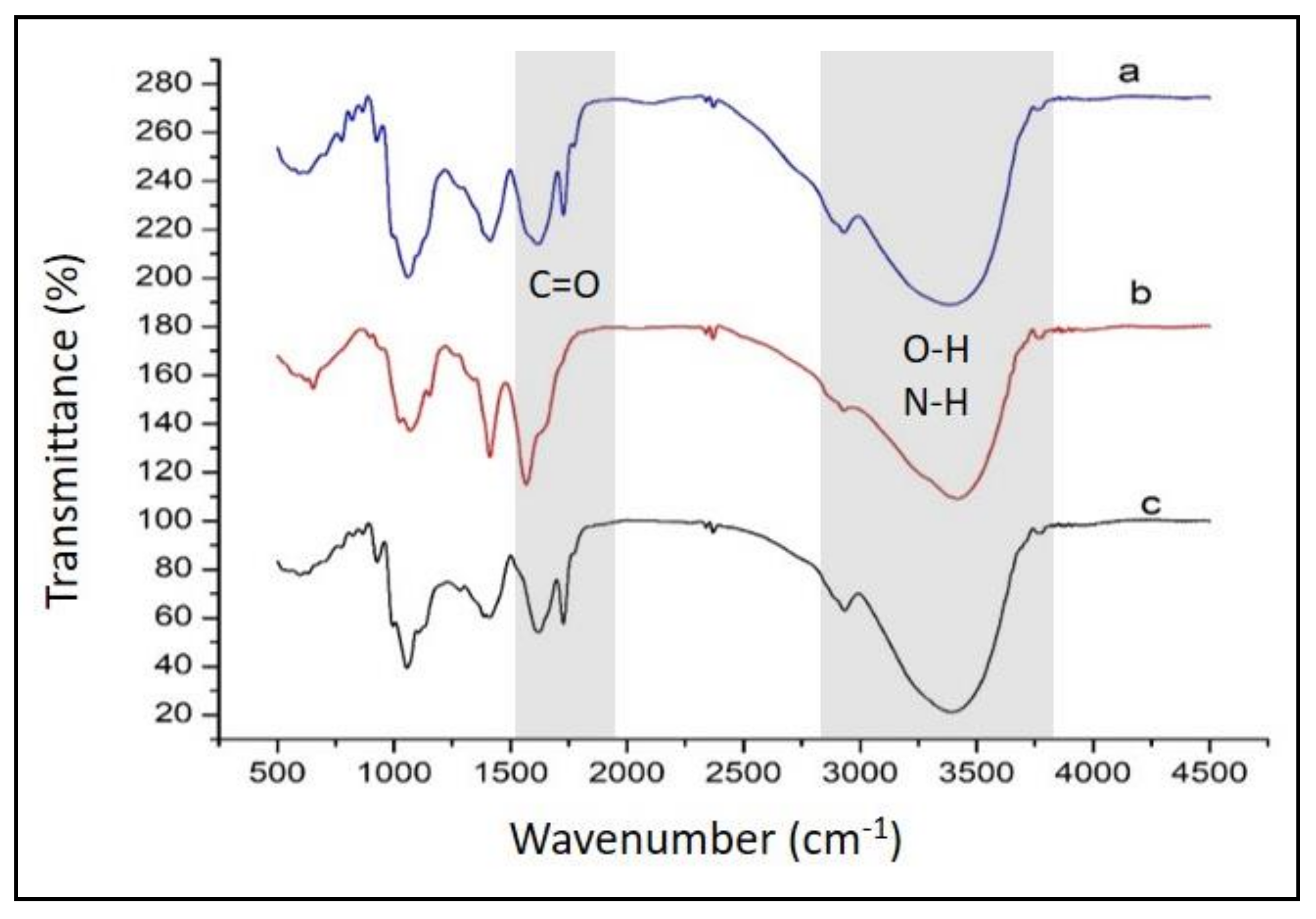
4.1. Synthesis and FTIR Characterization of the Investigated Systems
4.2. Morphological Properties
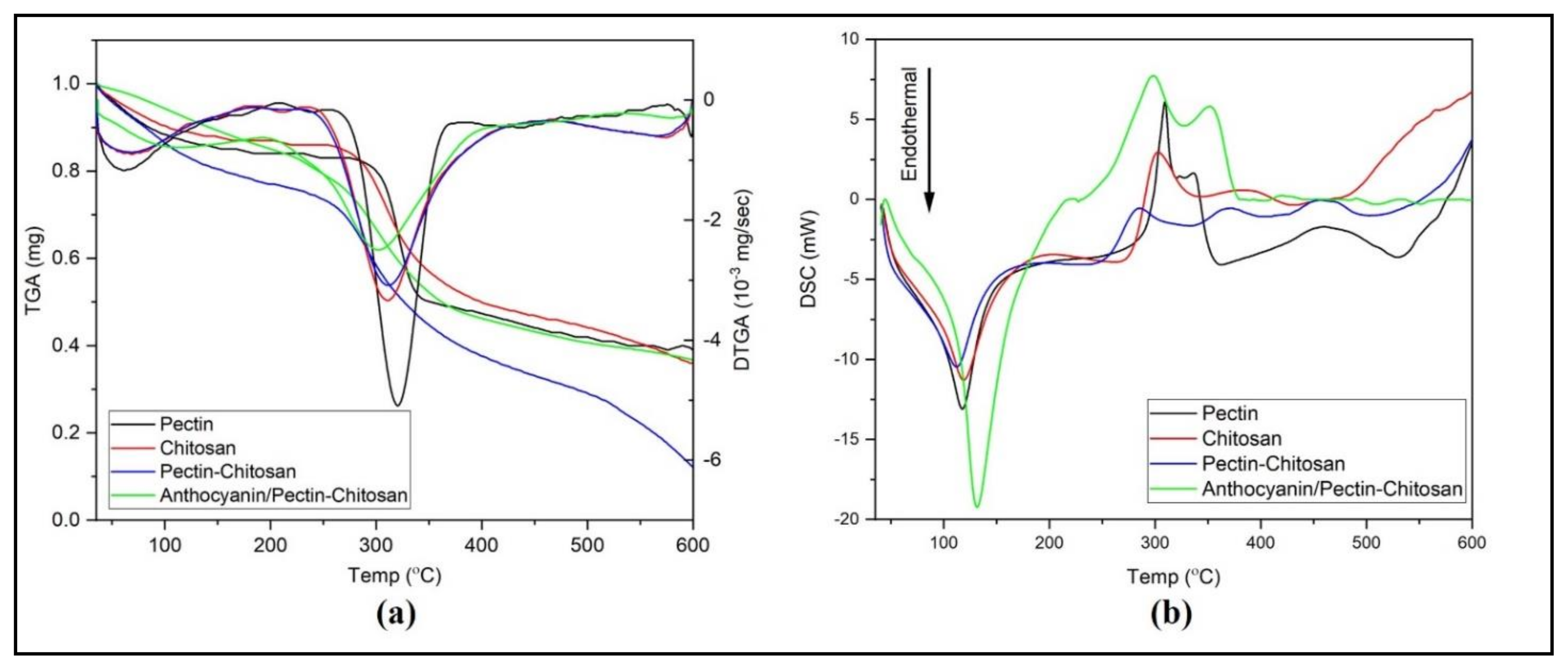
4.3. Thermal Characteristics
4.4. Optical pH Sensor Optimization
Effect of the Anthocyanin Concentration on Sensor Sensitivity
4.5. Characterization of Optical pH Sensor
4.5.1. Response Time
4.5.2. Reproducibility of Optical pH Sensors
4.5.3. Lifetime Profile of Optical Sensor pH
5. Salivary pH Determination
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, L.; Singh, A.; Singh, A.V. Synthesis and characterization of pectin-chitosan conjugate for biomedical application. Int. J. Biol. Macromol. 2020, 153, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zeng, F.; Zhang, M.; Zheng, S.; Li, J.; Fei, P. Hydrophobic edible composite packaging membrane based on low-methoxyl pectin/chitosan: Effects of lotus leaf cutin. Food Packag. Shelf Life 2020, 26, 100592. [Google Scholar] [CrossRef]
- Xin, Y.; Jin, Z.; Chen, F.; Lai, S.; Yang, H. Effect of chitosan coatings on the evolution of sodium carbonate-soluble pectin during sweet cherry softening under non-isothermal conditions. Int. J. Biol. Macromol. 2020, 154, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Marlina, M.; Iqhrammullah, M.; Darmadi, D.; Mustafa, I.; Rahmi, R. The Application of Chitosan Modified Polyurethane Foam Adsorbent. Rasayan J. Chem. 2019, 12, 494–501. [Google Scholar] [CrossRef]
- Prashanth, K.H.; Tharanathan, R. Chitin/chitosan: Modifications and their unlimited application potential—an overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, S.; Chen, X. A visual pH sensing film using natural dyes from Bauhinia blakeana Dunn. Sens. Actuators B Chem. 2014, 198, 268–273. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Alves, V.D.; Coelhoso, I.M. Polysaccharide-Based Membranes in Food Packaging Applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Iqhrammullah, M.; Marlina, M.; Khalil, H.P.S.A.; Kurniawan, K.H.; Suyanto, H.; Hedwig, R.; Karnadi, I.; Olaiya, N.G.; Abdullah, C.K.; Abdulmadjid, S.N. Characterization and Performance Evaluation of Cellulose Acetate–Polyurethane Film for Lead II Ion Removal. Polymers 2020, 12, 1317. [Google Scholar] [CrossRef]
- Hao, L.; Ding, J.; Yuan, N.; Xu, J.; Zhou, X.; Dai, S.; Chen, B. Visual and flexible temperature sensor based on a pectin-xanthan gum blend film. Org. Electron. 2018, 59, 243–246. [Google Scholar] [CrossRef]
- Gabriele, F.; Donnadio, A.; Casciola, M.; Germani, R.; Spreti, N. Ionic and covalent crosslinking in chitosan-succinic acid membranes: Effect on physicochemical properties. Carbohydr. Polym. 2021, 251, 117106. [Google Scholar] [CrossRef]
- Al-Kharkhi, A.; Phuah, E.T.; Nadiah, W.A.W.; Rosma, A.; Wong, W.W.; Easa, A.M.; Liong, M.T. Biosorbent ingredients from durian rind waste. In Proceedings of the International Conference on Environmental Research and Technology (ICERT 08), Parkroyal Hotel, Pulau Pinang, Malaysia, 28–30 May 2008. [Google Scholar]
- Hasanah, U.; Sani, N.D.M.; Heng, L.Y.; Idroes, R.; Safitri, E. Construction of a Hydrogel Pectin-Based Triglyceride Optical Biosensor with Immobilized Lipase Enzymes. Biosensors 2019, 9, 135. [Google Scholar] [CrossRef]
- Hasanah, U.; Setyowati, M.; Efendi, R.; Muslem, M.; Sani, N.D.M.; Safitri, E.; Heng, L.Y.; Idroes, R. Preparation and Characterization of a Pectin Membrane-Based Optical pH Sensor for Fish Freshness Monitoring. Biosensors 2019, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Rashidova, S.S.; Milusheva, R.Y.; Semenova, L.N.; Mukhamedjanova, M.Y.; Voropaeva, N.L.; Vasilyeva, S.; Faizieva, R.; Ruban, I.N. Characteristics of Interactions in the Pectin? Chitosan System. Chromatographia 2004, 59, 779–782. [Google Scholar] [CrossRef]
- Khan, P.M.A.; Farooqui, M. Analytical Applications of Plant Extract as Natural pH Indicator: A Review. J. Adv. Sci. Res. 2011, 2, 20–27. [Google Scholar]
- Safitri, E.; Afifah, N.; Khairi; Lelifajri; Nazaruddin; Susilawati; Sani, N.D. Ruellia tuberosa L. Anthocyanin extract as a pH sensitive substance. IOP Conf. Ser. Earth Environ. Sci. 2019, 364, 012015. [Google Scholar] [CrossRef]
- Maciel, V.B.V.; Yoshida, C.M.; Franco, T.T. Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr. Polym. 2015, 132, 537–545. [Google Scholar] [CrossRef]
- Sato, T.P. A pH curve of human resting saliva sampled with a small paper slip and its medical application. Pathophysiology 2002, 8, 283–290. [Google Scholar] [CrossRef]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461–465. [Google Scholar] [CrossRef]
- Cohen, M.; Khalaila, R. Saliva pH as a biomarker of exam stress and a predictor of exam performance. J. Psychosom. Res. 2014, 77, 420–425. [Google Scholar] [CrossRef]
- Le, X.T.; Huynh, M.T.; Pham, T.N.; Than, V.T.; Toan, T.Q.; Bach, L.G.; Trung, N.Q. Optimization of Total Anthocyanin Content, Stability and Antioxidant Evaluation of the Anthocyanin Extract from Vietnamese Carissa Carandas L. Fruits. Processes 2019, 7, 468. [Google Scholar] [CrossRef]
- De Queiroz Antonino, R.; Lia Fook, B.; De Oliveira Lima, V.; De Farias Rached, R.; Lima, E.; Da Silva Lima, R.; Peniche Covas, C.; Lia Fook, M. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Majekodunmi, S.O.; Olorunsola, E.O.; Uzoaganobi, C.C. Comparative physicochemical characterization of chitosan from shells of two bivalved mollusks from two different continents. Am. J. Polym. Sci. 2017, 7, 15–22. [Google Scholar]
- Limsitthichaikoon, S.; Saodaeng, K.; Priprem, A.; Damrongrungruang, T. Anthocyanin complex: Characterization and cytotoxicity studies. Int. J. Chem. Mol. Eng. 2015, 9, 147–153. [Google Scholar]
- Iqhrammullah, M.; Marlina; Hedwig, R.; Karnadi, I.; Kurniawan, K.H.; Olaiya, N.G.; Haafiz, M.K.M.; Khalil, H.P.S.A.; Abdulmadjid, S.N. Filler-Modified Castor Oil-Based Polyurethane Foam for the Removal of Aqueous Heavy Metals Detected Using Laser-Induced Breakdown Spectroscopy (LIBS) Technique. Polymers 2020, 12, 903. [Google Scholar] [CrossRef] [PubMed]
- Pantić, M.; Horvat, G.; Knez, Ž.; Novak, Z. Preparation and Characterization of Chitosan-Coated Pectin Aerogels: Curcumin Case Study. Molecules 2020, 25, 1187. [Google Scholar] [CrossRef]
- Neufeld, L.; Bianco-Peled, H. Pectin–chitosan physical hydrogels as potential drug delivery vehicles. Int. J. Biol. Macromol. 2017, 101, 852–861. [Google Scholar] [CrossRef]
- He, X.-L.; Li, X.-L.; Lv, Y.-P.; He, Q. Composition and color stability of anthocyanin-based extract from purple sweet potato. Food Sci. Technol. 2015, 35, 468–473. [Google Scholar] [CrossRef]
- Johnson, K.Y.; Lulich, J.P.; Osborne, C.A. Evaluation of the reproducibility and accuracy of pH-determining devices used to measure urine pH in dogs. J. Am. Veter Med. Assoc. 2007, 230, 364–369. [Google Scholar] [CrossRef]
- Giannobile, W.V. Salivary diagnostics for periodontal diseases. J. Am. Dent. Assoc. 2012, 143, 6S–11S. [Google Scholar] [CrossRef]
- Fejerskov, O.; Kidd, E. Dental Caries: The Disease and Its Clinical Management; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 1444309285. [Google Scholar]

| Anthocyanin Concentration (mg/L) | Citrate Buffer | Phosphate Buffer | ||||
|---|---|---|---|---|---|---|
| pH Range | Sensitivity (AU/pH Unit) | R2 | pH Range | Sensitivity (AU/pH Unit) | R2 | |
| 0.025 | 4.0–6.0 | 0.0423 ± 0.003 | 0.933 | 4.8–9.0 | 0.0785 ± 0.001 | 0.9830 |
| 0.0375 | 4.0–5.5 | 0.0795 ± 0.004 | 0.999 | 4.8–9.0 | 0.0744 ± 0.001 | 0.9679 |
| 0.05 | 5.0–7.5 | 0.0128 ± 0.001 | 0.969 | 4.8–7.5 | 0.0730 ± 0.003 | 0.9711 |
| Phosphate Buffer | |||
|---|---|---|---|
| Concentration (M) | pH Range | Sensitivity (AU/pH Unit) | R2 |
| 0.01 | 7.0–9.5 | 0.0588 ± 0.0145 | 0.9760 |
| 0.03 | 7.0–9.5 | 0.0623 ± 0.0070 | 0.9616 |
| 0.05 | 6.5–9.5 | 0.0682 ± 0.009 | 0.9614 |
| 0.075 | 4.8–9.5 | 0.056 ± 0.02 | 0.9746 |
| 0.1 | 4.8–9.5 | 0.0786 ± 0.001 | 0.9838 |
| No. | Optical pH Sensor | Absorbance (AU) |
|---|---|---|
| 1 | Sensor A | 0.461 |
| 2 | Sensor B | 0.450 |
| 3 | Sensor C | 0.401 |
| 4 | Sensor D | 0.413 |
| 5 | Sensor E | 0.460 |
| 6 | Sensor F | 0.463 |
| 7 | Sensor G | 0.513 |
| 8 | Sensor H | 0.471 |
| 9 | Sensor I | 0.436 |
| 10 | Sensor J | 0.516 |
| Average | 0.458 | |
| SD | 0.0352 | |
| RSD (%) | 7.687 | |
| No. | Age of People from Whom Saliva Samples Were Tested before Meals (Years) | pH Determined by Optical pH Sensor | pH Determined by Ion Selective Electrode (ISE H+) | Tvalue | Ttable |
|---|---|---|---|---|---|
| 1. | 6 | 7.23 | 7.3 | 0.835 | 2.92 |
| 2. | 22 | 6.83 | 7.15 | 2.29 | |
| 3. | 56 | 6.50 | 6.8 | 1.42 |
| No. | Time after Meal (Min) | Optical pH Sensor | ISE H+ | Tvalue | Ttable |
|---|---|---|---|---|---|
| 1. | Before meal | 6.83 | 7.15 | 2.29 | 2.92 |
| 2. | 15 | 6.76 | 6.73 | 1.48 | |
| 3. | 30 | 6.62 | 6.63 | 0.539 | |
| 4. | 45 | 6.85 | 6.81 | 1.09 | |
| 5. | 60 | 6.96 | 6.91 | 1.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safitri, E.; Humaira, H.; Murniana, M.; Nazaruddin, N.; Iqhrammullah, M.; Md Sani, N.D.; Esmaeili, C.; Susilawati, S.; Mahathir, M.; Latansa Nazaruddin, S. Optical pH Sensor Based on Immobilization Anthocyanin from Dioscorea alata L. onto Polyelectrolyte Complex Pectin–Chitosan Membrane for a Determination Method of Salivary pH. Polymers 2021, 13, 1276. https://doi.org/10.3390/polym13081276
Safitri E, Humaira H, Murniana M, Nazaruddin N, Iqhrammullah M, Md Sani ND, Esmaeili C, Susilawati S, Mahathir M, Latansa Nazaruddin S. Optical pH Sensor Based on Immobilization Anthocyanin from Dioscorea alata L. onto Polyelectrolyte Complex Pectin–Chitosan Membrane for a Determination Method of Salivary pH. Polymers. 2021; 13(8):1276. https://doi.org/10.3390/polym13081276
Chicago/Turabian StyleSafitri, Eka, Hani Humaira, Murniana Murniana, Nazaruddin Nazaruddin, Muhammad Iqhrammullah, Nor Diyana Md Sani, Chakavak Esmaeili, Susilawati Susilawati, Muhammad Mahathir, and Salsabilla Latansa Nazaruddin. 2021. "Optical pH Sensor Based on Immobilization Anthocyanin from Dioscorea alata L. onto Polyelectrolyte Complex Pectin–Chitosan Membrane for a Determination Method of Salivary pH" Polymers 13, no. 8: 1276. https://doi.org/10.3390/polym13081276
APA StyleSafitri, E., Humaira, H., Murniana, M., Nazaruddin, N., Iqhrammullah, M., Md Sani, N. D., Esmaeili, C., Susilawati, S., Mahathir, M., & Latansa Nazaruddin, S. (2021). Optical pH Sensor Based on Immobilization Anthocyanin from Dioscorea alata L. onto Polyelectrolyte Complex Pectin–Chitosan Membrane for a Determination Method of Salivary pH. Polymers, 13(8), 1276. https://doi.org/10.3390/polym13081276







