Synthesis of Poly(l-lactide-co-ε-caprolactone) Copolymer: Structure, Toughness, and Elasticity
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PLCL
2.3. Preparation of PLCL Films
2.4. Characterization of PLCL
2.4.1. 1HNMR
2.4.2. FT-IR
2.4.3. GPC
2.4.4. DSC
2.4.5. Tensile Test
2.4.6. Rheological Test
2.4.7. DMA
2.4.8. AFM
3. Results and Discussion
3.1. Synthesis of PLCL
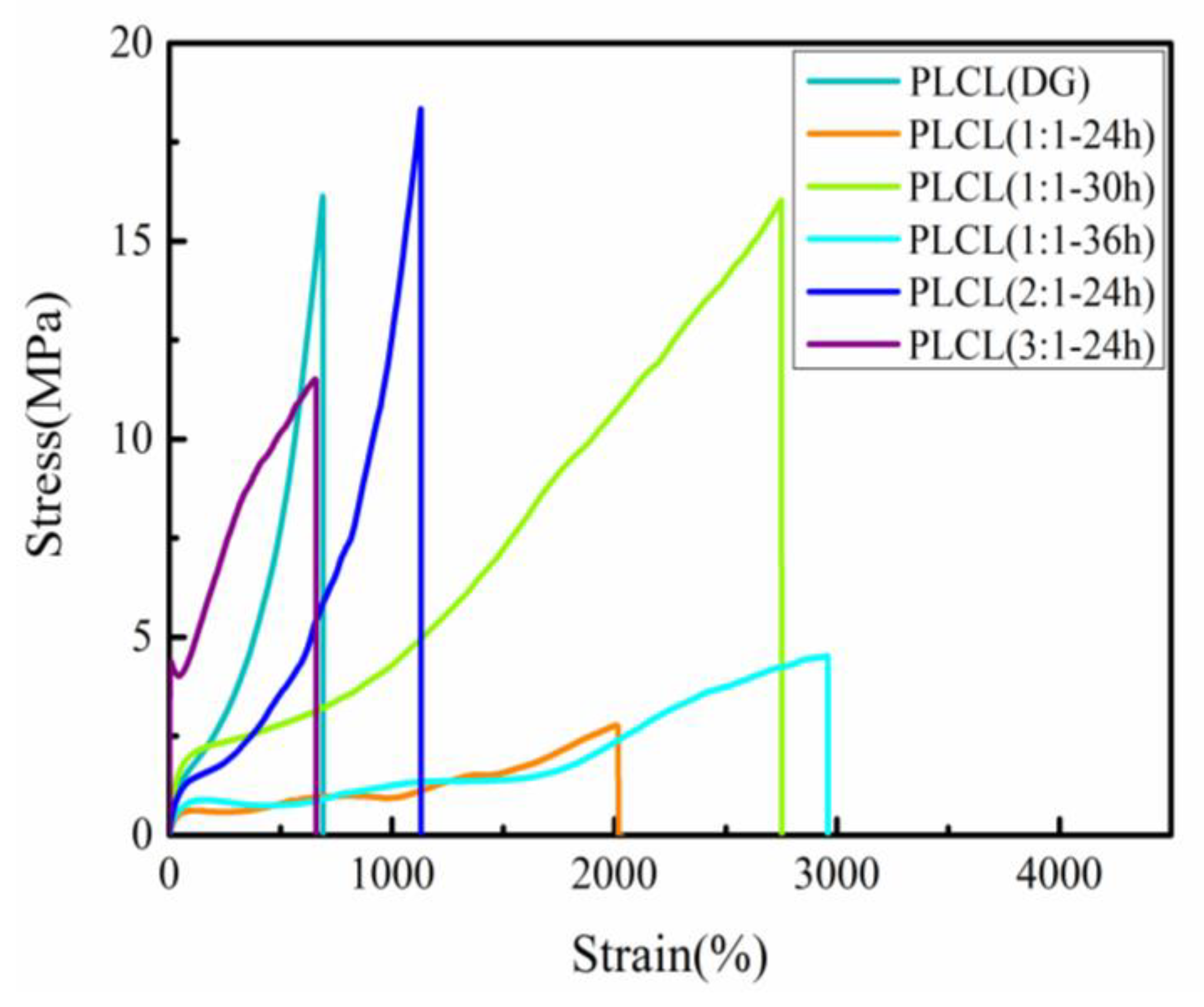
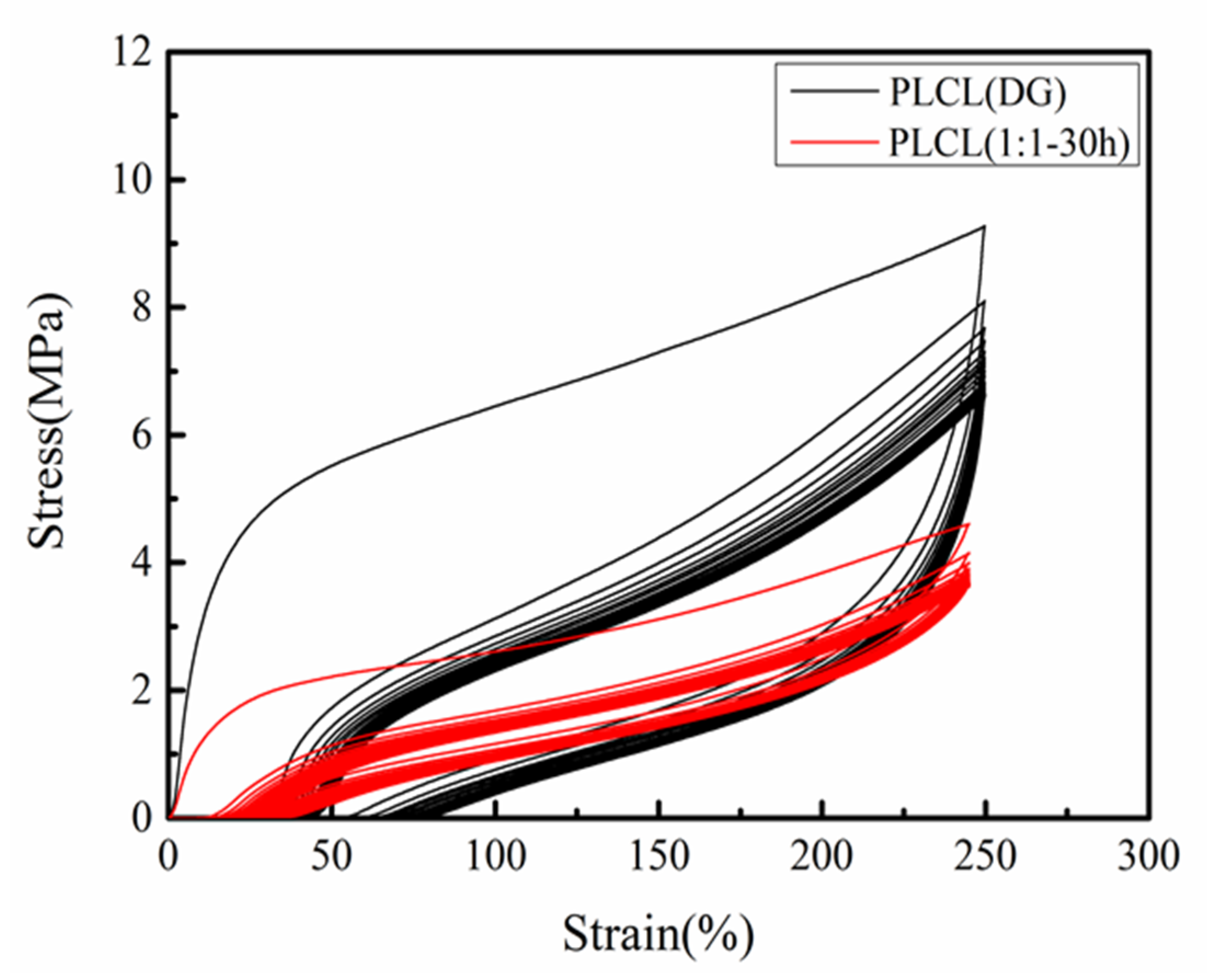
3.2. Mechanical Properties
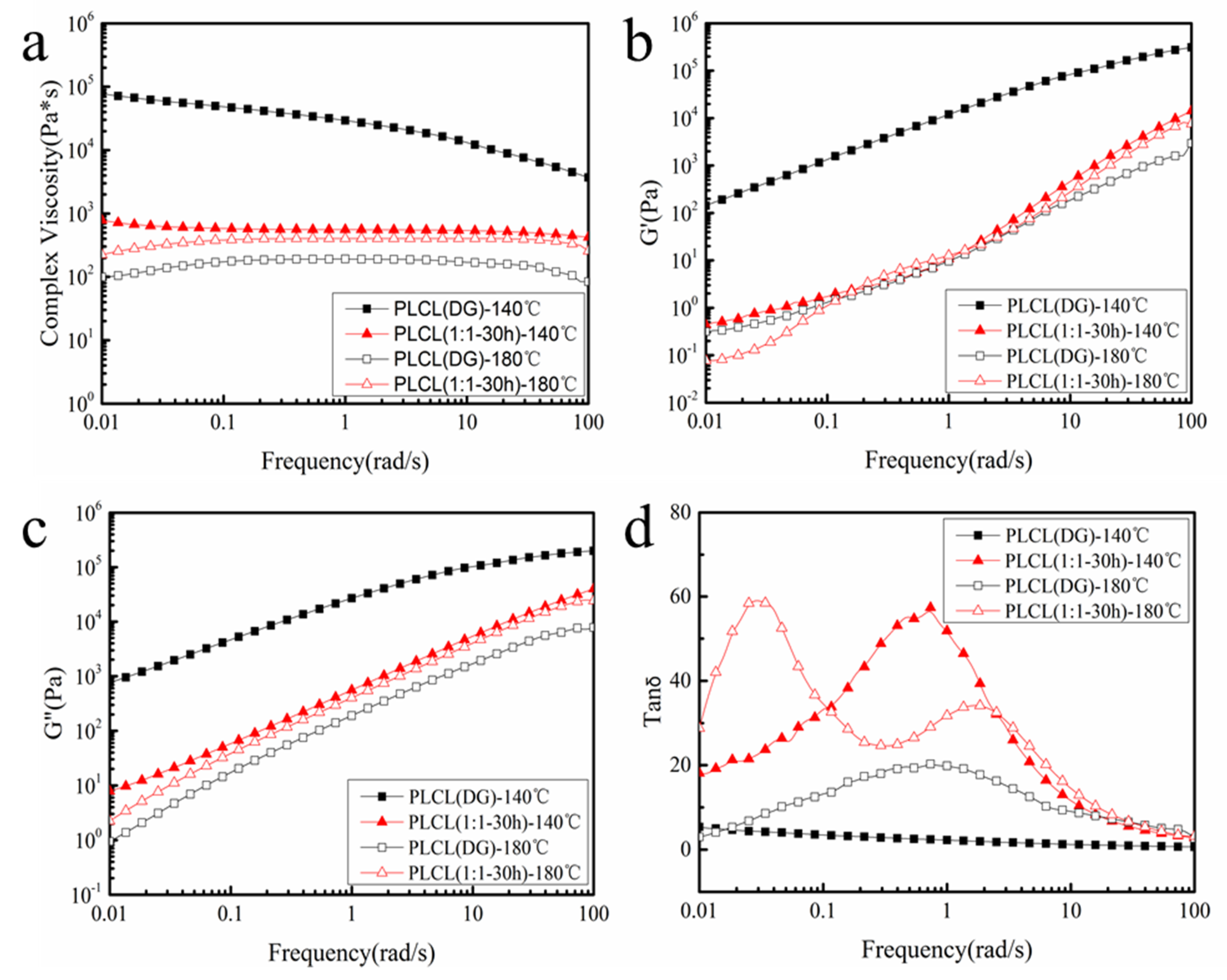
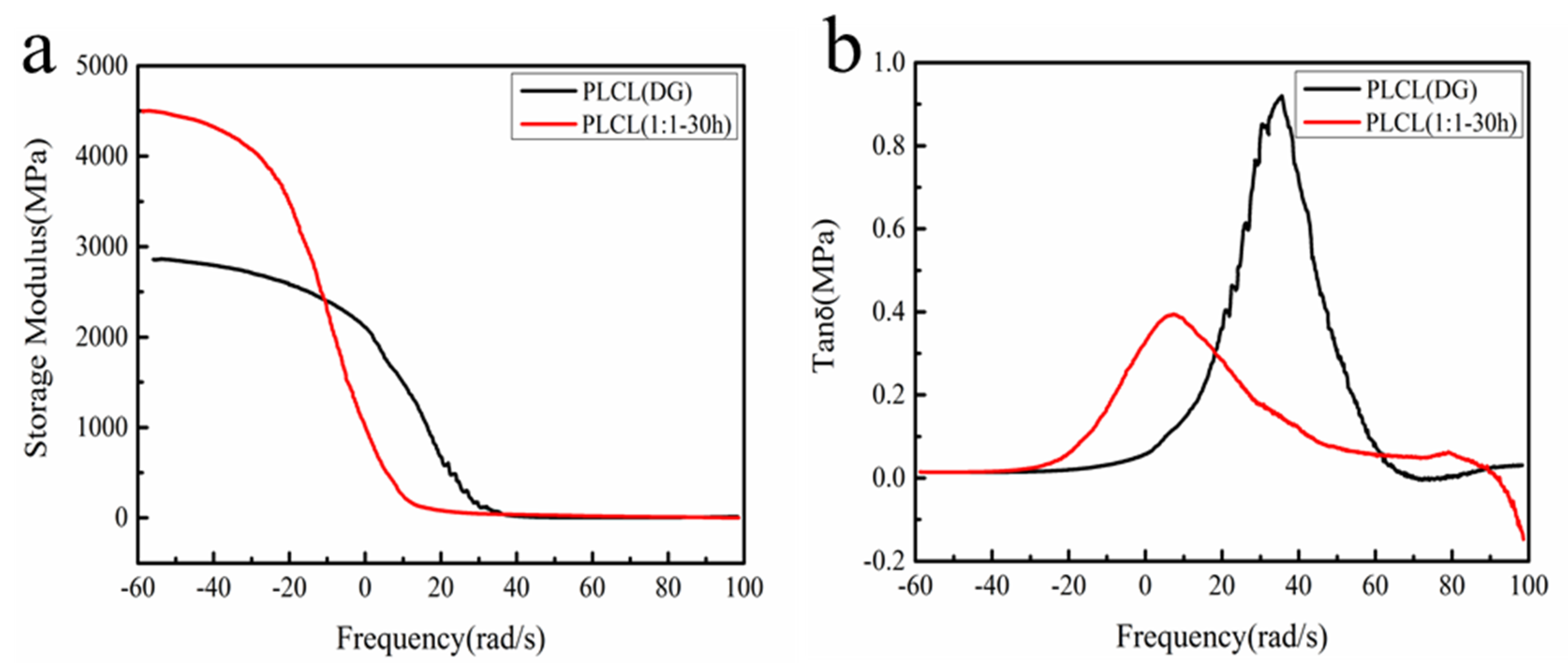
3.3. Thermodynamic Properties
3.4. Surface Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Santoro, O.; Zhang, X.; Redshaw, C. Synthesis of Biodegradable Polymers: A Review on the Use of Schiff-Base Metal Complexes as Catalysts for the Ring Opening Polymerization (ROP) of Cyclic Esters. Catalysts 2020, 10, 800. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kwon, T.-Y.; Kim, K.-H.; Kwon, S.-T.; Cho, D.-H.; Jang, S.H.; Son, J.S.; Lee, K.-B. Anti-inflammatory drug releasing absorbable surgical sutures using poly(lactic-co-glycolic acid) particle carriers. Polym. Bull. 2014, 71, 1933–1946. [Google Scholar] [CrossRef]
- Kharaziha, M.; Nikkhah, M.; Shin, S.-R.; Annabi, N.; Masoumi, N.; Gaharwar, A.K.; Camci-Unal, G.; Khademhosseini, A. PGS:Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues. Biomaterials 2013, 34, 6355–6366. [Google Scholar] [CrossRef]
- Ugartemendia, J.; Larrañaga, A.; Amestoy, H.; Etxeberria, A.; Sarasua, J. Tougher biodegradable polylactide system for bone fracture fixations: Miscibility study, phase morphology and mechanical properties. Eur. Polym. J. 2018, 98, 411–419. [Google Scholar] [CrossRef]
- Bee, S.-L.; Hamid, Z.A.A.; Mariatti, M.; Yahaya, B.H.; Lim, K.; Sin, L.T. Approaches to Improve Therapeutic Efficacy of Biodegradable PLA/PLGA Microspheres: A Review. Polym. Rev. 2018, 58, 495–536. [Google Scholar] [CrossRef]
- Lasprilla, A.J.; Martinez, G.A.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Crawford, K.; Gorman, C.B. Effects of Temperature and pH on the Degradation of Poly(lactic acid) Brushes. Macromolecules 2011, 44, 4777–4782. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Song, C.; Cui, X.; Wang, P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials 2006, 27, 1735–1740. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Becker, J.M.; Pounder, R.J.; Dove, A.P. Synthesis of Poly(lactide)s with Modified Thermal and Mechanical Properties. Macromol. Rapid Commun. 2010, 31, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Salhi, S.; Mahfoudh, J.; Abid, S.; Atanase, L.; Popa, M.; Delaite, C. Random poly(ε-caprolactone-L-alanine) by direct melt copolymerization. Polym. Int. 2020, 69, 1161–1168. [Google Scholar] [CrossRef]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liang, S.; Thouas, G.A. Elastomeric biomaterials for tissue engineering. Prog. Polym. Sci. 2013, 38, 584–671. [Google Scholar] [CrossRef]
- Jeong, S.I.; Kim, S.H.; Kim, Y.H.; Jung, Y.; Kwon, J.H.; Kim, B.-S.; Lee, Y.M. Manufacture of elastic biodegradable PLCL scaffolds for mechano-active vascular tissue engineering. J. Biomater. Sci. Polym. Ed. 2004, 15, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Baśko, M.; Kubisa, P. Cationic copolymerization of ε-caprolactone and L,L-lactide by an activated monomer mechanism. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 7071–7081. [Google Scholar] [CrossRef]
- Florczak, M.; Duda, A. Effect of the Configuration of the Active Center on Comonomer Reactivities: The Case of ε-Caprolactone/l,l-Lactide Copolymerization. Angew. Chem. Int. Ed. 2008, 47, 9088–9091. [Google Scholar] [CrossRef]
- Alamri, H.; Zhao, J.; Pahovnik, D.; Hadjichristidis, N. Phosphazene-catalyzed ring-opening polymerization of ε-caprolactone: Influence of solvents and initiators. Polym. Chem. 2014, 5, 5471–5478. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Wang, Y.; Guo, J.; Li, J. Ring-opening block copolymerization of ε-caprolactone with L-lactide catalyzed by N-heterocyclic carbenes: Synthesis, characteristics, mechanism. Macromol. Res. 2014, 22, 600–605. [Google Scholar] [CrossRef]
- Grijpma, D.W.; Pennings, A.J. Polymerization temperature effects on the properties of l-lactide and ε-caprolactone copolymers. Polym. Bull. 1991, 25, 335–341. [Google Scholar] [CrossRef]
- Fernandez, J.; Etxeberria, A.; Sarasua, J.-R. Synthesis, structure and properties of poly(L-lactide-co-caprolactone) statistical copolymers. J. Mech. Behav. Biomed. Mater. 2012, 9, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Meaurio, E.; Chaos, A.; Etxeberria, A.; Alonso-Varona, A.; Sarasua, J. Synthesis and characterization of poly (l-lactide/ε-caprolactone) statistical copolymers with well resolved chain microstructures. Polymers 2013, 54, 2621–2631. [Google Scholar] [CrossRef]
- Contreras, J.; Pestana, J.; López-Carrasquero, F.; Torres, C. Synthesis of ε-caprolactone-b-l-lactide block copolymers by mean sequential polymerization, using diphenylzinc as initiator. Polym. Bull. 2014, 71, 1661–1674. [Google Scholar] [CrossRef]
- Mezzasalma, L.; Harrisson, S.; Saba, S.; Loyer, P.; Coulembier, O.; Taton, D. Bulk Organocatalytic Synthetic Access to Statistical Copolyesters from l-Lactide and ε-Caprolactone Using Benzoic Acid. Biomacromolecules 2019, 20, 1965–1974. [Google Scholar] [CrossRef]
- McMahon, S.; Bertollo, N.; Cearbhaill, E.D.O.; Salber, J.; Pierucci, L.; Duffy, P.; Dürig, T.; Bi, V.; Wang, W. Bio-resorbable polymer stents: A review of material progress and prospects. Prog. Polym. Sci. 2018, 83, 79–96. [Google Scholar] [CrossRef]
- Averianov, I.V.; Korzhikov-Vlakh, V.A.; Moskalenko, Y.E.; Smirnova, V.E.; Tennikova, T.B. One-pot synthesis of poly(lactic acid) with terminal methacrylate groups for the adjustment of mechanical properties of biomaterials. Mendeleev Commun. 2017, 27, 574–576. [Google Scholar] [CrossRef]
- Jeong, S.I.; Kim, B.-S.; Lee, Y.M.; Ihn, K.J.; Kim, S.H.; Kim, Y.H. Morphology of Elastic Poly(l-lactide-co-ε-caprolactone) Copolymers and in Vitro and in Vivo Degradation Behavior of Their Scaffolds. Biomacromolecules 2004, 5, 1303–1309. [Google Scholar] [CrossRef]
- Suganuma, K.; Matsuda, H.; Cheng, H.; Iwai, M.; Nonokawa, R.; Asakura, T. NMR analysis and tacticity determination of poly(lactic acid) in C5D5N. Polym. Test. 2014, 38, 35–39. [Google Scholar] [CrossRef]
- Zheng, W.; Li, J.; Zheng, Y. Preparation of poly(l-lactide) and its application in bioelectrochemistry. J. Electroanal. Chem. 2008, 621, 69–74. [Google Scholar] [CrossRef]
- Phillipson, K.; Hay, J.; Jenkins, M. Thermal analysis FTIR spectroscopy of poly(ε-caprolactone). Thermochim. Acta 2014, 595, 74–82. [Google Scholar] [CrossRef]
- Lipik, V.T.; Widjaja, L.K.; Liow, S.S.; Abadie, M.J.; Venkatraman, S.S. Effects of transesterification and degradation on properties and structure of polycaprolactone–polylactide copolymers. Polym. Degrad. Stab. 2010, 95, 2596–2602. [Google Scholar] [CrossRef]
- Marek, A.A.; Verney, V. Photochemical reactivity of PLA at the vicinity of glass transition temperature. The photo-rheology method. Eur. Polym. J. 2016, 81, 239–246. [Google Scholar] [CrossRef]
- Harrison, K.L.; Jenkins, M.J. The effect of crystallinity and water absorption on the dynamic mechanical relaxation behaviour of polycaprolactone. Polym. Int. 2004, 53, 1298–1304. [Google Scholar] [CrossRef]
- Ning, N.; Ji, L.; Zhang, L.; Liu, J.; Lu, Y.; Wu, S.; Zou, H.; Tian, M.; Chan, T.W. High elasticity and conductivity of elastomer composites with arrayed carbon nanotubes as nanosprings. Compos. Sci. Technol. 2015, 118, 78–84. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, X.; Meng, X.; Guo, X.; Jiang, Y.; Xu, Y.; Li, Q.; Shen, C. Controllable fiber orientation and nonlinear elasticity of electrospun nanofibrous small diameter tubular scaffolds for vascular tissue engineering. Biomed. Mater. 2019, 14, 035006. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, J.; Wang, J.; Liu, X.; Zhang, R.; Hu, G.-H.; Na, H.; Zhu, J. Soft segment free thermoplastic polyester elastomers with high performance. J. Mater. Chem. A 2015, 3, 13637–13641. [Google Scholar] [CrossRef]
- Alessandrini, A.; Facci, P. AFM: A versatile tool in biophysics. Meas. Sci. Technol. 2005, 16, R65–R92. [Google Scholar] [CrossRef]


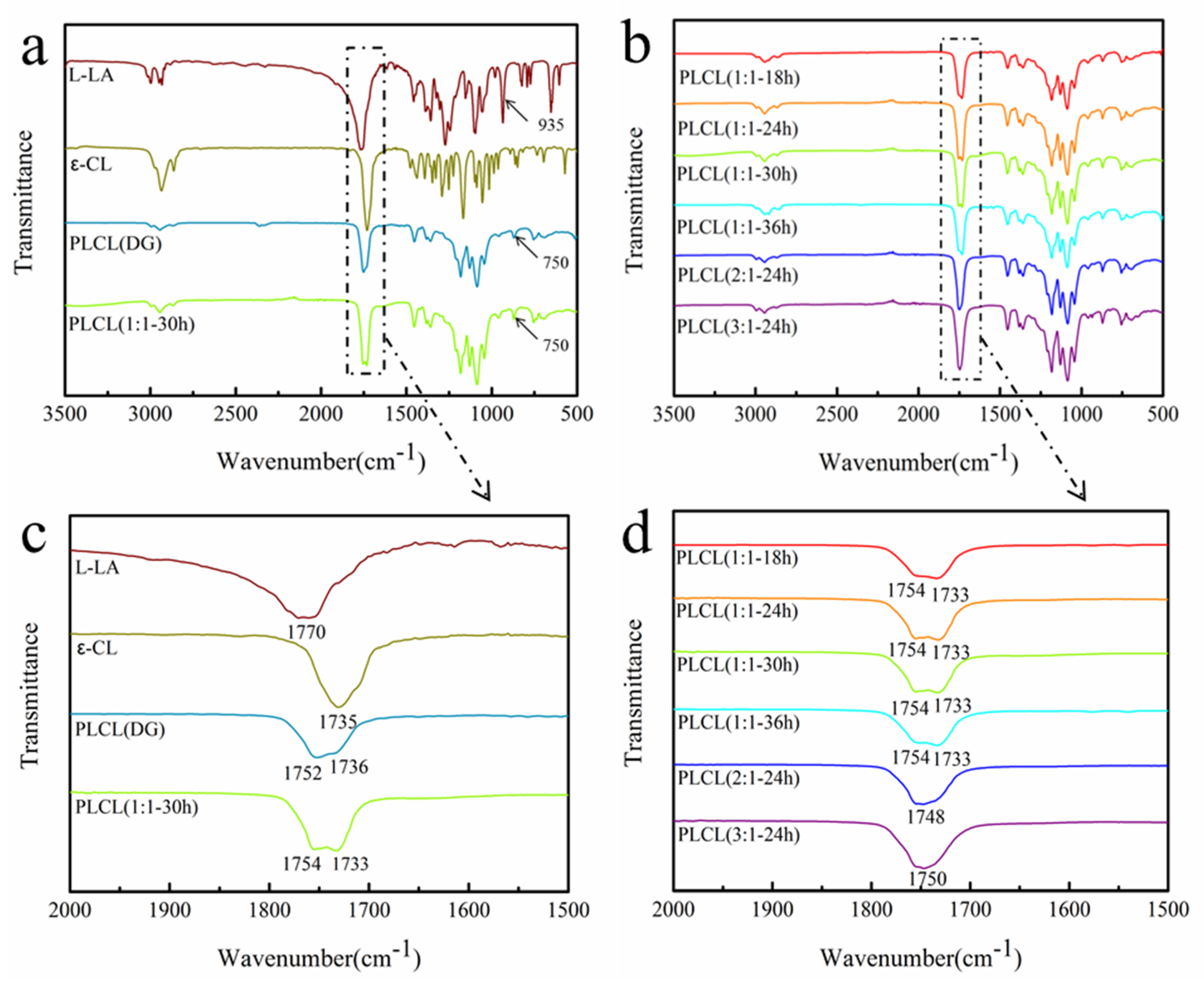



| Sample | Feed Molar Ratio | Copolymer Composit Molar Ratio | Yield | Mn (×103) | Mw (×103) | Ð | Tg |
|---|---|---|---|---|---|---|---|
| LA:CL | LA:CL | % | g/mol | g/mol | °C | ||
| PLCL (DG) | 1:1 | 3.18:1 | / | 54.46 | 231.2 | 4.25 | 9.14 |
| PLCL (1:1-18 h) | 1:1 | 2.18:1 | 76.44 | 20.33 | 43.79 | 2.15 | −12.77 |
| PLCL (1:1-24 h) | 1:1 | 2.28:1 | 88.78 | 29.85 | 57.81 | 1.94 | −4.39 |
| PLCL (1:1-30 h) | 1:1 | 2.46:1 | 87.34 | 53.81 | 120.0 | 2.23 | 1.98 |
| PLCL (1:1-36 h) | 1:1 | 2.22:1 | 82.75 | 37.97 | 78.85 | 2.08 | −11.03 |
| PLCL (2:1-24 h) | 2:1 | 5.44:1 | 92.16 | 41.67 | 90.80 | 2.18 | 11.07 |
| PLCL (3:1-24 h) | 3:1 | 9.90:1 | 88.57 | 33.88 | 67.36 | 1.99 | 18.51 |
| Sample | Elastic Modulus (MPa) | Tensile Strength (MPa) | Elongation at the Breaking Point (%) |
|---|---|---|---|
| PLCL(DG) | 12.0 ± 1.0 | 16.1 ± 3.2 | 689.5 ± 21.2 |
| PLCL(1:1-18 h) | / | / | / |
| PLCL(1:1-24 h) | 1.3 ± 0.3 | 2.7 ± 0.2 | 2611.7 ± 572.4 |
| PLCL(1:1-30 h) | 8.5 ± 0.6 | 15.1 ± 0.9 | 2661.3 ± 575.9 |
| PLCL(1:1-36 h) | 2.1 ± 0.4 | 3.8 ± 1.0 | 2860.0 ± 135.8 |
| PLCL(2:1-24 h) | 2.5 ± 0.6 | 18.9 ± 2.9 | 1131.7 ± 175.1 |
| PLCL(3:1-24 h) | 46.2 ± 5.9 | 12.1 ± 0.8 | 705.1 ± 70.7 |
| Sample | Rr(1) (%) | Rr(5) (%) | Rr(10) (%) | Rr(15) (%) | Rr(20) (%) |
|---|---|---|---|---|---|
| PLCL(DG) | 79.8 | 74.4 | 72.5 | 71.1 | 69.9 |
| PLCL(1:1-30 h) | 93.5 | 90.7 | 89.5 | 88.9 | 88.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Chang, Z.; Wang, X.; Li, Q. Synthesis of Poly(l-lactide-co-ε-caprolactone) Copolymer: Structure, Toughness, and Elasticity. Polymers 2021, 13, 1270. https://doi.org/10.3390/polym13081270
Zhang M, Chang Z, Wang X, Li Q. Synthesis of Poly(l-lactide-co-ε-caprolactone) Copolymer: Structure, Toughness, and Elasticity. Polymers. 2021; 13(8):1270. https://doi.org/10.3390/polym13081270
Chicago/Turabian StyleZhang, Mengyuan, Zhonghua Chang, Xiaofeng Wang, and Qian Li. 2021. "Synthesis of Poly(l-lactide-co-ε-caprolactone) Copolymer: Structure, Toughness, and Elasticity" Polymers 13, no. 8: 1270. https://doi.org/10.3390/polym13081270
APA StyleZhang, M., Chang, Z., Wang, X., & Li, Q. (2021). Synthesis of Poly(l-lactide-co-ε-caprolactone) Copolymer: Structure, Toughness, and Elasticity. Polymers, 13(8), 1270. https://doi.org/10.3390/polym13081270








