bioORMOCER®—Compostable Functional Barrier Coatings for Food Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Tosylation of the Tamarind Hemicellulose Glyate®
2.2. Amination of the Tosylated Glyate®
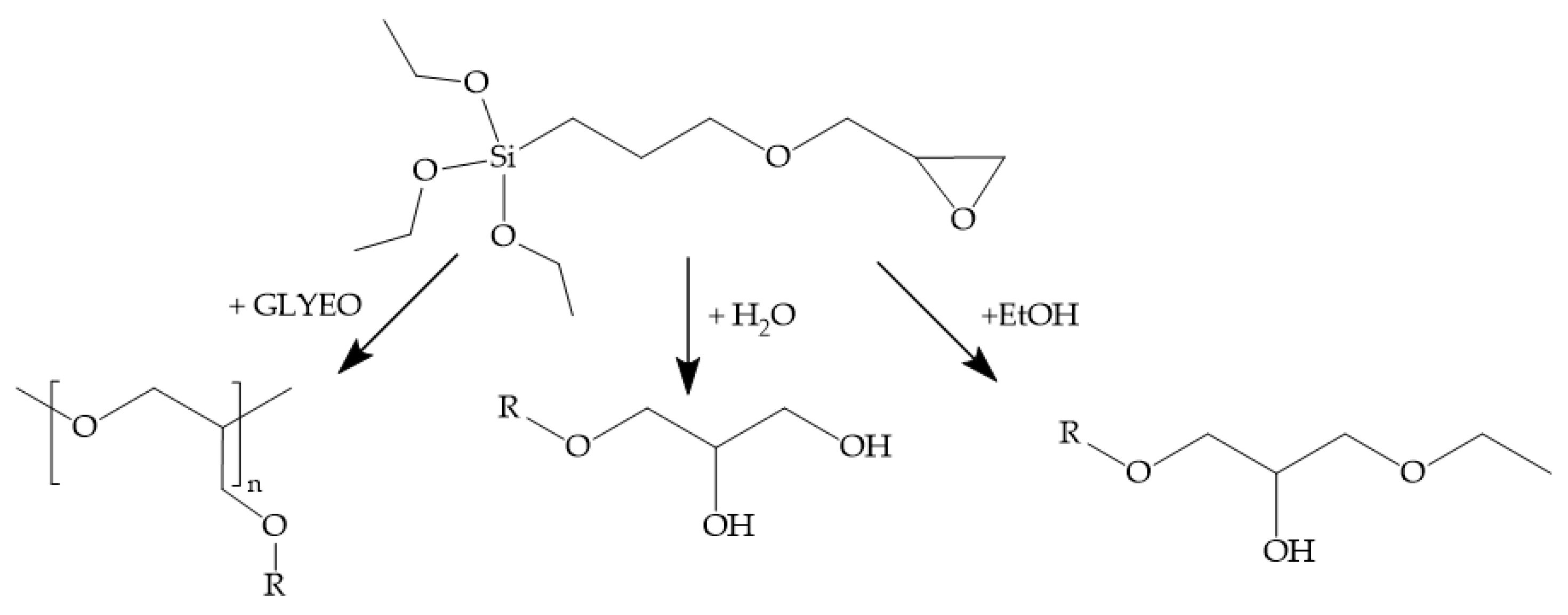
2.3. ORMOCER® and bioORMOCER®-Based Lacquers: Synthesis and Coating
2.4. Measurements
2.4.1. FT-IR Spectroscopy
2.4.2. Raman Spectroscopy
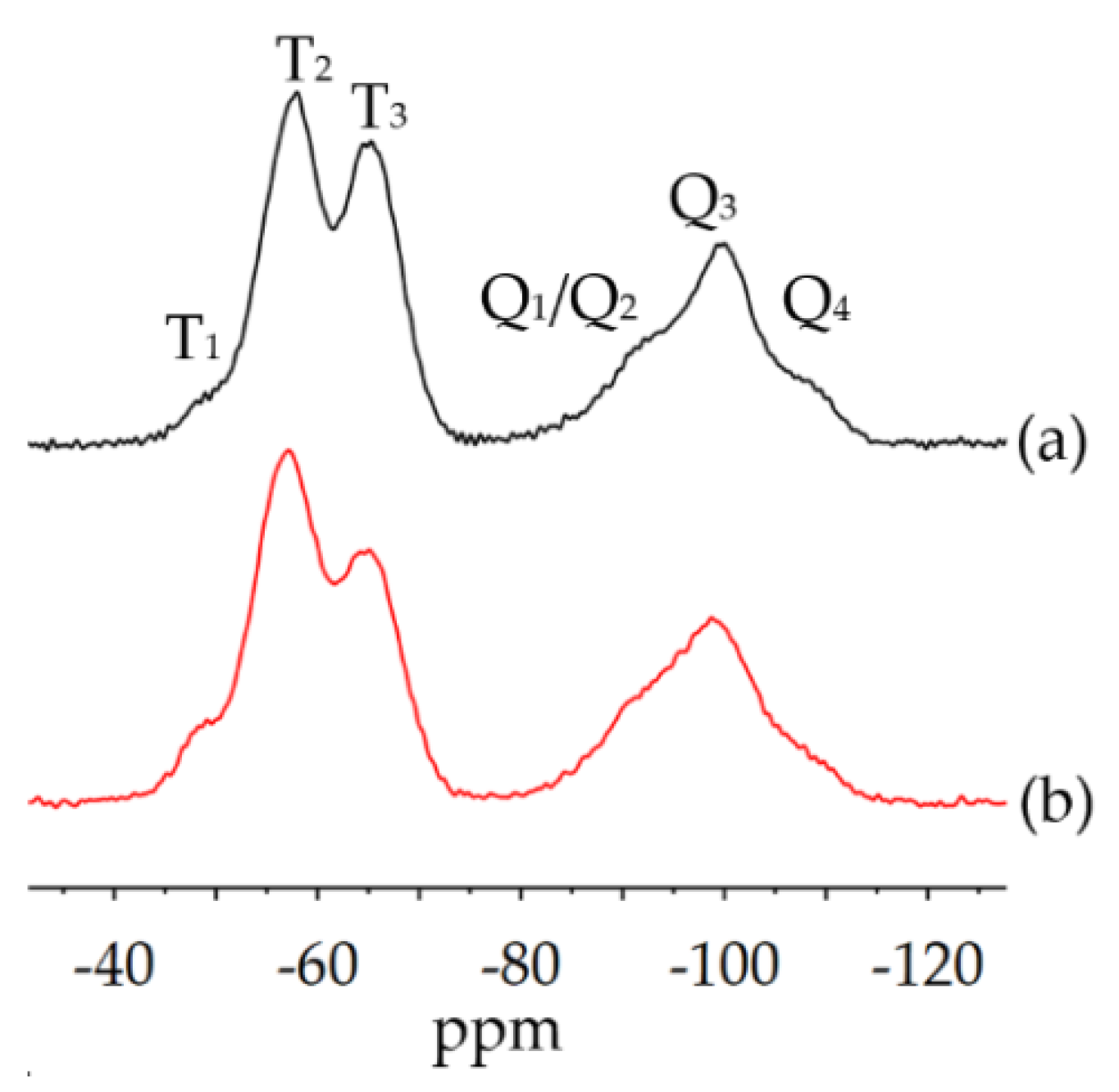
2.4.3. Solid-State-NMR Spectroscopy
2.4.4. Elemental Analysis
2.4.5. Adhesion Test
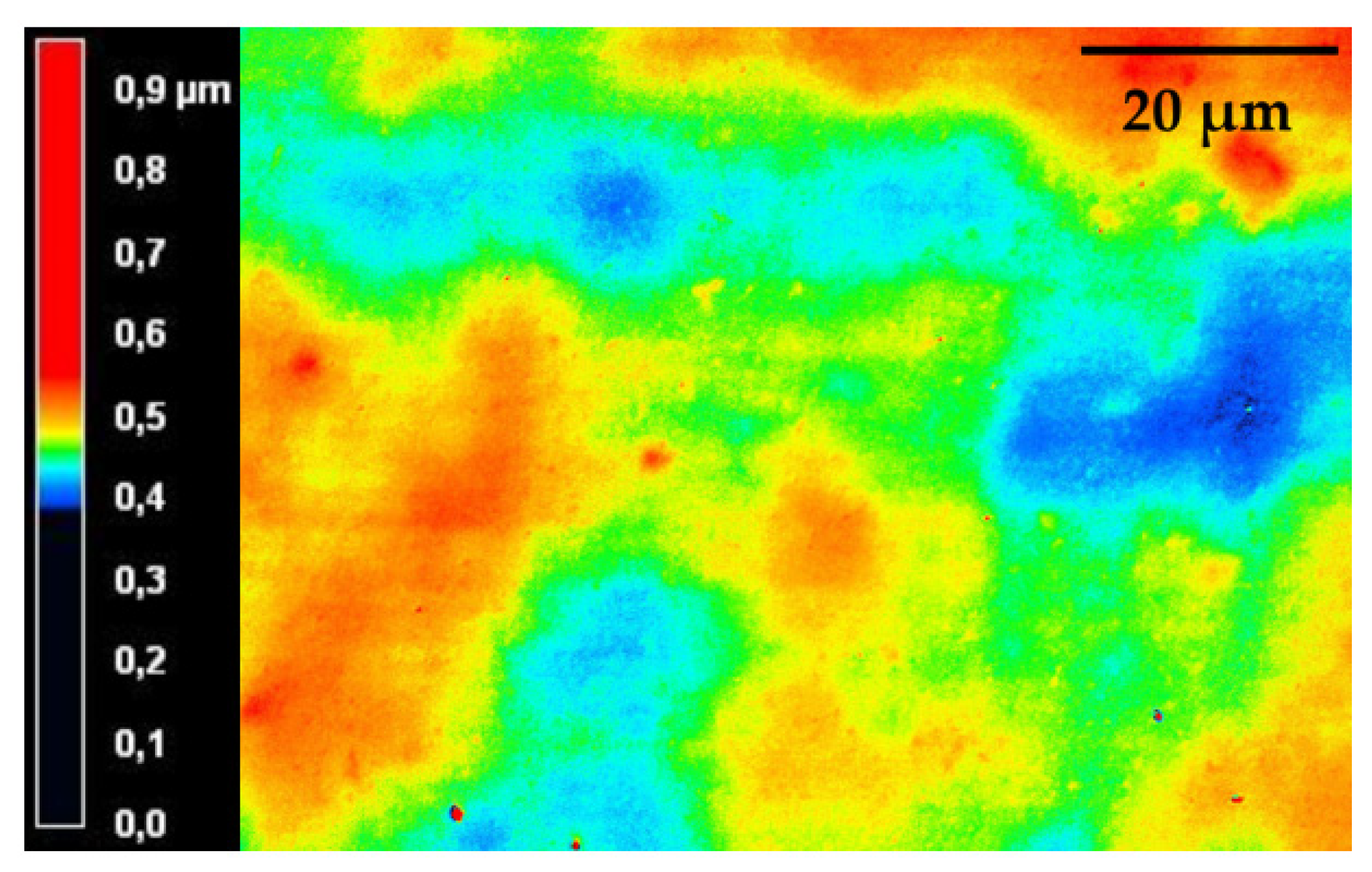
2.4.6. Laser Scanning Microscopy
2.4.7. Hardness and E-Module Measurements
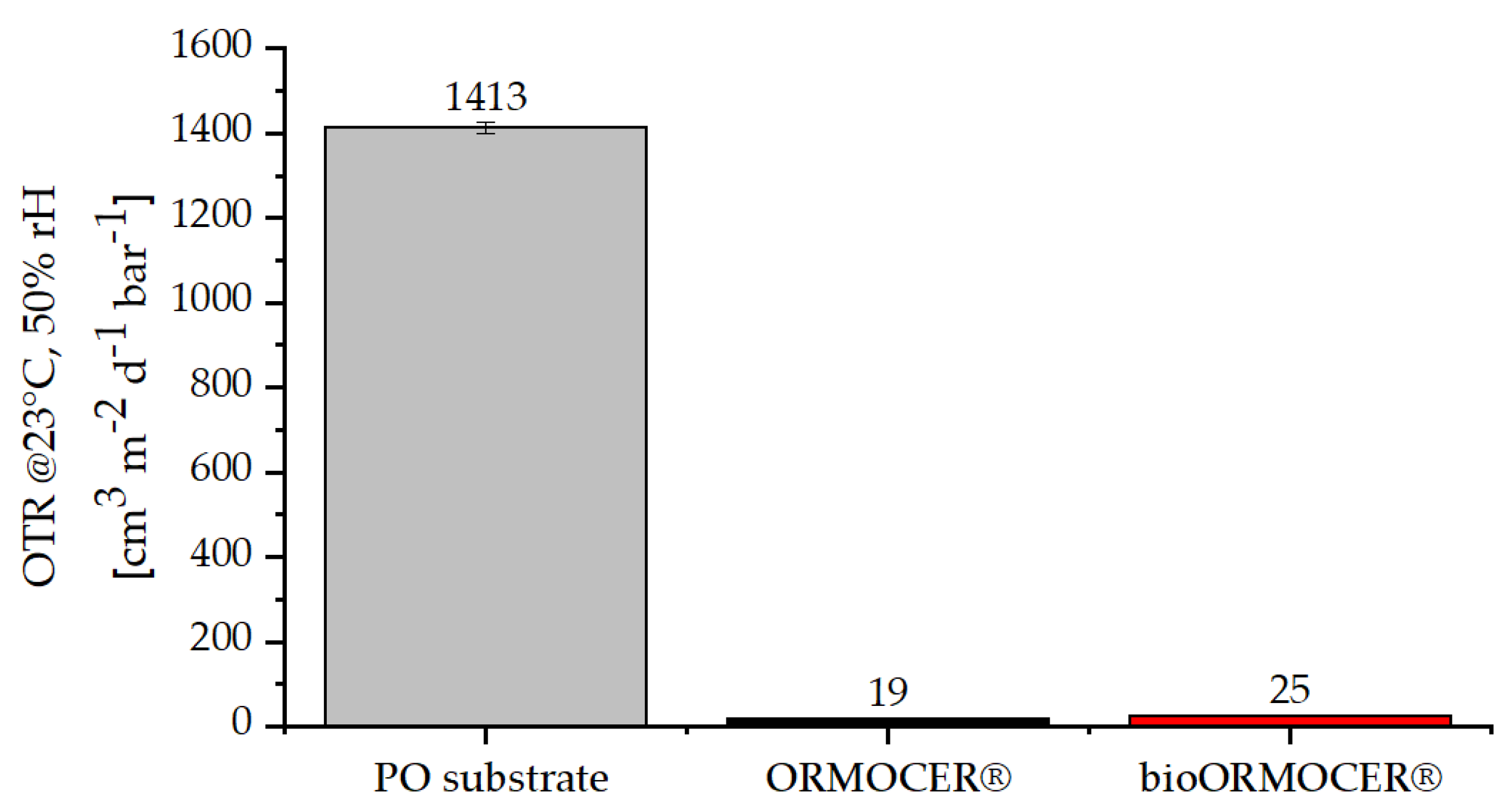
2.4.8. Oxygen Transmission Rates Measurement
2.4.9. Compostability
2.4.10. Biodegradability
2.4.11. Disintegration
2.4.12. Ecotoxicity
- -
- Lighting for 16 h a day at 3000 lx minimum (with light at a wavelength suitable for photosynthesis), at 25 °C temperature and 70% relative humidity;
- -
- Dark phase of 8 h a day, at 20 °C temperature and 80% relative humidity;
- -
- incubation period: 14–21 days from the time when 50% of the control plants have germinated.
- -
- The number of plants per pot (germination);
- -
- The fresh (wet) weight of biomass per pot;
- -
- The dry weight of biomass per pot (after a drying period of 1 day at 60 °C).
2.4.13. Overall Migration
2.4.14. Antimicrobial Properties
3. Results and Discussion
3.1. Functionalization of Glyate®
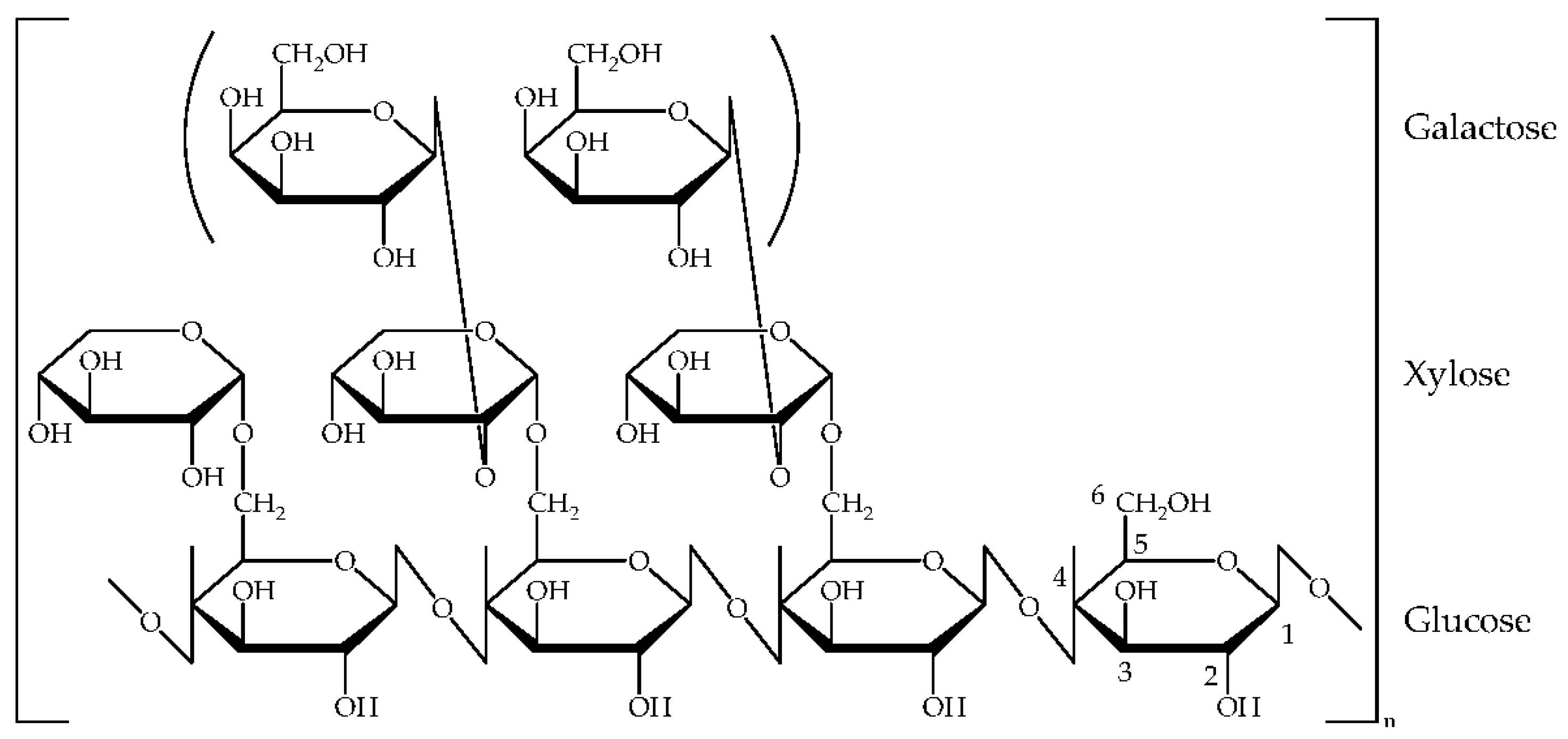
3.1.1. Structure and Properties of Glyate®
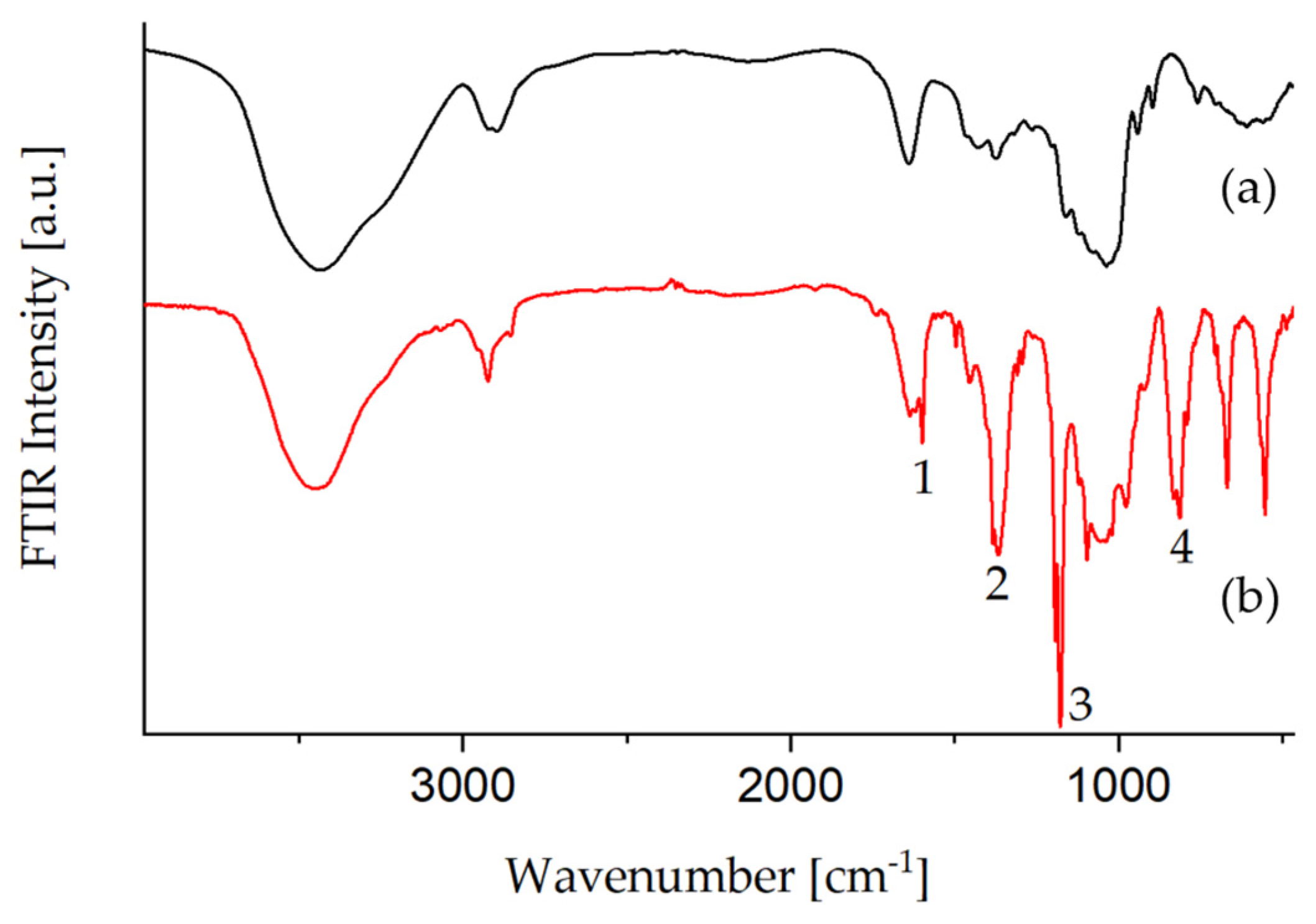
3.1.2. Tosylation of Glyate®
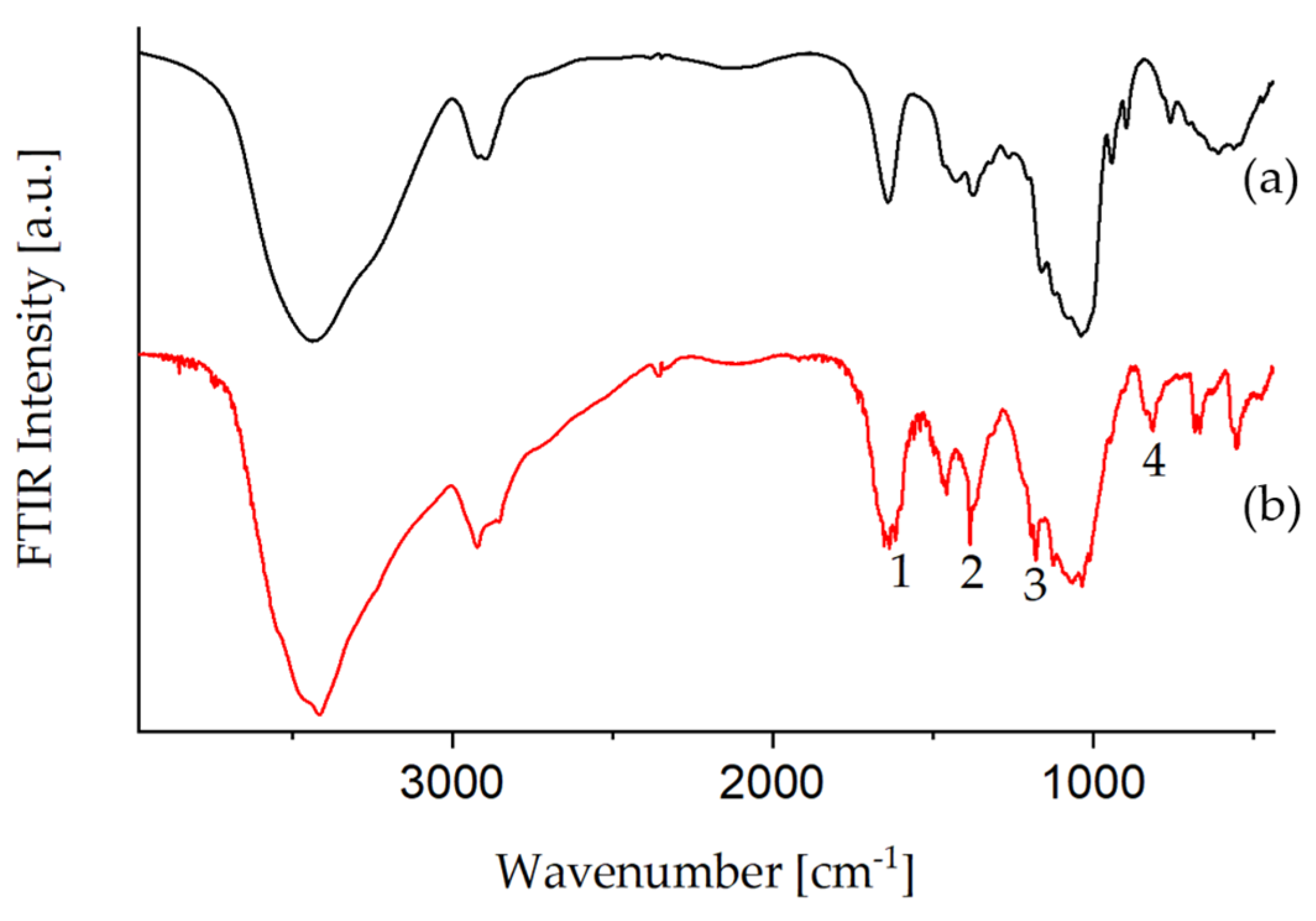
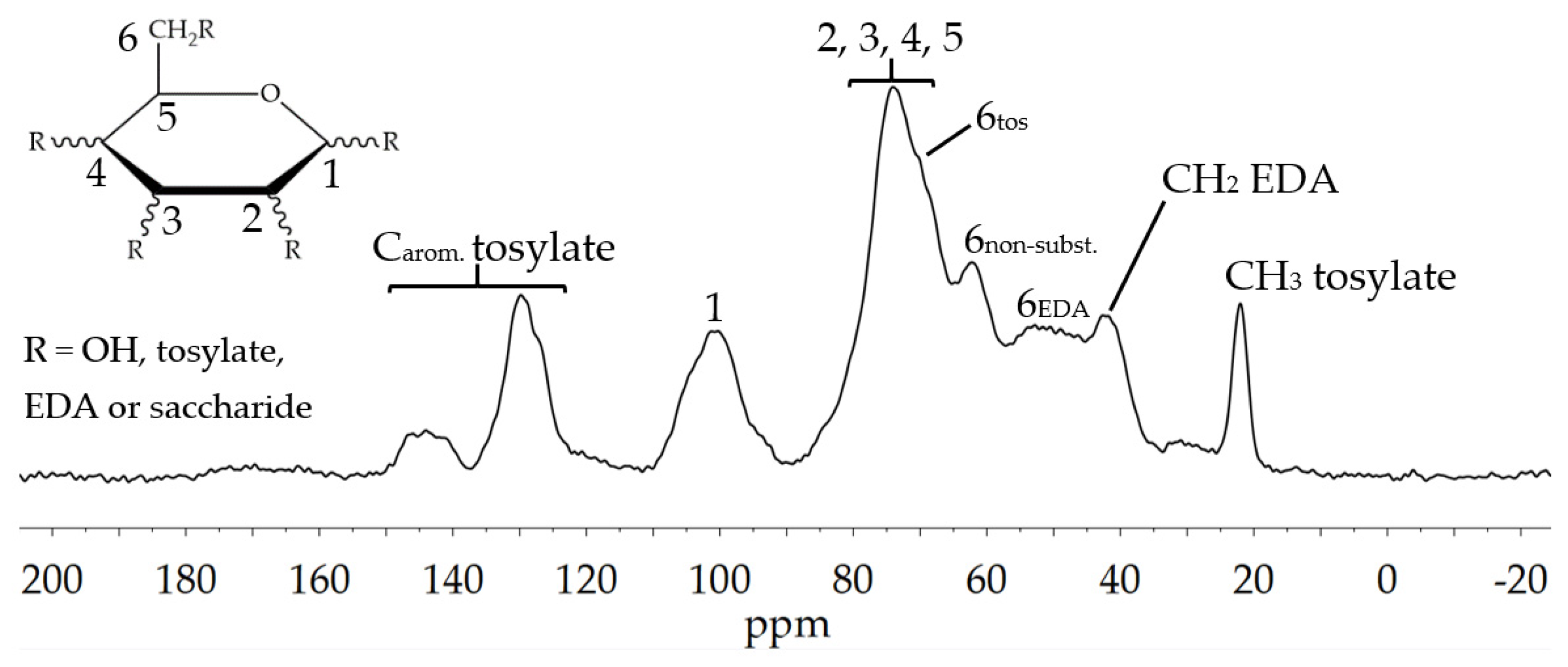
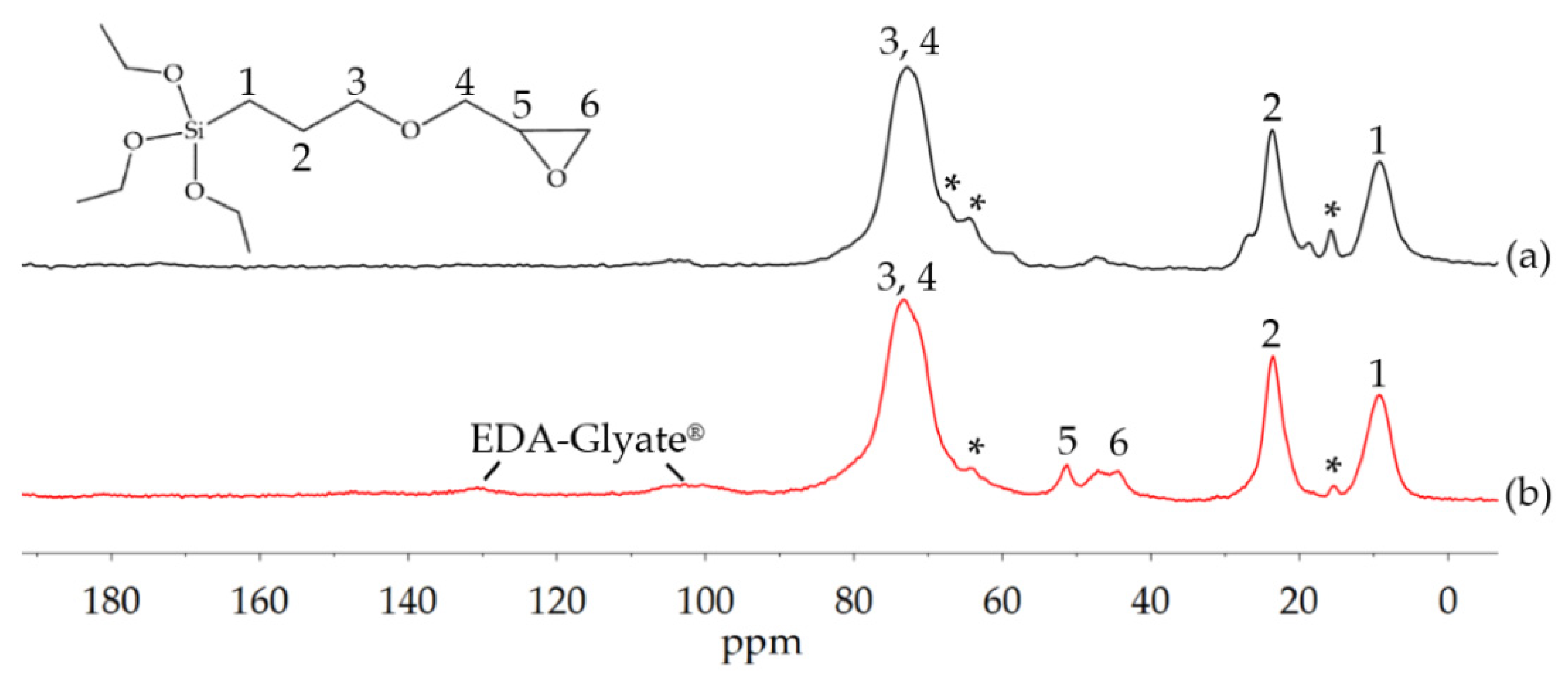
3.1.3. Amination of Glyate®
3.2. Development of Barrier Coatings with EDA-Glyate®
3.2.1. bioORMOCER® Synthesis
3.2.2. Physical-Chemical Properties of the Cured bioORMOCER® Coatings
3.3. Evaluation of the Compostability, Antimicrobial Properties and Migration of Cured bioORMOCER® Coatings
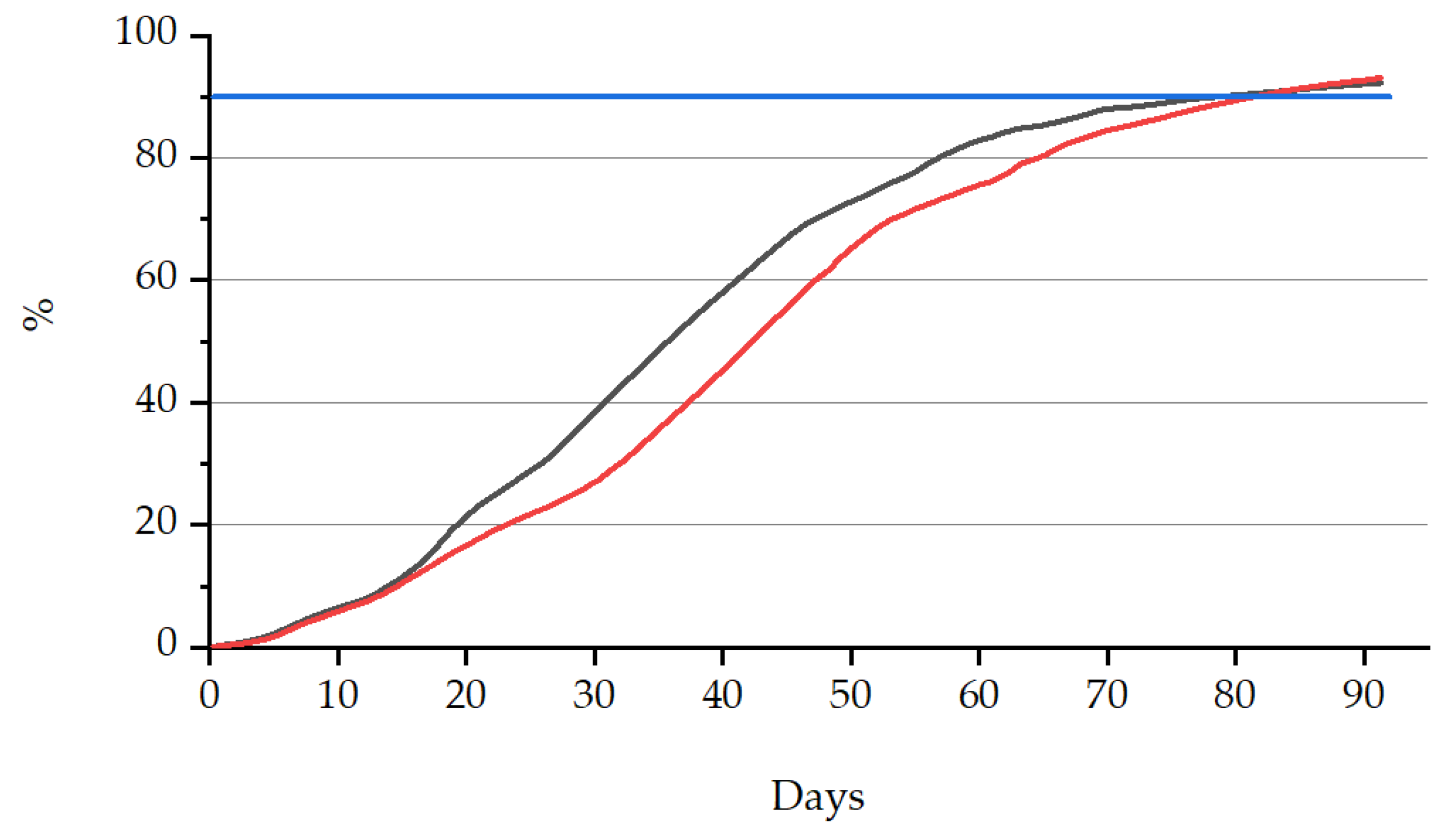
3.3.1. Biodegradability
3.3.2. Disintegration
3.3.3. Ecotoxicity
3.3.4. Antimicrobial Properties
3.3.5. Overall Migration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pira, S. The Future of Flexible Packaging to 2022. 2017. Available online: https://www.smitherspira.com/industry-market-reports/packaging/flexible-packaging-to-2022 (accessed on 19 March 2021).
- Horodytska, O.; Valdés, F.J.; Fullana, A. Plastic flexible films waste management—A state of art review. Waste Manag. 2018, 77, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Parker, L. Here’s How Much Plastic Trash Is Littering the Earth. Available online: https://www.nationalgeographic.com/science/article/plastic-produced-recycling-waste-ocean-trash-debris-environment (accessed on 19 March 2021).
- World Economic Forum, Ellen MacArthur Foundation and McKinsey & Company. The New Plastics Economy—Rethinking the Future of Plastics. 2016. Available online: www.ellenmacarthurfoundation.org/publications (accessed on 19 March 2021).
- European Environment Agency. Litter in Our Seas. Available online: https://www.eea.europa.eu/signals/signals-2014/close-up/litter-in-our-seas (accessed on 19 March 2021).
- Bertling, J.; Bertling, R.; Hamann, L. Kunststoffe in der Umwelt: Mikro-und Makroplastik: Ursachen, Mengen, Umweltschicksale, Wirkungen, Lösungsansätze, Empfehlungen. Kurzfassung der Konsortialstudie, Oberhausen. 2018. Available online: https://www.umsicht.fraunhofer.de/content/dam/umsicht/de/dokumente/publikationen/2018/kunststoffe-id-umwelt-konsortialstudie-mikroplastik.pdf (accessed on 19 March 2021).
- Hollman, P.C.H.; Bouwmeester, H.; Peters, R.J.B. Microplastics in the Aquatic Food Chain: Sources, Measurement, Occurrence and Potential Health Risks; Report; Wageningen University and Research Centre: Wageningen, The Netherlands, 2013. [Google Scholar]
- Muncke, J.; Andersson, A.-M.; Backhaus, T.; Boucher, J.M.; Carney Almroth, B.; Castillo Castillo, A.; Chevrier, J.; Demeneix, B.A.; Emmanuel, J.A.; Fini, J.-B.; et al. Impacts of food contact chemicals on human health: A consensus statement. Environ. Health 2020, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Detzel, A.; Bodrogi, F.; Kauertz, B.; Bick, C.; Welle, F.; Schmid, M.; Schmitz, K.; Müller, K.; Käb, H. Biobasierte Kunststoffe als Verpackung von Lebensmitteln; Bundesministerium für Ernährung und Landwirtschaft (Auftraggeber), Fachagentur für Nachwachsende Rohstoffe (Projektträger): Heidelberg, Germany; Freising, Germany; Berlin, Germany, 2018; Available online: https://www.ifeu.de/wp-content/uploads/Endbericht-Bio-LVp_20180612.pdf (accessed on 19 March 2021).
- Endres, H.-J.; Siebert-Raths, A. Technische Biopolymere: Rahmenbedingungen, Marktsituation, Herstellung, Aufbau und Eigenschaften; Hanser: München, Germany, 2009; ISBN 3446416838. [Google Scholar]
- Schüler, K. Aufkommen und Verwertung von Verpackungsabfällen in Deutschland im Jahr 2017; Umweltbundesamt: Dessau-Roßlau, Germany, 2019; Available online: www.umweltbundesamt.de (accessed on 19 March 2021).
- Amberg-Schwab, S. Handbook of Sol-Gel Science and Technology; Kluwer Academic Publishers: Norwell, Plymouth, 2004; p. 455. [Google Scholar]
- Amberg-Schwab, S.; Collin, D.; Schwaiger, J. Protection for Bioplastics. Eur. Coat. J. 2005, 12, 32–36. [Google Scholar]
- Amberg-Schwab, S. Functional barrier coatings on the basis of hybrid polymers. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 1–21. ISBN 978-3-319-19454-7. [Google Scholar]
- European Committee for Standardization. Packaging—Requirements for Packaging Recoverable through Composting and Biodegradation—Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging; CEN-EN 13432: 2000; European Committee for Standardization: Brussels, Belgium, 2000. [Google Scholar]
- Schmidt, S.; Liebert, T.; Heinze, T. Synthesis of soluble cellulose tosylates in an eco-friendly medium. Green Chem. 2014, 16, 1941–1946. [Google Scholar] [CrossRef]
- Popall, M.; Schulz, J.; Schmidt, H.K. ORMOCERe: Neue Werkstoffe für die Elektronik; Erstes Werkstoffsymposium Neue Polymere: Regensburg, Germany, 1989. [Google Scholar]
- Haas, K.-H.; Amberg-Schwab, S.; Rose, K.; Schottner, G. Functionalized coatings based on inorganic–organic polymers (ORMOCER®s) and their combination with vapor deposited inorganic thin films. Surf. Coat. Technol. 1999, 111, 72–79. [Google Scholar] [CrossRef]
- Gericke, M.; Heinze, T. Homogeneous tosylation of agarose as an approach toward novel functional polysaccharide materials. Carbohydr. Polym. 2015, 127, 236–245. [Google Scholar] [CrossRef]
- International Organization for Standardization. Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide—Part 1: General Method; ISO 14855-1:2012; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- International Organization for Standardization. Plastics—Determination of the Degree of Disintegration of Plastic Materials under Defined Composting Conditions in a Pilot-Scale Test; ISO 16929:2019; International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- Organisation for Economic Co-Operation and Development. Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test; OECD 208:2006; Organisation for Economic Co-Operation and Development: Paris, France, 2006. [Google Scholar]
- European Committee for Standardization. Materials and Articles in Contact with Foodstuffs—Plastics—Part 1: Guide to the Selection of Conditions and Test Methods for Overall Migration; EN 1186-1:2002; European Committee for Standardization: Brussels, Belgium, 2002. [Google Scholar]
- International Organization for Standardization. Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces; ISO 22196:2011; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Koschella, A.; Heinze, T. Novel Regioselectively 6-Functionalized Cationic Cellulose Polyelectrolytes Prepared via Cellulose Sulfonates. Macromol. Biosci. 2001, 1, 178–184. [Google Scholar] [CrossRef]
- Berlin, P.; Klemm, D.; Jung, A.; Liebegott, H.; Tiller, J.C. Film-Forming Aminocellulose Derivatives as Enzyme-Compatible Support Matrices for Biosensor Developments. Cellulose 2003, 10, 343–367. [Google Scholar] [CrossRef]
- Elchinger, P.-H.; Faugeras, P.-A.; Zerrouki, C.; Montplaisir, D.; Brouillette, F.; Zerrouki, R. Tosylcellulose synthesis in aqueous medium. Green Chem. 2012, 14, 3126. [Google Scholar] [CrossRef]
- DSP GOKYO Food & Chemical. For Healthy Life with Better Food—GLYOID®-Tamarind Gum; Food Additives; DSP GOKYO Food & Chemical: Osaka, Japan, 2014; Available online: https://www.dsp-gokyo-fc.co.jp/en/pickup/glyloid/food/ (accessed on 22 March 2021).
- van der Vorm, S.; van Hengst, J.M.A.; Bakker, M.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. Mapping the Relationship between Glycosyl Acceptor Reactivity and Glycosylation Stereoselectivity. Angew. Chem. 2018, 130, 8372–8376. [Google Scholar] [CrossRef]
- Fox, S.C.; Li, B.; Xu, D.; Edgar, K.J. Regioselective esterification and etherification of cellulose: A review. Biomacromolecules 2011, 12, 1956–1972. [Google Scholar] [CrossRef]
- Fernandes Diniz, J.M.B.; Gil, M.H.; Castro, J.A.A.M. Hornification–its origin and interpretation in wood pulps. Wood Sci. Technol. 2004, 37, 489–494. [Google Scholar] [CrossRef]
- Obst, M.; Heinze, T. Simple Synthesis of Reactive and Nanostructure Forming Hydrophobic Amino Cellulose Derivatives. Macromol. Mater. Eng. 2016, 301, 65–70. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S. Effects of Sodium Hydroxide Pretreatment on Structural Components of Biomass. Trans. ASABE 2014, 1187–1198. [Google Scholar] [CrossRef]
- Loow, Y.-L.; Wu, T.Y.; Md. Jahim, J.; Mohammad, A.W.; Teoh, W.H. Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 2016, 23, 1491–1520. [Google Scholar] [CrossRef]
- European Comission, Group of Chief Scientific Advisors. Biodegradability of Plastics in the Open Environment. Sci. Opin. 2020, 10, 1–42. [Google Scholar]
- Gigant, K. Raman-Spektroskopie hybridpolymerer Sol-Gel-Materialien: Vom Sol bis zur Schicht. Ph.D. Thesis, Universität Würzburg, Würzburg, Germany, 2005. [Google Scholar]
- Templin, M.; Wiesner, U.; Spiesss, H.W. Multinuclear solid-state-NMR studies of hybrid organic-inorganic materials. Adv. Mater. 1997, 9, 814–817. [Google Scholar] [CrossRef]
- Gigant, K.; Posset, U.; Schottner, G.; Baia, L.; Kiefer, W.; Popp, J. Inorganic-Organic Cross-Linking in UV Curable Hard Coats Based Upon Vinyltriethoxysilane-Tetraethoxysilane-Polyfunctional Acrylate Hybrid Polymers: A Raman Spectroscopic Study. J. Sol-Gel Sci. Technol. 2003, 26, 369–373. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: Boston, MA, USA, 1991; ISBN 0124511600. [Google Scholar]
- Artaki, I.; Bradley, M.; Zerda, T.W.; Jonas, J. NMR and Raman study of the hydrolysis reaction in sol-gel processes. J. Phys. Chem. 1985, 89, 4399–4404. [Google Scholar] [CrossRef]
- Ilić, D. Metrology in the 3rd Millennium: XVII IMEKO World Congress; 22–27 June 2003, Dubrovnik, Croatia; Proceedings; International Measurement Confederation; Hrvatsko Mjeriteljsko Društvo: Dubrovnik, Croatia, 2003; ISBN 9537124002. [Google Scholar]
- de Ferri, L.; Lorenzi, A.; Lottici, P.P. OctTES/TEOS system for hybrid coatings: Real-time monitoring of the hydrolysis and condensation by Raman spectroscopy. J. Raman Spectrosc. 2016, 47, 699–705. [Google Scholar] [CrossRef]
- Osswald, T.; Baur, E.; Rudolph, N. Plastics Handbook: The Resource for Plastics Engineers, 5th ed.; Hanser Publications: Cincinnati, OH, USA, 2018; ISBN 1569905592. [Google Scholar]
- Plackett, D.V.; Holm, V.K.; Johansen, P.; Ndoni, S.; Væggemose Nielsen, P.; Sipilainen-Malm, T.; Södergård, A.; Verstichel, S. Characterization of l-polylactide and l-polylactide-polycaprolactone co-polymer films for use in cheese-packaging applications. Packag. Technol. Sci. 2006, 19, 1–24. [Google Scholar] [CrossRef]
- Jahn, A.; Thümmler, K.; Gebke, S.; Kahl, M.; Aubel, I.; Fischer, S.; Bertau, M. Utilization of Hemicelluloses as Example for Holistic Recovery of Agricultural Residues. Chem. Ing. Tech. 2020, 92, 1764–1771. [Google Scholar] [CrossRef]
- Lahtela, V.; Silwal, S.; Kärki, T. Re-Processing of Multilayer Plastic Materials as a Part of the Recycling Process: The Features of Processed Multilayer Materials. Polymers 2020, 12, 2517. [Google Scholar] [CrossRef]
| Simulant | Test Conditions | Overall Migration Test |
|---|---|---|
| Distilled water | 10 days, 60 °C and 40 °C, total immersion | EN 1186-3 |
| Ethanol 10% v/v | 10 days, 60 °C and 40 °C, total immersion | EN 1186-3 |
| Acetic acid 3% w/v | 10 days, 60 °C and 40 °C, total immersion | EN 1186-3 |
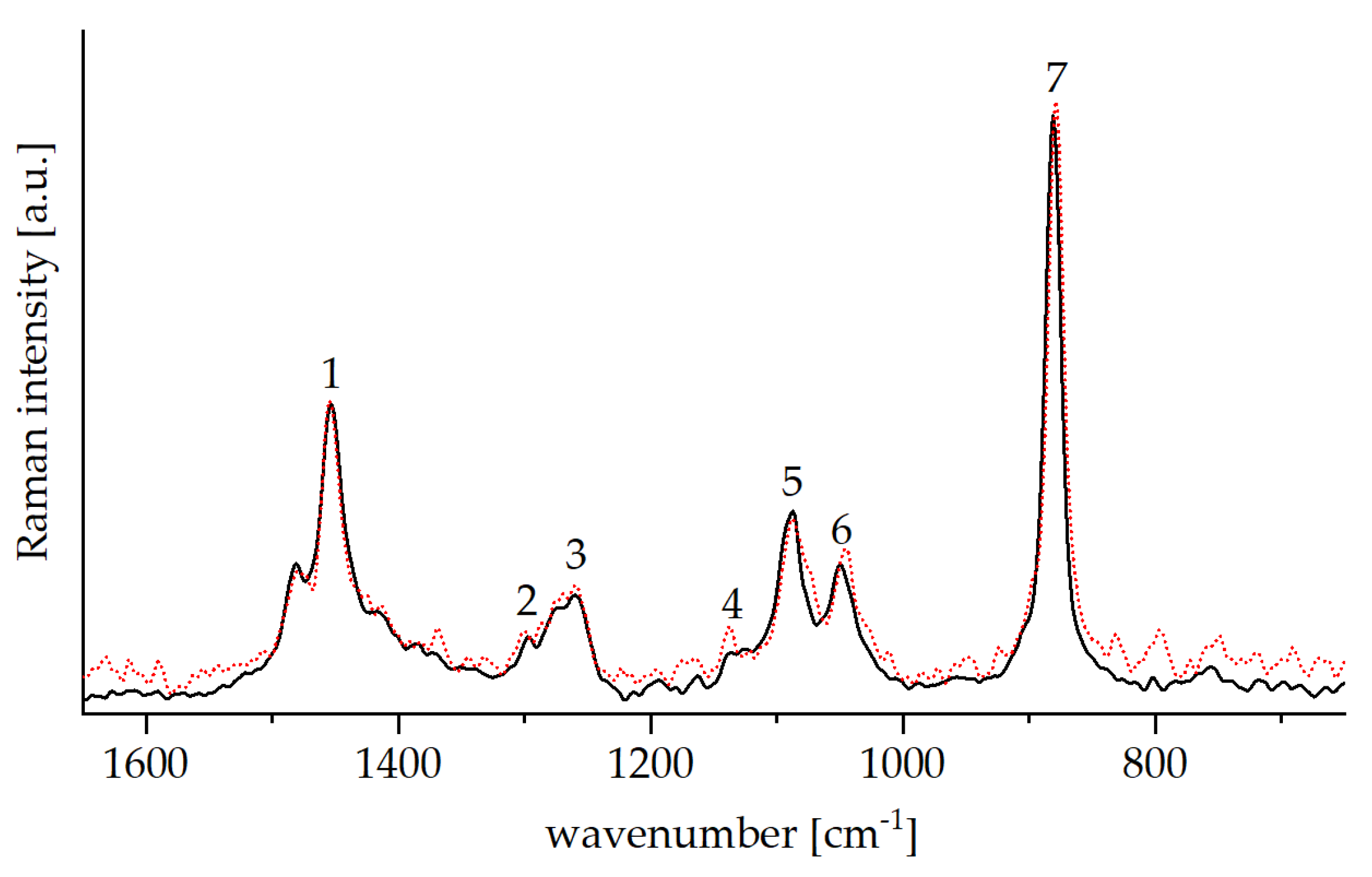
| Nr. | Wavenumber (cm−1) | Assignment |
|---|---|---|
| 1 | 1598 | ν (C=Caromatic) |
| 2 | 1364 | νas(SO2) |
| 3 | 1177 | νs (SO2) |
| 4 | 812 | ν ( S–O–C) |
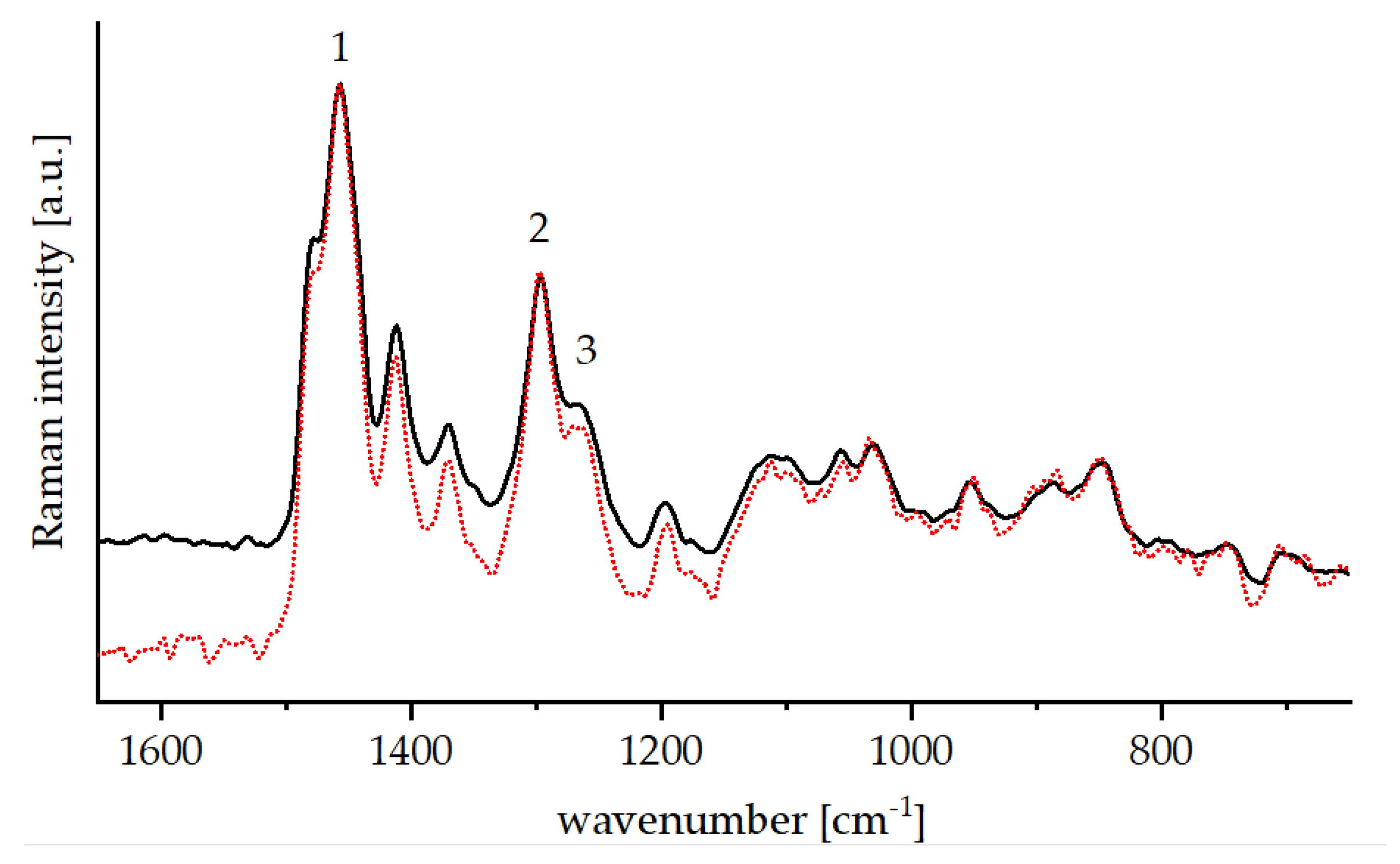
| No. | Wavenumber (cm−1) | Assignment |
|---|---|---|
| 1 | 1453 | νasym (CH3),νasym (CH2), δ(CH2) in ethanol, TEOS, GLYEO |
| 2 | 1298 | τ (CH2) in TEOS, GLYEO |
| 3 | 1259 | νip(Epoxy ring) in GLYEO |
| 4 | 1136 | νasym (COC) acetylacetone |
| 5 | 1087 | νasym (CCO) in ethanol |
| 6 | 1049 | ν (CO) in ethanol |
| 7 | 880 | νsym (CCO) in ethanol |
| Coating | Martens Hardness (HM) (N/mm2) | E-Module (GPa) |
|---|---|---|
| ORMOCER® | 406.08 ± 6.16 | 6.95 ± 0.09 |
| bioORMOCER® | 287.23 ± 7.51 | 5.30 ± 0.21 |
| Sample Name | Biodegradability (%) | Time (Elapsed Days) |
|---|---|---|
| PLA + bioORMOCER® | 92.9 | 91 |
| PLA/PBAT + bioORMOCER® | 93.0 | 91 |
| Value limit (EN 13432) | >90 | <180 |
| Sample Name | Disintegration (%) |
|---|---|
| PLA + bioORMOCER® | 100 |
| PLA/PBAT + bioORMOCER® | 100 |
| Value limit (EN 13432) | >90 after 12 weeks |
| Sample | Sample (%) | Sample (g) | Substrate (g) | Germination (Number) | Fresh Weight (g) |
|---|---|---|---|---|---|
| Control compost | 25 | 112.5 | 337.5 | 95 | 40.86 |
| 25 | 112.5 | 337.5 | 97 | 41.33 | |
| 25 | 112.5 | 337.5 | 92 | 41.00 | |
| 50 | 225 | 225 | 92 | 39.95 | |
| 50 | 225 | 225 | 91 | 38.69 | |
| 50 | 225 | 225 | 94 | 40.43 | |
| Test compost MIX+ bioORMOCER® | 25 | 112.5 | 337.5 | 88 | 38.32 |
| 25 | 112.5 | 337.5 | 94 | 37.80 | |
| 25 | 112.5 | 337.5 | 90 | 37.42 | |
| 50 | 225 | 225 | 91 | 36.79 | |
| 50 | 225 | 225 | 93 | 37.14 | |
| 50 | 225 | 225 | 96 | 37.62 |
| Sample | Sample (%) | Sample (g) | Substrate (g) | Germination (Number) | Fresh Weight (g) |
|---|---|---|---|---|---|
| Control compost | 25 | 60 | 180 | 94 | 8.25 |
| 25 | 60 | 180 | 90 | 7.32 | |
| 25 | 60 | 180 | 92 | 7.90 | |
| 50 | 120 | 120 | 89 | 7.10 | |
| 50 | 120 | 120 | 91 | 7.41 | |
| 50 | 120 | 120 | 95 | 8.09 | |
| Test compost MIX+ bioORMOCER® | 25 | 60 | 180 | 90 | 7.70 |
| 25 | 60 | 180 | 88 | 7.80 | |
| 25 | 60 | 180 | 86 | 8.06 | |
| 50 | 120 | 120 | 88 | 7.51 | |
| 50 | 120 | 120 | 91 | 7.64 | |
| 50 | 120 | 120 | 91 | 7.26 |
| Barley | Concentration (%) | Germination (Number) | Fresh Weight (g) | ||
|---|---|---|---|---|---|
| AVG | STD | AVG | STD | ||
| Test compost dilution (MIX+ bioORMOCER®) | 25 | 90.67 | 3.0 | 37.84 | 0.45 |
| 50 | 93.33 | 2.51 | 37.18 | 0.41 | |
| Cress | |||||
| Test compost dilution (MIX+ bioORMOCER®) | 25 | 88.0 | 2.0 | 7.85 | 0.19 |
| 50 | 90.0 | 1.73 | 7.47 | 0.19 | |
| Concentration (%) | Germination Rate (%) | Fresh Weight (%) | |
|---|---|---|---|
| Barley | |||
| Test compost dilution (MIX+ bioORMOCER®) | 25 | 96.1 | 92.2 |
| 50 | 102 | 93.7 | |
| Cress | |||
| Test compost dilution (MIX+ bioORMOCER®) | 25 | 96.1 | 100 |
| 50 | 97.9 | 99.2 |
| Recovery after Inoculation (cells/cm2) | Recovery after 24 h Incubation (cells/cm2) | |||
|---|---|---|---|---|
| S. aureus | E. coli | S. aureus | E. coli | |
| Uncoated PO | 21,875 | 21,250 | 20,625 | 20,000 |
| PO + bioORMOCER® (Run 1) | - | - | 213 | 1750 |
| PO + bioORMOCER® (Run 2) | - | - | 300 | 2938 |
| Antibacterial Activity (R) | ||
|---|---|---|
| S. aureus | E. coli | |
| PO + bioORMOCER® (Run 1) | 1.98 | 1.06 |
| PO + bioORMOCER® (Run 2) | 1.83 | 0.84 |
| Water (mg/dm2) | Ethanol 10% (mg/dm2) | Acetic Acid 3% (mg/dm2) | |
|---|---|---|---|
| PO film | 12 | 15 | 4.2 |
| PO film + bioORMOCER® | <0.1 | <0.1 | 7.7 |
| PLA film | 1.7 | 3.1 | 3.2 |
| PLA + bioORMOCER® | 9.5 | 5.9 | 11.8 |
| PLA/PBAT + bioORMOCER® | 5.7 | 3.1 | 6.4 |
| Water (mg/dm2) | Ethanol 10% (mg/dm2) | Acetic Acid 3% (mg/dm2) | |
|---|---|---|---|
| PLA + bioORMOCER® | 6.4 | 8.8 | 7.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmert, K.; Amberg-Schwab, S.; Braca, F.; Bazzichi, A.; Cecchi, A.; Somorowsky, F. bioORMOCER®—Compostable Functional Barrier Coatings for Food Packaging. Polymers 2021, 13, 1257. https://doi.org/10.3390/polym13081257
Emmert K, Amberg-Schwab S, Braca F, Bazzichi A, Cecchi A, Somorowsky F. bioORMOCER®—Compostable Functional Barrier Coatings for Food Packaging. Polymers. 2021; 13(8):1257. https://doi.org/10.3390/polym13081257
Chicago/Turabian StyleEmmert, Katharina, Sabine Amberg-Schwab, Francesca Braca, Agostino Bazzichi, Antonio Cecchi, and Ferdinand Somorowsky. 2021. "bioORMOCER®—Compostable Functional Barrier Coatings for Food Packaging" Polymers 13, no. 8: 1257. https://doi.org/10.3390/polym13081257
APA StyleEmmert, K., Amberg-Schwab, S., Braca, F., Bazzichi, A., Cecchi, A., & Somorowsky, F. (2021). bioORMOCER®—Compostable Functional Barrier Coatings for Food Packaging. Polymers, 13(8), 1257. https://doi.org/10.3390/polym13081257






