New Trends in the Use of Volatile Compounds in Food Packaging
Abstract
1. Introduction
2. Volatile Chemical Compounds Used in Food Packaging
2.1. Terpenes
2.2. Sulfur Compounds
2.3. Aldehydes
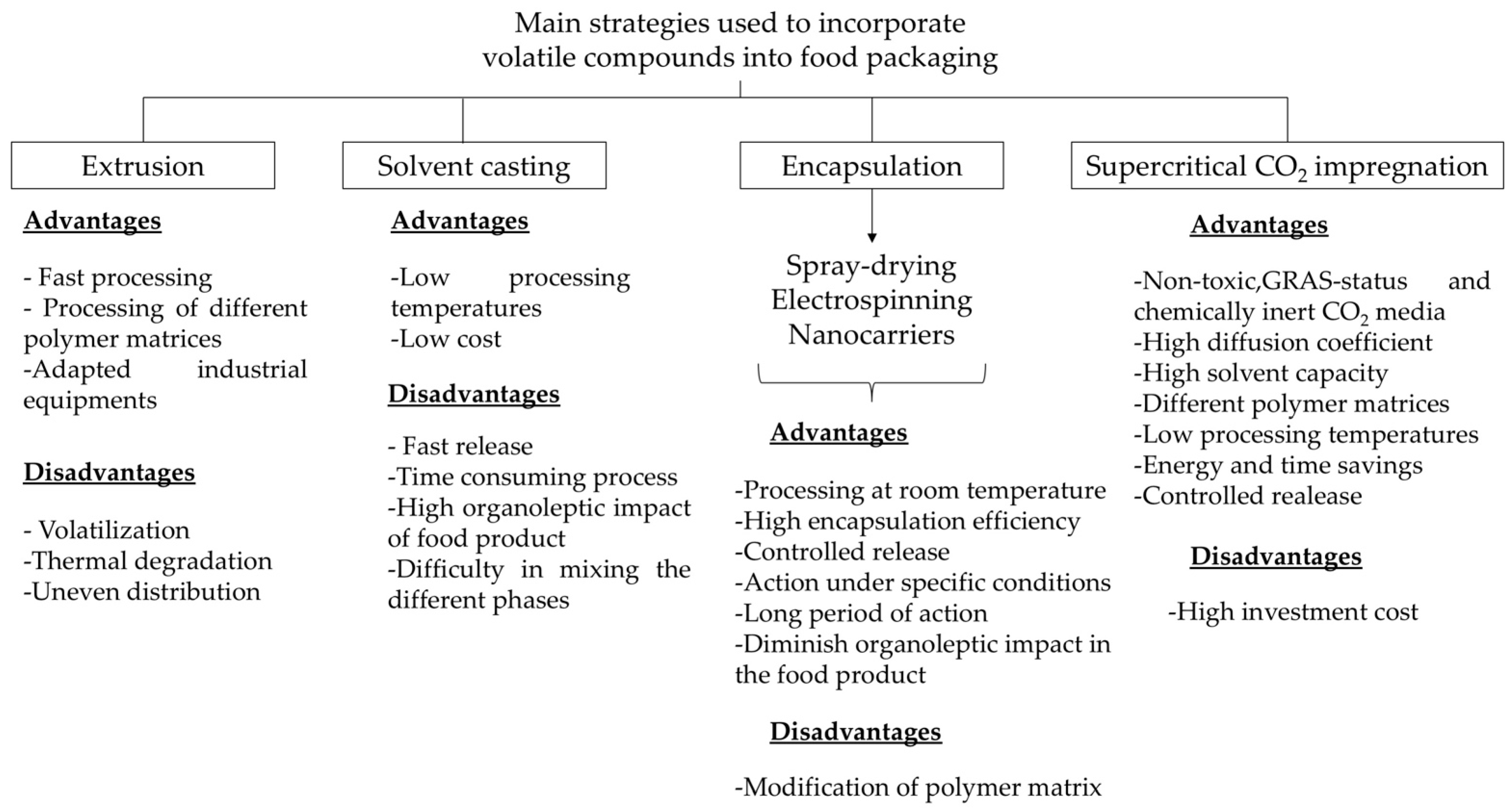
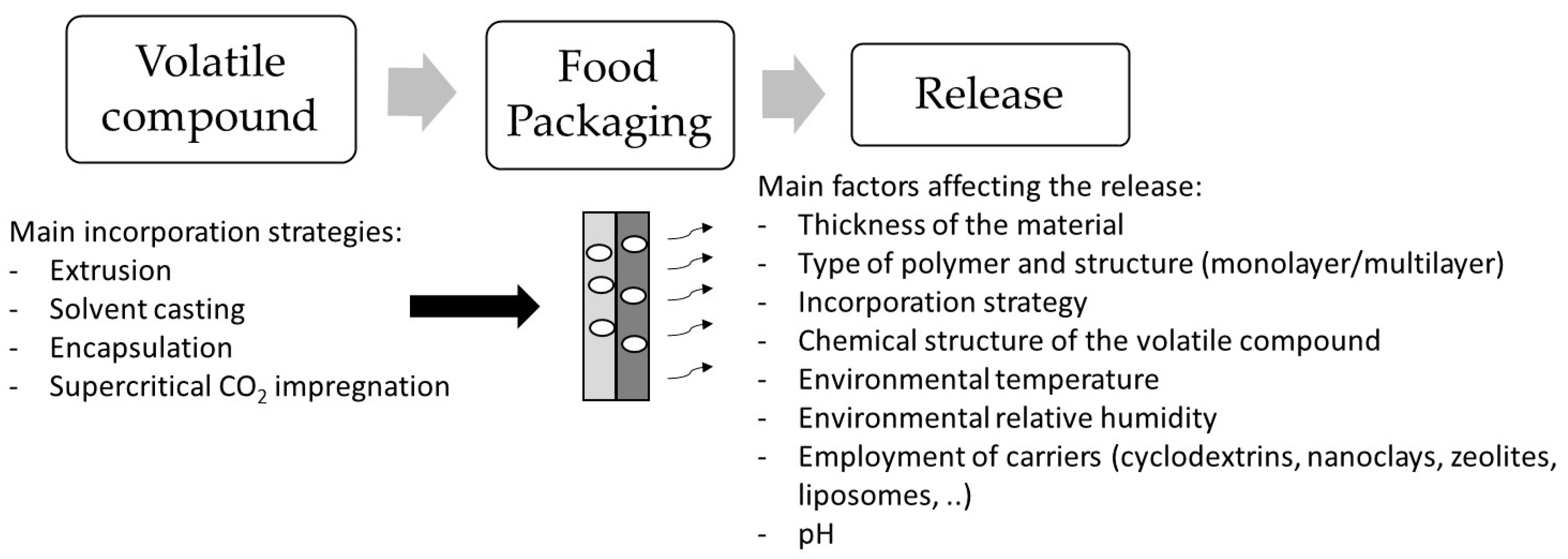
3. Incorporation Strategies of Volatile Compounds into Food Packaging
3.1. Extrusion Technique
3.2. Solvent Casting
3.3. Encapsulation
| Volatile Compound | Food Packaging | Processing Method | Concentration | Activity | Food Product | Reference |
|---|---|---|---|---|---|---|
| Eugenol | Polyvinyl pyrrolidone (PVP)/shellac fibrous film | Encapsulation followed by electrospinning | 3, 6, 9, and 12% w/w | Antifungal | Strawberry | [9] |
| Poly(3-hydroxybutyrate) (PHB)/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) film | Electrospinning and annealing | 2.5–25% w/w | Antimicrobial | NA | [10] | |
| Gelatin nanofibers/poly (lactic acid) (PLA) film | Electrospinning and annealing | 2–4 mg g−1 gelatin | Antioxidant, antimicrobial | NA | [11] | |
| Poly(3-hydroxybutyrate) (PHB)-thermoplastic starch (TPS)/organically modified montmorillonite (OMMT) bionanocomposites | Extrusion and melt blending | 3% w/w | NA | NA | [79] | |
| Starch film | Encapsulation followed by compression molding | 1.2–1.6% w/w | Antioxidant | NA | [33] | |
| PHBV film | Encapsulation followed by electrospinning and annealing | 2.5–20% w/w | Antimicrobial | NA | [32] | |
| Starch film | Casting technique | 1, 3, and 5% w/w | Antimicrobial | Pork | [31] | |
| Cellulose acetate (CA) or acrylic component/hydrophobically modified starch (AC/S) coatings on corona-treated oriented polypropylene film (OPP) | Casting of CA on corona-treated commercial OPP | 12.5 and 25% w/v | Antioxidant | Beef | [30] | |
| Thymol | PLA/poly(ε-caprolactone) (PCL) blended films | Solvent casting method followed by supercritical CO2 impregnation of thymol | 35.8% w/w | Antimicrobial | NA | [94] |
| PLA/nanoclay C30B | Extrusion of PLA with organo-modified montmorillonite C30B followed by CO2 supercritical impregnation of thymol | 17% w/w | Antimicrobial | NA | [95] | |
| PLA/PCL | Solvent casting method followed by CO2 impregnation of thymol | 21.49% w/w | Antioxidant | NA | [96] | |
| Thymol | Soybean protein isolate (SPI) | Solution casting method by adding thymol/diatomite complex | 12.5% w/w | Antimicrobial | NA | [97] |
| PLA/poly(butylene-succinate-co-adipate) (PBSA) | Blow film extrusion technique | 3 and 6% w/w | Antifungal | Bread | [76] | |
| Starch/chitosan | Casting method followed by supercritical CO2 impregnation | 27.3% w/w | Antimicrobial | NA | [98] | |
| PLA | Solvent casting | 10% w/w | Antimicrobial | Chicken | [89] | |
| Poly(lactide-co-glycolide) (PLGA) nanofibers | Encapsulation of thymol in PLGA fiber via coaxial electrospinning | Encapsulation of 75% w/w | Antibacterial | Strawberry | [99] | |
| PLA | PLA extrusion followed by supercritical CO2 impregnation of thymol | 20.5% w/w | NA | NA | [100] |
3.3.1. Electrospinning
3.3.2. Nanocarriers
3.3.3. Other Encapsulation Methodologies
3.4. Supercritical CO2 Impregnation
| Volatile Compound | Food Packaging | Processing Method | Concentration | Activity | Food Product | Reference |
|---|---|---|---|---|---|---|
| Carvacrol | Polyvinyl alcohol (PVA) | Electrospinning followed by casting method | 15% w/w | NA | NA | [101] |
| Flaxseed gum films | Solvent casting method by sonication | 0.05, 0.1, and 0.2% w/w | Antioxidant and antimicrobial | NA | [91] | |
| Thermoplastic starch (TPS) | Montmorillonite encapsulation followed by casting method | 4.5, 9, and 15% w/w | Antimicrobial | NA | [103] | |
| Sodium alginate | Encapsulation with β-cyclodextrin followed by solvent casting | 15, 30, and 60 g L−1 | Antifungal | Mushrooms | [104] | |
| Vanillin | Chitosan | Covalent immobilization and casting method | 6.2 mmol | Antimicrobial | Fresh-cut melon | [105] |
| Xanthan gum-lignin hydrogel film | Hydrogel mixing followed by freeze-drying | 0.9% w/w | Antimicrobial | NA | [110] | |
| PCL | Encapsulation in nanoparticles followed by extrusion and melting in a hot press | 5 mL per gram of substrate | Antimicrobial | NA | [13] | |
| Allyl isothiocyanate | Halloysite nanotubes (HNTs) coated with sodium polyacrylate (PA) | HNT encapsulation with PA by stirring and centrifugation | 10 mg of HNTs per mL of allyl isothiocyanate oil | Antimicrobial | NA | [68] |
| 1,8-Cineole | Chitosan | Nanoencapsulation and casting method | 0.2% w/w | Antimicrobial and antioxidant | Ground beef meat | [107] |
| Citral | Cashew gum/gelatin | Casting method | 10% w/w | Antimicrobial | Bread | [86] |
| Cellulose acetate | Casting method | 10% w/w | Improvement of physical properties | Coelho cheese | [87] | |
| R-(−)-carvone | Low density polyethylene (LDPE) | Supercritical CO2-assisted impregnation | 0.8 mg g−1 CO2 | NA | NA | [109] |
| Cinnamaldehyde | PLA | Supercritical CO2-assisted impregnation | 8 to 13% w/w | Antimicrobial | NA | [95] |
| Hexanal | PLA and ethylcellulose (EC) | Electrospinning and electrosprying | Hexanal into the polymer at 1:9 (w/w) ratio | Antimicrobial | NA | [74] |
| Galactoglucomannans (GGM) | Hydrogel mixing followed by freeze-drying | 1–100 mg g−1 | Antimicrobial | Blueberries and cherry tomatoes | [75] | |
| Octanal, nonanal, decanal, d-limonene, and eugenol | Epoxy, polyolefin, and acrylate can lining polymers | Empty cans exposition | 4.1–4.2 ppb | NA | NA | [111] |
| d-Limonene | Gluten and ι-carrageenans | Casting method | 0.5 mL per gram of solution | NA | NA | [88] |
3.5. Others Technologies
4. Retention Capacity and Controlled Release of Volatile Compounds from Food Packaging
4.1. Retention Capacity of Volatile Compounds in Food Packaging
4.2. Release of Volatile Compounds from Food Packaging during Different Storage Conditions
5. Legislation Remarks
6. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, A.; Valente, A.J.; Jiménez, A.; Garrigós, M.C. Characterization of poly(ε-caprolactone)-based nanocomposites containing hydroxytyrosol for active food packaging. J. Agric. Food Chem. 2014, 12, 2244–2252. [Google Scholar] [CrossRef]
- Ramos, M.; Beltrán, A.; Fortunati, E.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled Release of Thymol from Poly(Lactic Acid)-Based Silver Nanocomposite Films with Antibacterial and Antioxidant Activity. Antioxidants 2020, 9, 395. [Google Scholar] [CrossRef]
- Wicochea-Rodríguez, J.D.; Chalier, P.; Ruiz, T.; Gastaldi, E. Active Food Packaging Based on Biopolymers and Aroma Compounds: How to Design and Control the Release. Front Chem. 2019, 7, 398. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Development of novel nano-biocomposite antioxidant films based on poly (lactic acid) and thymol for active packaging. Food Chem. 2014, 162, 149–155. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Encapsulation Systems for Antimicrobial Food Packaging Components: An Update. Molecules 2020, 25, 1134. [Google Scholar] [CrossRef]
- Chen, Q.; Duncan, M.; Zhong, F.; Wen, J.; Young Quek, S. Co-encapsulation of fish oil with phytosterol esters and limonene by milk proteins. J. Food Eng. 2013, 117, 505–512. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein-gallic acid system. Food Chem. 2013, 15, 1013–1021. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Q.; Chen, J.; Li, L. Effects of coaxial electrospun eugenol loaded core-sheath PVP/shellac fibrous films on postharvest quality and shelf life of strawberries. Postharvest Biol. Technol. 2020, 159, 111028. [Google Scholar] [CrossRef]
- Figueroa-López, K.J.; Cabedo, L.; Lagarón, J.M.; Torres-Giner, S. Development of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Monolayers Containing Eugenol and Their Application in Multilayer Antimicrobial Food Packaging. Front. Nutr. 2020, 7, 140. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Fabrication of eugenol loaded gelatin nanofibers by electrospinning technique as active packaging material. LWT Food Sci. Technol. 2021, 139, 110800. [Google Scholar] [CrossRef]
- Higueras, L.; López-Carballo, G.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Antimicrobial packaging of chicken fillets based on the release of carvacrol from chitosan/cyclodextrin films. Int. J. Food Microbiol. 2014, 188, 53–59. [Google Scholar] [CrossRef]
- Stanzione, M.; Gargiulo, N.; Caputo, D.; Liguori, B.; Cerruti, P.; Amendola, E.; Lavorgna, M.; Buonocore, G.G. Peculiarities of vanillin release from amino-functionalized mesoporous silica embedded into biodegradable composites. Eur. Polym. J. 2017, 89, 88–100. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Livinalli, N.F.; Baldasso, C.; Tessaro, I.C. Pervaporation in the separation of essential oil components: A review. Trends Food Sci. Technol. 2019, 93, 42–52. [Google Scholar] [CrossRef]
- Ghadiriasli, R.; Mahmoud, M.A.A.; Wagenstaller, M.; van de Kuilen, J.-W.; Buettner, A. Molecular and sensory characterization of odorants in Cembran pine (Pinus cembra L.) from different geographic regions. Talanta 2020, 220, 121380. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Perez-Cacho, P.; Rouseff, R. Processing and storage effects on orange juice aroma: A review. J. Agric. Food Chem. 2008, 56, 9785–9796. [Google Scholar] [CrossRef]
- Schreiner, L.; Bauer, J.; Ortner, E.; Buettner, A. Structure-Odor Activity Studies on Derivatives of Aromatic and Oxygenated Monoterpenoids Synthesized by Modifying p-Cymene. J. Nat. Prod. 2020, 83, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; An, K.; Su, S.; Yu, Y.; Wu, J.; Xiao, G.; Xu, Y. Aromatic characterizati on of mangoes (Mangifera indica L.) Using solid phase extraction coupled with gas chromatography-mass spectrometry and olfactometry and sensory analyses. Foods 2020, 9, 75. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Chen, C.; Tong, H.; Liang, G.; Gmitter, F.G., Jr. Juice volatile composition differences between Valencia orange and its mutant Rohde Red Valencia are associated with carotenoid profile differences. Food Chem. 2018, 245, 223–232. [Google Scholar] [CrossRef]
- Sato, S.; Sekine, Y.; Kakumu, Y.; Hiramoto, T. Measurement of diallyl disulfide and allyl methyl sulfide emanating from human skin surface and influence of ingestion of grilled garlic. Sci. Rep. 2020, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Xiao, X.; Wei, L.; Li, L.; Li, G.; Liu, F.; Xie, J.; Yu, J.; Zhong, Y. Development and comprehensive HS-SPME/GC–MS analysis optimization, comparison, and evaluation of different cabbage cultivars (Brassica oleracea L. var. capitata L.) volatile components. Food Chem. 2021, 340, 128166. [Google Scholar] [CrossRef] [PubMed]
- Schaller, T.; Schieberle, P. Comparison of the Key Aroma Compounds in Fresh, Raw Ginger (Zingiber officinale Roscoe) from China and Roasted Ginger by Application of Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2020, 68, 15292–15300. [Google Scholar] [CrossRef]
- Wang, N.; Zhou, Z.; Chen, S. Aging status characterization of Chinese rice wine based on key aging-marker profiles combined with principal components analysis and partial least-squares regression. Eur. Food Res. Technol. 2020, 246, 1283–1296. [Google Scholar] [CrossRef]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Natural Plant-Derived Chemical Compounds as Listeria monocytogenes Inhibitors In Vitro and in Food Model Systems. Pathogens 2021, 10, 12. [Google Scholar] [CrossRef]
- Murakami, Y.; Shoji, M.; Hanazawa, S.; Tanaka, S.; Fujisawa, S. Preventive effect of bis-eugenol, a eugenol ortho dimer, on lipopolysaccharide-stimulated nuclear factor kappaB activation and inflammatory cytokine expression in macrophages. Biochem. Pharmacol. 2003, 66, 1061–1066. [Google Scholar] [CrossRef]
- Murakami, Y.; Shoji, M.; Hirata, A.; Tanaka, S.; Yokoe, I.; Fujisawa, S. Dehydrodiisoeugenol, an isoeugenol dimer, inhibits lipopolysaccharide-stimulated nuclear factor kappaB activation and cyclooxygenase-2 expression in macrophages. Arch. Biochem. Biophys. 2005, 434, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Rao, A.S.; Nandal, A.; Kumar, S.; Yadav, S.S.; Ganaie, S.A.; Narasimhan, B. Phytochemical and pharmacological review of Cinnamomum verum J. Presl-a versatile spice used in food and nutrition. Food Chem. 2021, 338, 127773. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Sankaran, V.; Ramar, M.; Chellappan, D.R. Chemical analysis of leaf essential oil of Cinnamomum verum from Palni hills, Tamil Nadu. J. Chem. Pharmac. Sci. 2015, 8, 476–479. [Google Scholar]
- Hassan, H.A.; Genaidy, M.M.; Kamel, M.S.; Abdelwahab, S.F. Synergistic antifungal activity of mixtures of clove, cumin and caraway essential oils and their major active components. J. Herb. Med. 2020, 24, 100399. [Google Scholar] [CrossRef]
- Navikaite-Snipaitiene, V.; Ivanauskas, L.; Jakstas, V.; Rüegg, N.; Rutkaite, R.; Wolfram, E.; Yildirim, S. Development of antioxidant food packaging materials containing eugenol for extending display life of fresh beef. Meat Sci. 2018, 145, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, H.; Kang, S.; Xia, L.; Jiang, S.; Chen, M.; Jiang, S. An active packaging film based on yam starch with eugenol and its application for pork preservation. Food Hydrocoll. 2019, 96, 546–554. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Figueroa-López, K.J.; Bernandos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; Lagarón, J.M. Electrospun Antimicrobial Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Containing Eugenol Essential Oil Encapsulated in Mesoporous Silica Nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Talón, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Eugenol incorporation into thermoprocessed starch films using different encapsulating materials. Food Packag. Shelf Life 2019, 21, 100326. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 5th ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The bioactivity and toxicological actions of Carvacrol. Crit. Rev. Food Sci. 2015, 55, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Del Nobile, M.A.; Conte, A.; Incoronato, A.L.; Panza, O. Antimicrobial efficacy and release kinetics of thymol from zein films. J. Food Eng. 2008, 89, 57–63. [Google Scholar] [CrossRef]
- Horvathova, E.; Navarova, J.; Galova, E.; Sevcovicova, A.; Chodakova, L.; Snahnicanova, Z.; Melusova, M.; Kozics, K.; Slamenova, D. Assessment of antioxidative, chelating, and DNAProtective effects of selected essential oil components (Eugenol, Carvacrol, Thymol, Borneol, Eucalyptol) of plants and intact rosmarinus officinalis oil. J. Agric. Food Chem. 2014, 62, 6632–6639. [Google Scholar] [CrossRef]
- Chew, S.C.; Nyam, K.L. Microencapsulation of kenaf seed oil by co-extrusion technology. J. Food Eng. 2016, 175, 43–50. [Google Scholar] [CrossRef]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Sharma, N.; Tripathi, A. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol. Res. J. Int. 2008, 163, 337–344. [Google Scholar] [CrossRef]
- Soottitantawat, A.; Yoshii, H.; Furuta, T.; Ohgawara, M.; Forssell, P.; Partanen, R.; Poutanen, K.; Linko, P. Effect of water activity on the release characteristics and oxidative stability of D-limonene encapsulated by spray drying. J. Agric. Food Chem. 2004, 52, 1269–1276. [Google Scholar] [CrossRef]
- Jin, L.; Liu, X.; Bian, C.; Sheng, J.; Song, Y.; Zhu, Y. Fabrication linalool-functionalized hollow mesoporous silica spheres nanoparticles for efficiently enhance bactericidal activity. Chin. Chem. Lett. 2020, 31, 2137–2141. [Google Scholar] [CrossRef]
- Amiri, P.; Shahpiri, A.; Asadollahi, M.A.; Momenbeik, F.; Partow, S. Metabolic engineering of Saccharomyces cerevisiae for linalool production. Biotechnol. Lett. 2016, 38, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, H.; El-Ghorab, A.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Terzi, V. Chapter 35: Carvone (Mentha spicata L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 309–316. [Google Scholar] [CrossRef]
- Ceylan, E.; Fung, D.Y.C. Antimicrobial activity of spices. J. Rapid Methods Autom. Microbiol. 2004, 12, 1–55. [Google Scholar] [CrossRef]
- Neri, M.; Brigati, S.; Bertolini, P. Control of Neofabraea alba by plant volatile compounds and hot water. Postharvest Biol. Technol. 2009, 51, 425–430. [Google Scholar] [CrossRef]
- Ekpenyong, C.E.; Akpan, E.; Nyoh, A. Ethnopharmacology, phytochemistry, and biological activities of Cymbopogon citratus (DC.) Stapf extracts. Chin. J. Nat. Med. 2015, 13, 321–337. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Gottardi, D.; Malik, A.; Guerzoni, M.E. Chemical composition, in vitro anti-yeast activity and fruit juice preservation potential of lemon grass oil. LWT Food Sci. Technol. 2014, 57, 731–737. [Google Scholar] [CrossRef]
- Machado, T.F.; Pereira, R.C.A.; Sousa, C.T.; Batista, V.C.V. Atividade antimicrobiana do oleo esscencial do capim limao (Cymbopogon citratus) e sua interaçao com os componentes dos alimentos. Bol. Cent. Pesqui. Process. Aliment. 2015, 33, 30–38. [Google Scholar] [CrossRef]
- Philis, J.G. The S1 ← S0 spectrum of jet-cooled p-cymene. Spectrochim. Acta A 2005, 61, 1239–1241. [Google Scholar] [CrossRef]
- Tian, F.; Woo, S.Y.; Lee, S.Y.; Chun, H.S. p-Cymene and its derivatives exhibit antiaflatoxigenic activities against Aspergillus flavus through multiple modes of action. Appl. Biol. Chem. 2018, 61, 489–497. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Barbieri, R.; Silva, A.S.; Nabavi, S.F.; Tsetegho Sokeng, A.J.; Izadi, M.; Jafari, N.J.; Suntar, I.; Daglia, M.; et al. Update on Monoterpenes as Antimicrobial Agents: A Particular Focus on p-Cymene. Materials 2017, 10, 947. [Google Scholar] [CrossRef]
- Pavoni, L.; Maggi, F.; Mancianti, F.; Nardoni, S.; Ebani, V.V.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Microemulsions: An effective encapsulation tool to enhance the antimicrobial activity of selected Eos. J. Drug Deliv. Sci. Technol. 2019, 53, 101101. [Google Scholar] [CrossRef]
- Reichardt, S.; Budahn, H.; Lamprecht, D.; Riewe, D.; Ulrich, D.; Dunemann, F.; Kopertekh, L. The carrot monoterpene synthase gene cluster on chromosome 4 harbours genes encoding flavour-associated sabinene synthases. Hortic. Res. 2020, 7, 190. [Google Scholar] [CrossRef]
- Llana-Ruíz-Cabello, M.; Pichardo, S.; Jiménez-Morillo, N.T.; Bermúdez, J.M.; Aucejo, S.; González-Vila, F.J.; Cameán, A.M.; González-Pérez, J.A. Molecular characterisation of a bio-based active packaging containing Origanum vulgare L. essential oil using pyrolysis gas chromatography-mass spectrometry. J. Sci. Food Agric. 2016, 96, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, S.; Kim, E.; Kim, Y.-N.; Lee, J.; Lee, D.-U. Effects of Pulsed Electric Field and Thermal Treatments on Microbial Reduction, Volatile Composition, and Sensory Properties of Orange Juice, and Their Characterization by a Principal Component Analysis. Appl. Sci. 2021, 11, 186. [Google Scholar] [CrossRef]
- Buettner, A.; Schieberle, P. Evaluation of key aroma compounds in handsqueezed grapefruit juice (Citrus paradisi Macfayden) by quantitation and flavor reconstitution experiments. J. Agric. Food Chem. 2001, 49, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- van Willige, R.W.G.; Linssen, J.P.H.; Legger-Huysman, A.; Voragen, A.G.J. Influence of flavour absorption by food-packaging materials (low-density polyethylene, polycarbonate and polyethylene terephthalate) on taste perception of a model solution and orange juice. Food Addit. Contam. 2003, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Hori, Y.; Myoda, T. Characterization of key aroma compounds in aged garlic extract. Food Chem. 2020, 312, 126081. [Google Scholar] [CrossRef] [PubMed]
- Saud, S.M.; Li, W.; Gray, Z.; Matter, M.S.; Colburn, N.H.; Young, M.R.; Kim, Y.S. Diallyl disulfide (DADS), a constituent of garlic, inactivates NF-kB and prevents colitis-induced colorectal cancer by inhibiting GSK3b. Cancer Prev. Res. 2016, 9, 607–615. [Google Scholar] [CrossRef]
- Wu, Y.-R.; Li, L.; Sun, X.-C.; Wang, J.; Ma, C.-Y.; Zhang, Y.; Qu, H.-L.; Xu, R.-X.; Li, J.-J. Diallyl disulfide improves lipid metabolism by inhibiting PCSK9 expression and increasing LDL uptake via PI3K/Akt-SREBP2 pathway in HepG2 cells. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.A.; Yoshii, H. Encapsulation of allyl sulfide with middle-chain triglyceride oil and cyclodextrin by spray drying. Jpn. J. Food Eng. 2017, 18, 35–42. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J. Constituents of the Essential Oils of Garlic and Citronella and Their Vapor-phase Inhibition Mechanism against S. aureus. Food Sci. Technol. Res. 2019, 25, 65–74. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Rajkumar, G.; Shanmugam, S.; Galvao, M.D.S.; Sandes, R.D.D.; Neta, M.T.S.L.; Narain, N.; Mujumdar, A.S. Comparative evaluation of physical properties and volatiles profile of cabbages subjected to hot air and freeze drying. Food Sci. Technol. 2017, 80, 501–509. [Google Scholar] [CrossRef]
- Maruthupandy, M.; Seo, J. Allyl isothiocyanate encapsulated halloysite covered with polyacrylate as a potential antibacterial agent against food spoilage bacteria. Mater. Sci. Eng. 2019, 105, 110016. [Google Scholar] [CrossRef] [PubMed]
- Sangsuwan, J.; Sutthasupa, S. Effect of chitosan and alginate beads incorporated with lavender, clove essential oils, and vanillin against Botrytis cinerea and their application in fresh table grapes packaging system. Packag. Technol. Sci. 2019, 32, 595–605. [Google Scholar] [CrossRef]
- Buslovich, A.; Horev, B.; Rodov, V.; Gedanken, A.; Poverenov, E. One-step surface grafting of organic nanoparticles: In situ deposition of antimicrobial agents vanillin and chitosan on polyethylene packaging films. J. Mater. Chem. B 2017, 5, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Misran, A.; Padmanabhan, P.; Sullivan, J.A.; Khanizadeh, S.; Paliyath, G. Composition of phenolics and volatiles in strawberry cultivars and influence of preharvest hexanal treatment on their profiles. Can. J. Plant Sci. 2015, 95, 115–126. [Google Scholar] [CrossRef]
- Sholberg, P.L.; Randall, P. Fumigation of stored pome fruit with hexanal reduces blue and gray mold decay. HortScience 2007, 42, 611–616. [Google Scholar] [CrossRef]
- Jash, A.; Lim, L. Triggered release of hexanal from an imidazolidine precursor encapsulated in poly(lactic acid) and ethylcellulose carriers. J. Mater. Sci. 2018, 53, 2221–2235. [Google Scholar] [CrossRef]
- Lehtonen, M.; Kekäläinen, S.; Nikkilä, I.; Kilpeläinen, P.; Tenkanen, M.; Mikkonen, K. Active food packaging through controlled in situ production and release of hexanal. Food Chem. X 2020, 5, 100074. [Google Scholar] [CrossRef]
- Suwanamornlert, P.; Kerddonfag, N.; Sane, A.; Chinsirikul, W.; Zhou, W.; Chonhenchob, V. Poly(lactic acid)/poly(butylene-succinate-co-adipate) (PLA/PBSA) blend films containing thymol as alternative to synthetic preservatives for active packaging of bread. Food Packag. Shelf Life 2020, 25, 100515. [Google Scholar] [CrossRef]
- Boonruang, K.; Chinsirikul, W.; Hararak, B.; Kerddonfag, N.; Chonhenchob, V. Antifungal Poly(lactic acid) Films Containing Thymol and Carvone. MATEC Web Conf. 2016, 67, 06107. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Conte, A.; Buonocore, G.G.; Incoronato, A.L.; Massaro, A.; Panza, O. Active packaging by extrusion processing of recyclable and biodegradable polymers. J. Food Eng. 2009, 93, 1–6. [Google Scholar] [CrossRef]
- Garrido-Miranda, K.A.; Rivas, B.L.; Pérez-Rivera, M.; Fernández-Blázquez, J.P.; Monclús, M.; Peña-Farfal, C. Mechanical and morphological properties of Poly(3-hudroxybutyrate)-thermoplastic starch/clay/eugenol bionanocomposites. J. Chil. Chem. Soc. 2020, 65, 4992–4997. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, F.E.; Di Salvo, C.; Arcarisi, A. Bilayer biodegradable films prepared by co-extrusion film blowing: Mechanical performance, release kinetics of an antimicrobial agent and hydrolytic degradation. Compos. Part A Appl. Sci. Manuf. 2020, 132, 105836. [Google Scholar] [CrossRef]
- Raouche, S.; Mauricio-Iglesias, M.; Peyron, S.; Guillard, V.; Gontard, N. Combined effect of high pressure treatment and anti-microbial bio-sourced materials on microorganisms’ growth in model food during storage. Innov. Food Sci. Emerg. Technol. 2011, 12, 426–434. [Google Scholar] [CrossRef]
- Brüster, B.; Adjoua, Y.-O.; Dieden, R.; Grysan, P.; Federico, C.E.; Berthé, V.; Addiego, F. Plasticization of Polylactide with Myrcene and Limonene as Bio-Based Plasticizers: Conventional vs. Reactive Extrusion. Polymers 2019, 11, 1363. [Google Scholar] [CrossRef]
- Suppakul, P.; Sonneveld, K.; Bigger, S.W.; Miltz, J. Loss of AM additives from antimicrobial films during storage. J. Food Eng. 2011, 105, 270–276. [Google Scholar] [CrossRef]
- Jagadish, R.S.; Raj, B.; Asha, M.R. Blending of low-density polyethylene with vanillin for improved barrier and aroma-releasing properties in food packaging. J. Appl. Polym. Sci. 2009, 113, 3732–3741. [Google Scholar] [CrossRef]
- Srisa, A.; Harnkarnsujarit, N. Antifungal films from trans-cinnamaldehyde incorporated poly(lactic acid) and poly(butylene adipate-co-terephthalate) for bread packaging. Food Chem. 2020, 333, 127537. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Gonzaga, M.; Bastos, M.; Magalhaes, H.; Benevides, S.; Furtado, R.; Zambelli, R.; Garruti, D. Packaging with cashew gum/gelatin/essential oil for bread: Release potential of the citral. Food Packag. Shelf Life 2020, 23, 100431. [Google Scholar] [CrossRef]
- Oliveira, M.; Bastos, M.; Magalhaes, H.; Garruti, D.; Benevides, S.; Furtado, R.; Egito, A. α,β-citral from Cymbopogon citratus on cellulosic film: Release potential and quality of coalho cheese. LWT-Food Sci. Technol. 2017, 85, 246–251. [Google Scholar] [CrossRef]
- Marcuzzo, E.; Debeaufort, F.; Sensidoni, A.; Tat, L.; Beney, L.; Hambleton, A.; Peressini, D.; Voilley, A. Release Behavior and Stability of Encapsulated D‑Limonene from Emulsion-Based Edible Films. J. Agric. Food Chem. 2012, 60, 12177–12185. [Google Scholar] [CrossRef]
- Mohamad, N.; Mazlan, M.; Amin Tawakkal, I.; Talib, R.A.; Kian, L.K.; Fouad, H.; Jawaid, M. Development of active agents filled polylactic acid films for food packaging application. Int. J. Biol. Macromol. 2020, 163, 1451–1457. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, L.J.; Xue, J. Effects of high pressure homogenization on rheological properties of flaxseed gum. Carbohydr. Polym. 2011, 83, 489–494. [Google Scholar] [CrossRef]
- Fang, S.; Qiu, W.; Mei, J.; Xie, J. Effect of Sonication on the Properties of Flaxseed Gum Films Incorporated with Carvacrol. Int. J. Mol. Sci. 2020, 21, 1637. [Google Scholar] [CrossRef] [PubMed]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Zanetti, M.; Carniel, T.K.; Dalcanton, F.; dos Anjos, R.S.; Gracher Riella, H.; de Araújo, P.H.H.; de Oliveira, D.; Antônio Fiori, M. Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends Food Sci. Technol. 2018, 81, 51–60. [Google Scholar] [CrossRef]
- Milovanovic, S.; Hollermann, G.; Errenst, C.; Pajnik, J.; Frerich, S.; Kroll, S.; Rezwan, K.; Ivanovic, J. Supercritical CO2 impregnation of PLA/PCL films with natural substances for bacterial growth control in food packaging. Food Res. Int. 2018, 107, 486–495. [Google Scholar] [CrossRef]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; López de Dicastillo, C.; Valenzuela, X.; Galorro, M.J.; Romero Guarda, A. Supercritical impregnation of cinnamaldehyde into polylactic acid as a route to develop antibacterial food packaging materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Wu, H.; Lu, J.; Xiao, D.; Yan, Z.; Li, S.; Li, T.; Wan, X.; Zhang, Z.; Liu, Y.; Shen, G.; et al. Development and characterization of antimicrobial protein films based on soybean protein isolate incorporating diatomite/thymol complex. Food Hydrocoll. 2021, 110, 106138. [Google Scholar] [CrossRef]
- Pajnik, J.; Lukic, I.; Dikic, J.; Asanin, J.; Gordic, M.; Misic, S.; Zizovic, I.; Korzeniowska, M. Application of Supercritical Solvent Impregnation for Production of Zeolite Modified Starch-Chitosan Polymers with Antibacterial Properties. Molecules 2020, 25, 4717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Zhu, Z.; Jiao, X.; Shang, Y.; Wen, Y. Encapsulation of Thymol in Biodegradable Nanofiber via Coaxial Eletrospinning and Applications in Fruit Preservation. J. Agric. Food Chem. 2019, 67, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of processing conditions on the physical, chemical and transport properties of polylactic acid films containing thymol incorporated by supercritical impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Tampau, A.; González-Martinez, C.; Chiralt, A. Polyvinyl alcohol-based materials encapsulating carvacrol obtained by solvent casting and electrospinning. React. Funct. Polym. 2020, 153, 104603. [Google Scholar] [CrossRef]
- Buchs, B.; Godin, G.; Trachsel, A.; de Saint Laumer, J.; Lehn, J.; Hermann, A. Reversible aminal formation: Controlling the evaporation of bioactive volatiles by dynamic combinatorial/covalent chemistry. Eur. J. Org. Chem. 2011, 4, 681–695. [Google Scholar] [CrossRef]
- De Souza, A.G.; dos Santos, N.M.; da Silva Torin, R.F.; dos Santos Rosa, D. Synergic antimicrobial properties of Carvacrol essential oil and montmorillonite in biodegradable starch films. Int. J. Biol. Macromol. 2020, 164, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Wang, J.; Zhang, R.; Kong, R.; Lu, W.; Wang, X. Characterization and application of the microencapsulated carvacrol/ sodium alginate films as food packaging materials. Int. J. Biol. Macromol. 2019, 141, 259–267. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Cohen, Y.; Saidi, L.; Porat, R.; Poverenov, E. Covalent linkage of bioactive volatiles to a polysaccharide support as a potential approach for preparing active edible coatings and delivery systems for food products. Food Chem. 2021, 338, 127822. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z. Mesoporous silica-based nanodevices for biological applications. RSC Adv. 2014, 36, 18961. [Google Scholar] [CrossRef]
- Oliveira, L.; Sganzerla, W.G.; Bachega, G.; Goncalves, C.; Agostinetto, L.; Lima, A.P.; Czemainski, L.; Amadeu, G.; Dalla, M.; Cleber, F.; et al. Chitosan packaging functionalized with Cinnamodendron dinisii essential oil loaded zein: A proposal for meat conservation. Int. J. Biol. Macromol. 2021, 169, 183–193. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Braga, M.E.; Seabra, I.J.; Ferreira, P.; Gil, M.H.; De Sousa, H.C. Development of natural-based wound dressings impregnated with bioactive compounds and using supercritical carbon dioxide. Int. J. Pharm. 2011, 408, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Goñi, M.L.; Gañán, N.A.; Martini, R.E.; Andreatta, A.E. Carvone-loaded LDPE films for active packaging: Effect of supercritical CO2- assisted impregnation on loading, mechanical and transport properties of the films. J. Supercrit. Fluid 2018, 133, 278–290. [Google Scholar] [CrossRef]
- Raschip, I.E.; Paduraru-Mocanu, O.; Nita, L.E.; Dinu, M.V. Antibacterial porous xanthan-based films containing flavoring agents evaluated by near infrared chemical imaging technique. J. Appl. Polum. Sci. 2020, 137, 49111. [Google Scholar] [CrossRef]
- You, X.; O’Keefe, S.F. Binding of volatile aroma compounds to can linings with different polymeric characteristics. Food Sci. Nutr. 2017, 6, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. RSC Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef]
- Zizovic, I. Potential of Supercritical Solvent Impregnation for Development of Materials with Antibacterial Properties. Int. Arch. Med. Microbiol. 2017, 1, 1–6. [Google Scholar] [CrossRef][Green Version]
- Kurek, M.; Guinault, A.; Voilley, A.; Galić, K.; Debeaufort, F. Effect of relative humidity on carvacrol release and permeation properties of chitosan based films and coatings. Food Chem. 2014, 144, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar] [CrossRef]
- Gao, H.; Fang, X.; Chen, H.; Qin, Y.; Xu, F.; Jin, T.Z. Physiochemical properties and food application of antimicrobial PLA film. Food Control 2017, 73, 1522–1531. [Google Scholar] [CrossRef]
- Stroescu, M.; Stoica-Guzun, A.; Mihaela Jipa, I. Vanillin release from poly(vinyl alcohol)-bacterial cellulose mono and multilayer films. J Food Eng. 2013, 114, 153–157. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Pinto, L.; Cefola, M.A.; Bonifacio, S.; Cometa, C.; Boccino, B.; Pace, B.; De Gligli, E.; Palumbo, M.; Sada, A.; Logrieco, A.F.; et al. Effect of red thyme oil (Thymus vulgaris L.) vapours on fungal decay, quality parameters and shelf-life of oranges during cold storage. Food Chem. 2021, 336, 127590. [Google Scholar] [CrossRef] [PubMed]
- European Comission webpage. Regulation (EC) No 1334/2008 on Flavourings and Certain FOOD Ingredients with Flavouring Properties for Use in/on Foods. Available online: https://ec.europa.eu/food/safety/food_improvement_agents/flavourings/eu_rules_en (accessed on 23 February 2021).

| Volatile Compound | Chemical Class | Main Sources | Odor Quality | Reference |
|---|---|---|---|---|
| Eugenol | Monoterpene | Clove and cinnamon | Clove-like | [14] |
| Thymol | Monoterpene | Thyme | Thyme and rosemary | [14] |
| Carvacrol | Monoterpene | Oregano, thyme, and marjoram | Oregano, wood, and pencil-like | [15] |
| d-Limonene | Monoterpene | Citrus fruit peel | Lemon | [14] |
| Linalool | Monoterpene | Camphor tree and basil | Floral, sweet | [14] |
| R-(−)-carvone | Monoterpene | Caraway seeds, mint, and dill | Minty and caraway | [16] |
| Citral | Monoterpene | Lemon, orange, tomato, and lemongrass | Citrus and lemon | [16] |
| p-Cymene | Monoterpene | Thyme and horsemint | Wood and citrus | [17] |
| γ-Terpinene | Monoterpene | Variety of plants such as thyme | Turpentine-like and fruity odor | [18] |
| Valencene | Sesquiterpene | Citrus, mainly orange | Citrus | [19] |
| 1,8-Cineole | Monoterpene | Cinnamondendon dinissi leaves and eucalyptus oil | Minty and herbal notes | [20] |
| Allyl sulfide | Sulfur compound | Garlic | Garlic | [20] |
| Diallyl disulfide | Sulfur compound | Garlic | Garlic | [20] |
| Allyl isothiocyanate | Sulfur compound | Cruciferous vegetables and black mustard seeds | Mustard-like odor | [21] |
| Vanillin | Phenolic aldehyde | Bean or pod of tropical vanilla orchid | Vanilla, sweet | [22] |
| Cinnamaldehyde | Aldehyde | Cinnamon tree | Cinnamon | [23] |
| Hexanal | Aldehyde | Edible oils such as sunflower | Green, grassy, soapy | [15] |
| Octanal | Aldehyde | Citrus oils | Green, citrus, orange peel | [16] |
| Nonanal | Aldehyde | Natural oils | Citrus, soapy | [16] |
| Decanal | Aldehyde | Citrus, buckwheat, and coriander essential oil | Green, citrus, fatty | [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán Sanahuja, A.; Valdés García, A. New Trends in the Use of Volatile Compounds in Food Packaging. Polymers 2021, 13, 1053. https://doi.org/10.3390/polym13071053
Beltrán Sanahuja A, Valdés García A. New Trends in the Use of Volatile Compounds in Food Packaging. Polymers. 2021; 13(7):1053. https://doi.org/10.3390/polym13071053
Chicago/Turabian StyleBeltrán Sanahuja, Ana, and Arantzazu Valdés García. 2021. "New Trends in the Use of Volatile Compounds in Food Packaging" Polymers 13, no. 7: 1053. https://doi.org/10.3390/polym13071053
APA StyleBeltrán Sanahuja, A., & Valdés García, A. (2021). New Trends in the Use of Volatile Compounds in Food Packaging. Polymers, 13(7), 1053. https://doi.org/10.3390/polym13071053







