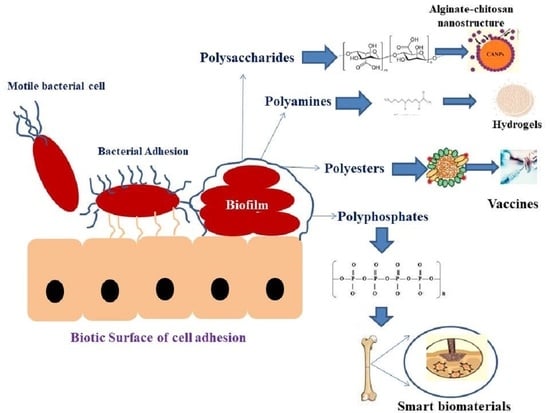
Bacterial Biopolymer: Its Role in Pathogenesis to Effective Biomaterials
Abstract
1. Introduction
2. Types of Bacterial Biopolymers
2.1. Polysaccharides
2.2. Polyamides
2.3. Polyesters
2.4. Polyphosphates
2.5. Other Bacterial Polymers
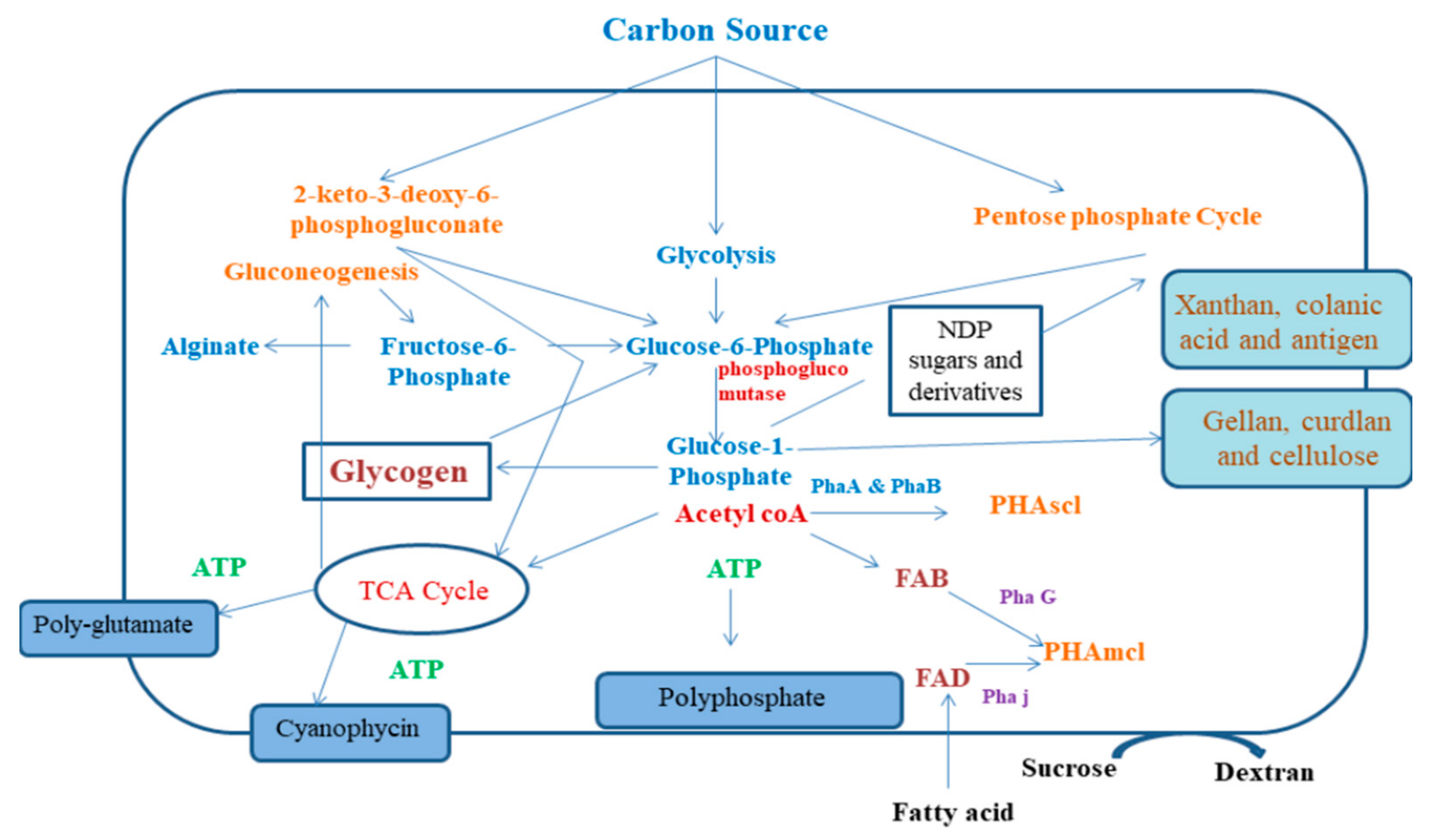
3. Synthesis and Production of Bacterial Biopolymers
3.1. PHA Production
3.2. Bioplastic Production
3.3. Xanthan Production
3.4. Gellan Production
3.5. Hyaluronate Production
3.6. Cellulose Production by Bacteria
3.7. Levan and Dextran Production
4. Enhancement in Biopolymer Production
4.1. Enhancement of Biopolymer Production by Alteration in Synthesis Mechanism
4.2. Enhancement in Biopolymer Production by the Use of Artificial Neural Network (ANN) and Machine Learning
4.3. Enhancement in Biopolymer Production by the Use of Genetic Engineering Technique
5. Applications of Bacterial Biopolymers
5.1. Biomedical Applications of Bacterial Biopolymers
5.1.1. Applications of bacterial cellulose
- Devices for targeted drug delivery: Within solid tablets, cellulose ether leads to a swelling-controlled drug release, since the tablet itself comes in contact with the physiological fluids. This cellulose ether over the surfaces of the tablets starts swelling, thereby forming a chain-like entrapment or a physical hydrogel. As the swelling starts expanding and penetrating from the surfaces to the glass-like core of the tablets, the drug readily gets dissolved in water, diffusing out through the network of polymers [103].
- Scaffolds used in regenerative medicines: Since BC is extensively biocompatible and has excellent mechanical features, so cellulose as well as its derivatives can be widely used as biomaterials for designing and fabrication of scaffolds used in tissue engineering [104].
- Dressings of wounds: BC has a unique property for wound healing because of its high water holding capacity and purity [105].
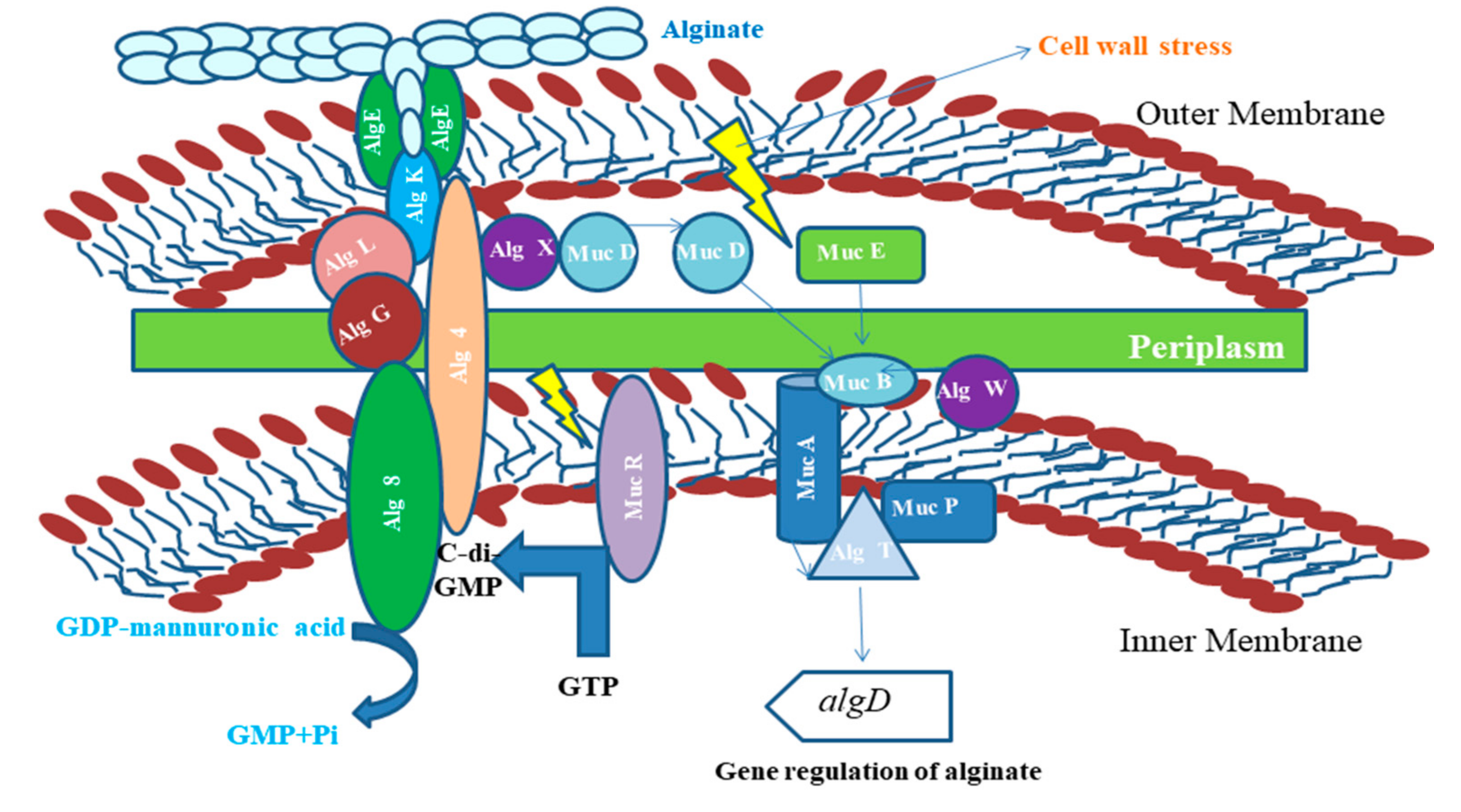
5.1.2. Applications of Alginates
- Dressings of wounds: A mixture of calcium alginate with sodium serves as an effective remedy for alginate dressing for the closure of wounds and haemostasis. They help in providing a moist condition at the site of wound achieving haemostasis and absorbing exudates [109]. They also aid in reduction of wound pain, decrease the biological burden of the wounded site, and absorb proteinases for reducing the odour [110]. Alginate-associated wound dressings include strategies such as electrospun mats, hydrogels, and sponges, offering numerous advantages, like gel-forming capability on absorbing wound exudates leading to haemostasis [111].
- Drug delivery system: Alginate potentially acts as a carrier for immobilization and encapsulation of drugs due to its biodegradability and biocompatibility [106].
5.1.3. Applications of Xanthan Gum
5.1.4. Applications of Hyaluronic Acid
- Epithelial regeneration
- Extracellular regeneration leading to wound healing
- Agent of viscosity in pulmonary diseases to achieve alveolar patency
- Topical medicinal treatment for treating Sjogren’s syndrome (dry eye syndrome) [115]
- Regenerative medicinal filler to cure cutaneous lines and wrinkles
- Commercial formulations to be used in intra-articular injections
5.1.5. Applications of PHB
- Scaffolds in tissue engineering: PHB has well known applications in the manufacture of scaffolds used in tissue engineering, because they have ideal materialistic properties like biocompatibility, can very well support growth of the cells, and also help in guiding and organizing cells.
- Growth of cells: PHB acts as a unique biomaterial for supporting the growth of different types of cells such as osteoblasts, fibroblasts, umbilical endothelial vein cells in humans, smooth muscle cells in aorta of rabbits, and chondrocytes derived from cartilage, because PHB can get readily metabolized in the cellular biosynthetic pathway [119].
- Reconstructive surgeries: In vivo and gradual biodegradability of PHB makes it an excellent and potential biopolymer to be used in reconstructive surgeries and to develop cardiovascular products like vascular grafts, pericardial patches, and heart valves [120].
- Controlled system for drug delivery and aids in surgeries: In a controlled drug delivery system, a carrier component that is potentially nonharmful to an organism and has essential mechanical, physical, and biomedical features like biodegradability in biological medium is required. As PHB along with its derivatives match these criteria, so they can be used in controlled drug delivery processes, manufacturing of sutures, surgical pins, swabs, wound healing, bone plates and replacements, orthopaedic applications, and remodeling of cartilage [121].
- Peripheral implants and substitutes: PHB is utilized as a pericardial replacement and blood vessel substituent, and it also serves the purpose of stimulating growth and healing of bones and acts as dental and cardiovascular implants because of its piezoelectric characteristics [122]. Sodian et al., 2000, also found that PHB can be successfully used in the fabrication of porous, biodegradable, three-dimensional heart valve scaffolds [122].
- Various disease treatments: The main product of biodegradation of PHB is 4-hydroxyl butyrate (HB), which is active pharmacologically and is a very promising compound for treating different diseases including narcolepsy, alcohol addiction withdrawal syndrome, cationic and chronic schizophrenia, chronic brain syndrome, atypical psychoses, drug addiction withdrawal, circulatory collapse, Parkinson’s disease, cancer, radiation exposure, and various other neuropharmacological diseases [123]. Units of HB are used to effectively treat narcolepsy, which is a sleeping disorder in humans that is detected during early adulthood causing paralysis, unanticipated sleep attacks, and in few cases, temporary muscle tone loss. HB can even act as a neurotransmitter in the central nervous system (CNS) of mammals, because it has a close chemical and structural homology with gamma-aminobutyric acid (GABA—a regulator of muscle tone), which functions on the receptors for GABA, thereby reducing narcolepsy and regulating the muscle tone [124].
5.2. Biopolymer Application in Nanotechnology and Nanoscience
5.3. Biopolymer Applications in Food Industries
5.4. Biopolymer Applications in Packaging Industries
5.5. Mechanical Applications of Biopolymers
5.5.1. Use of Biopolymers for Improving Surface Erosion Resistance
5.5.2. Biopolymer Applications as Construction Binder
5.6. Biopolymer Applications in Microbial Enhanced Oil Recovery (MEOR)
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Briscoe, W.H.; Queiroz, J. Polyethylenimine coated plasmid DNA–surfactant complexes as potential gene delivery systems. Colloids Surfaces B Biointerfaces 2015, 133, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Chatterji, A.; Dash, B.P. Chitosan from the carapace of Indian horseshoe crab (Tachypleus gigas, müller): Isolation and its characterization. Adv. Biores 2018, 9, 52–64. [Google Scholar] [CrossRef]
- Pati, S.; Chatterji, A.; Dash, B.P.; Nelson, B.R.; Sarkar, T.; Shahimi, S.; Edinur, H.A.; Abd Manan, T.S.B.; Jena, P.; Mohanta, Y.K.; et al. Structural characterization and antioxidant potential of chitosan by γ-irradiation from the carapace of horseshoe crab. Polymers 2020, 12, 2361. [Google Scholar] [CrossRef]
- Albuquerque, T.; Faria, R.; Sousa, Â.; Neves, A.R.; Queiroz, J.A.; Costa, D. Polymer-peptide ternary systems as a tool to improve the properties of plasmid DNA vectors in gene delivery. J. Mol. Liq. 2020, 309, 113157. [Google Scholar] [CrossRef]
- Costa, D.; Miguel, M.G.; Lindman, B. Responsive Polymer Gels: Double-Stranded versus Single-Stranded DNA. J. Phys. Chem. B 2007, 111, 10886–10896. [Google Scholar] [CrossRef]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.; Nayak, P.; Chakraborty, R. Storage study of mango leather in sustainable packaging condition. Mater. Today Proc. 2020, 22, 2001–2007. [Google Scholar] [CrossRef]
- Pati, S.; Jena, P.; Shahimi, S.; Nelson, B.R.; Acharya, D.; Dash, B.P.; Chatterji, A. Characterization dataset for pre- and post-irradiated shrimp waste chitosan. Data Brief 2020, 32, 106081. [Google Scholar] [CrossRef]
- Rehm, B.H.A. Bacterial polymers: Biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010, 8, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar] [CrossRef]
- Kiedrowski, M.R.; Kavanaugh, J.S.; Malone, C.L.; Mootz, J.M.; Voyich, J.M.; Smeltzer, M.S.; Bayles, K.W.; Horswill, A.R. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS ONE 2011, 6, e26714. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed]
- Bartell, J.A.; Sommer, L.M.; Haagensen, J.A.J.; Loch, A.; Espinosa, R.; Molin, S.; Johansen, H.K. Evolutionary highways to persistent bacterial infection. Nat. Commun. 2019, 10, 629. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Eyckmans, J.; Chen, C.S. Designer biomaterials for mechanobiology. Nat. Mater. 2017, 16, 1164–1168. [Google Scholar] [CrossRef]
- Gao, M.; Li, J.; Bao, Z.; Hu, M.; Nian, R.; Feng, D.; An, D.; Li, X.; Xian, M.; Zhang, H. A natural in situ fabrication method of functional bacterial cellulose using a microorganism. Nat. Commun. 2019, 10, 437. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Mondal, S.; Varenik, M.; Bloch, D.N.; Atsmon-Raz, Y.; Jacoby, G.; Adler-Abramovich, L.; Shimon, L.J.W.; Beck, R.; Miller, Y.; Regev, O.; et al. A minimal length rigid helical peptide motif allows rational design of modular surfactants. Nat. Commun. 2017, 8, 14018. [Google Scholar] [CrossRef]
- Bruchet, M.; Melman, A. Fabrication of patterned calcium cross-linked alginate hydrogel films and coatings through reductive cation exchange. Carbohydr. Polym. 2015, 131, 57–64. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Thongsomboon, W.; Serra, D.O.; Possling, A.; Hadjineophytou, C.; Hengge, R.; Cegelski, L. Phosphoethanolamine cellulose: A naturally produced chemically modified cellulose. Science 2018, 359, 334–338. [Google Scholar] [CrossRef]
- Tzeng, Y.-L.; Thomas, J.; Stephens, D.S. Regulation of capsule in Neisseria meningitidis. Crit. Rev. Microbiol. 2016, 42, 759–772. [Google Scholar] [CrossRef]
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef]
- Sambou, T.; Dinadayala, P.; Stadthagen, G.; Barilone, N.; Bordat, Y.; Constant, P.; Levillain, F.; Neyrolles, O.; Gicquel, B.; Lemassu, A.; et al. Capsular glucan and intracellular glycogen of Mycobacterium tuberculosis: Biosynthesis and impact on the persistence in mice. Mol. Microbiol. 2008, 70, 762–774. [Google Scholar] [CrossRef]
- Wilkening, R.V.; Federle, M.J. Evolutionary Constraints Shaping Streptococcus pyogenes-Host Interactions. Trends Microbiol. 2017, 25, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Mohan, T.; Rathner, R.; Reishofer, D.; Koller, M.; Elschner, T.; Spirk, S.; Heinze, T.; Stana-Kleinschek, K.; Kargl, R. Designing Hydrophobically Modified Polysaccharide Derivatives for Highly Efficient Enzyme Immobilization. Biomacromolecules 2015, 16, 2403–2411. [Google Scholar] [CrossRef]
- Kim, H.; Jeong, H.; Han, S.; Beack, S.; Hwang, B.W.; Shin, M.; Oh, S.S.; Hahn, S.K. Hyaluronate and its derivatives for customized biomedical applications. Biomaterials 2017, 123, 155–171. [Google Scholar] [CrossRef]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.D.; Carvalho, L.S.; Gomes, A.M.V.; Queiroz, L.R.; Magalhães, B.S.; Parachin, N.S. Genetic basis for hyper production of hyaluronic acid in natural and engineered microorganisms. Microb. Cell Fact. 2016, 15, 119. [Google Scholar] [CrossRef]
- Pauly, M.; Ramírez, V. New Insights Into Wall Polysaccharide O-Acetylation. Front. Plant Sci. 2018, 9, 1210. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Cho, M.; Lee, H.-R.; Cha, K.; Chun, J.-H.; Hong, K.-J.; Park, J.; Rhie, G.-E. Monoclonal antibody against the poly-gamma-D-glutamic acid capsule of Bacillus anthracis protects mice from enhanced lethal toxin activity due to capsule and anthrax spore challenge. Biochim. Biophys. Acta 2013, 1830, 2804–2812. [Google Scholar] [CrossRef]
- Moradali, M.F.; Rehm, B.H.A. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef]
- Watzer, B.; Forchhammer, K. Cyanophycin Synthesis Optimizes Nitrogen Utilization in the Unicellular Cyanobacterium Synechocystis sp. Strain PCC 6803. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef]
- Du, J.; Li, L.; Zhou, S. Microbial production of cyanophycin: From enzymes to biopolymers. Biotechnol. Adv. 2019, 37, 107400. [Google Scholar] [CrossRef] [PubMed]
- Ushimaru, K.; Hamano, Y.; Katano, H. Antimicrobial Activity of ε-Poly-l-lysine after Forming a Water-Insoluble Complex with an Anionic Surfactant. Biomacromolecules 2017, 18, 1387–1392. [Google Scholar] [CrossRef]
- Long, J.-Y.; Song, K.-L.; He, X.; Zhang, B.; Cui, X.-F.; Song, C.-F. Mutagenesis of PhaR, a Regulator Gene of Polyhydroxyalkanoate Biosynthesis of Xanthomonas oryzae pv. oryzae Caused Pleiotropic Phenotype Changes. Front. Microbiol. 2018, 9, 3046. [Google Scholar] [CrossRef] [PubMed]
- Winnacker, M. Polyhydroxyalkanoates: Recent Advances in Their Synthesis and Applications. Eur. J. Lipid Sci. Technol. 2019, 121, 1900101. [Google Scholar] [CrossRef]
- Akinmulewo, A.B.; Nwinyi, O.C. Polyhydroxyalkanoate: A biodegradable polymer (a mini review). J. Phys. Conf. Ser. 2019, 1378, 42007. [Google Scholar] [CrossRef]
- Wang, L.; Yan, J.; Wise, M.J.; Liu, Q.; Asenso, J.; Huang, Y.; Dai, S.; Liu, Z.; Du, Y.; Tang, D. Distribution Patterns of Polyphosphate Metabolism Pathway and Its Relationships with Bacterial Durability and Virulence. Front. Microbiol. 2018, 9, 782. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Tolba, E.; Ackermann, M.; Neufurth, M.; Wang, S.; Feng, Q.; Schröder, H.C.; Wang, X. Fabrication of amorphous strontium polyphosphate microparticles that induce mineralization of bone cells in vitro and in vivo. Acta Biomater. 2017, 50, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Lindner, S.N.; Knebel, S.; Pallerla, S.R.; Schoberth, S.M.; Wendisch, V.F. Cg2091 encodes a polyphosphate/ATP-dependent glucokinase of Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2010, 87, 703–713. [Google Scholar] [CrossRef]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Mondal, S.; Xue, B.; Shimon, L.J.W.; Cao, Y.; Gazit, E. Rigid helical-like assemblies from a self-aggregating tripeptide. Nat. Mater. 2019, 18, 503–509. [Google Scholar] [CrossRef]
- Gilbert, C.; Ellis, T. Biological Engineered Living Materials: Growing Functional Materials with Genetically Programmable Properties. ACS Synth. Biol. 2019, 8, 1–15. [Google Scholar] [CrossRef]
- Rehm, B.H.A. Synthetic biology towards the synthesis of custom-made polysaccharides. Microb. Biotechnol. 2015, 8, 19–20. [Google Scholar] [CrossRef]
- Moradali, M.F.; Donati, I.; Sims, I.M.; Ghods, S.; Rehm, B.H.A. Alginate Polymerization and Modification Are Linked in Pseudomonas aeruginosa. MBio 2015, 6, e00453-15. [Google Scholar] [CrossRef]
- Aljuraifani, A.A.; Berekaa, M.M.; Ghazwani, A.A. Bacterial biopolymer (polyhydroxyalkanoate) production from low-cost sustainable sources. Microbiologyopen 2019, 8, e00755. [Google Scholar] [CrossRef] [PubMed]
- Pagliano, G.; Ventorino, V.; Panico, A.; Pepe, O. Integrated systems for biopolymers and bioenergy production from organic waste and by-products: A review of microbial processes. Biotechnol. Biofuels 2017, 10, 113. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.-Q. Biosynthesis of polyhydroxybutyrate (PHB) and extracellular polymeric substances (EPS) by Ralstonia eutropha ATCC 17699 in batch cultures. Appl. Microbiol. Biotechnol. 2007, 75, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Marsudi, S.; Unno, H. Simultaneous production of polyhydroxyalkanoates and rhamnolipids by Pseudomonas aeruginosa. Biotechnol. Bioeng. 2002, 78, 699–707. [Google Scholar] [CrossRef]
- Guo, W.; Song, C.; Kong, M.; Geng, W.; Wang, Y.; Wang, S. Simultaneous production and characterization of medium-chain-length polyhydroxyalkanoates and alginate oligosaccharides by Pseudomonas mendocina NK-01. Appl. Microbiol. Biotechnol. 2011, 92, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Quagliano, J.C.; Miyazaki, S.S. Biosynthesis of poly-β-hydroxybutyrate and exopolysaccharides on azotobacter chroococcum strain 6B utilizing simple and complex carbon sources. Appl. Biochem. Biotechnol. 1999, 82, 199–208. [Google Scholar] [CrossRef]
- Sukan, A.; Roy, I.; Keshavarz, T. Dual production of biopolymers from bacteria. Carbohydr. Polym. 2015, 126, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Angelina, V.S.V.N. Microbial Biopolymers: The Exopolysaccharides. In Microbial Factories; Kalia, V., Ed.; Springer: New Delhi, India, 2015. [Google Scholar]
- Iukaszewicz, M.; Domzal- Kedzia, M.; Lewinska, A.; Weselski, M.; Wojtowicz, W.; Mlynarz, P. Microbial production of biopolymers using solid-state fermentation with Bacillus subtilis natto. J. Bacteriol. Parasitol. 2017, 8. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic Acid Bacteria Exopolysaccharides in Foods and Beverages: Isolation, Properties, Characterization, and Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cui, Y.; Yue, F.; Liu, L.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Exopolysaccharides produced by lactic acid bacteria and Bifidobacteria: Structures, physiochemical functions and applications in the food industry. Food Hydrocoll. 2019, 94, 475–499. [Google Scholar] [CrossRef]
- Escárcega-González, C.E.; Garza-Cervantes, J.A.; Vázquez-Rodríguez, A.; Morones-Ramírez, J.R. Bacterial Exopolysaccharides as Reducing and/or Stabilizing Agents during Synthesis of Metal Nanoparticles with Biomedical Applications. Int. J. Polym. Sci. 2018, 2018, 7045852. [Google Scholar] [CrossRef]
- Dhanarajan, G.; Rangarajan, V.; Bandi, C.; Dixit, A.; Das, S.; Ale, K.; Sen, R. Biosurfactant-biopolymer driven microbial enhanced oil recovery (MEOR) and its optimization by an ANN-GA hybrid technique. J. Biotechnol. 2017, 256, 46–56. [Google Scholar] [CrossRef]
- Dhanarajan, G.; Mandal, M.; Sen, R. A combined artificial neural network modeling–particle swarm optimization strategy for improved production of marine bacterial lipopeptide from food waste. Biochem. Eng. J. 2014, 84, 59–65. [Google Scholar] [CrossRef]
- Sarkar, T.; Salauddin, M.; Choudhury, T.; Um, J.-S.; Pati, S.; Hazra, S.K.; Chakraborty, R. Spatial optimisation of mango leather production and colour estimation through conventional and novel digital image analysis technique. Spat. Inf. Res. 2021, 29. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sarkar, T.; Dutta, B.; Ray, R.R. Antibiofilm Activity of α-Amylase from Bacillus subtilis and Prediction of the Optimized Conditions for Biofilm Removal by Response Surface Methodology (RSM) and Artificial Neural Network (ANN). Appl. Biochem. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Sutherland, I.W. Bioplastic and biopolymer production. Biotechnology 2007, 5, 152–176. [Google Scholar]
- Sutherland, I.W. Polysaccharide lyases. FEMS Microbiol. Rev. 1995, 16, 323–347. [Google Scholar] [CrossRef]
- Zikmanis, P.; Kolesovs, S.; Semjonovs, P. Production of biodegradable microbial polymers from whey. Bioresour. Bioprocess. 2020, 7, 36. [Google Scholar] [CrossRef]
- Nitschke, M.; Rodrigues, V.; Schinatto, L.F. Formulation of whey-based media for xanthan gum production by X. Campestris C7L isolate. Food Sci. Technol. 2001, 21, 82–85. [Google Scholar] [CrossRef]
- Barel, V.; Chalupowicz, L.; Barash, I.; Sharabani, G.; Reuven, M.; Dror, O.; Burdman, S.; Manulis-Sasson, S. Virulence and inplanta movement of Xanthomonas hortorum pv. pelargonii are affected by the diffusible signal factor (DSF)-dependent quorum sensing system. Mol. Plant Pathol. 2015, 16, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Fialho, A.M.; Moreira, L.M.; Granja, A.T.; Popescu, A.O.; Hoffmann, K.; Sá-Correia, I. Occurrence, production, and applications of gellan: Current state and perspectives. Appl. Microbiol. Biotechnol. 2008, 79, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, I.B.; Saudagar, P.S.; Singhal, R.S.; Pandey, A. Statistical approach to optimization of fermentative production of gellan gum from Sphingomonas paucimobilis ATCC 31461. J. Biosci. Bioeng. 2006, 102, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kim, H.; Lee, S.; Kim, D.-H.; Joe, M.-H. Improved gellan gum production by a newly-isolated Sphingomonas azotifigens GL-1 in a cheese whey and molasses based medium. Process Biochem. 2020, 95, 269–278. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Zhan, Y.; Xu, Y.; Deng, J.; Chen, J.; Cai, D.; Wang, Q.; Sheng, F.; Chen, S. Engineering Expression Cassette of pgdS for Efficient Production of Poly-γ-Glutamic Acids with Specific Molecular Weights in Bacillus licheniformis. Front. Bioeng. Biotechnol. 2020, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.B.; Oluwafemi, O.S.; Kalarikkal, N.; Thomas, S.; Songca, S. Biopolymers—Application in Nanoscience and Nanotechnology. Recent Adv. Biopolym. 2016, 1, 47–72. [Google Scholar]
- Mohan, N.; Balakrishnan, R.; Sivaprakasam, S. Optimization and effect of dairy industrial waste as media components in the production of hyaluronic acid by Streptococcus thermophilus. Prep. Biochem. Biotechnol. 2016, 46, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Ryngajłło, M.; Jacek, P.; Cielecka, I.; Kalinowska, H.; Bielecki, S. Effect of ethanol supplementation on the transcriptional landscape of bionanocellulose producer Komagataeibacter xylinus E25. Appl. Microbiol. Biotechnol. 2019, 103, 6673–6688. [Google Scholar] [CrossRef] [PubMed]
- Drozd, R.; Szymańska, M.; Żywicka, A.; Kowalska, U.; Rakoczy, R.; Kordas, M.; Konopacki, M.; Junka, A.F.; Fijałkowski, K. Exposure to non-continuous rotating magnetic field induces metabolic strain-specific response of Komagataeibacter xylinus. Biochem. Eng. J. 2021, 166, 107855. [Google Scholar] [CrossRef]
- Tsouko, E.; Kourmentza, C.; Ladakis, D.; Kopsahelis, N.; Mandala, I.; Papanikolaou, S.; Paloukis, F.; Alves, V.; Koutinas, A. Bacterial Cellulose Production from Industrial Waste and by-Product Streams. Int. J. Mol. Sci. 2015, 16, 14832–14849. [Google Scholar] [CrossRef] [PubMed]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Preparation and characterization of cellulose nanocrystals from bacterial cellulose produced in sugar beet molasses and cheese whey media. Int. J. Biol. Macromol. 2019, 122, 280–288. [Google Scholar] [CrossRef]
- Semjonovs, P.; Ruklisha, M.; Paegle, L.; Saka, M.; Treimane, R.; Skute, M.; Rozenberga, L.; Vikele, L.; Sabovics, M.; Cleenwerck, I. Cellulose synthesis by Komagataeibacter rhaeticus strain P 1463 isolated from Kombucha. Appl. Microbiol. Biotechnol. 2017, 101, 1003–1012. [Google Scholar] [CrossRef]
- Szymańska, M.; Karakulska, J.; Sobolewski, P.; Kowalska, U.; Grygorcewicz, B.; Böttcher, D.; Bornscheuer, U.T.; Drozd, R. Glycoside hydrolase (PelAh) immobilization prevents Pseudomonas aeruginosa biofilm formation on cellulose-based wound dressing. Carbohydr. Polym. 2020, 246, 116625. [Google Scholar] [CrossRef] [PubMed]
- Revin, V.V.; Novokuptsev, N.V.; Red’kin, N.A. Optimization of cultivation conditions for Azotobacter vinelandii D-08, producer of polysaccharide levan, for obtaining biocomposite materials. BioResources 2016, 11, 9661–9675. [Google Scholar] [CrossRef][Green Version]
- Cinti, G. Method for the Production of Dextran. Patent publication number: WO/2015/117624, International Application No: PCT/EP2014/000360 13 August 2015. [Google Scholar]
- Schwartz, R.D.; Bodie, E.A. Production of Viscous Dextran-Containing Whey-Sucrose Broths by Leuconostoc mesenteroides ATCC 14935. Appl. Environ. Microbiol. 1984, 48, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Noh, D.-H.; Ajo-Franklin, J.B.; Kwon, T.-H.; Muhunthan, B. P and S wave responses of bacterial biopolymer formation in unconsolidated porous media. J. Geophys. Res. Biogeosci. 2016, 121, 1158–1177. [Google Scholar] [CrossRef]
- Pohlmann, A.; Fricke, W.F.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Pötter, M.; Schwartz, E.; et al. Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat. Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef]
- Lütke-Eversloh, T.; Fischer, A.; Remminghorst, U.; Kawada, J.; Marchessault, R.H.; Bögershausen, A.; Kalwei, M.; Eckert, H.; Reichelt, R.; Liu, S.-J.; et al. Biosynthesis of novel thermoplastic polythioesters by engineered Escherichia coli. Nat. Mater. 2002, 1, 236–240. [Google Scholar] [CrossRef]
- Sarkar, T.; Salauddin, M.; Hazra, S.; Chakraborty, R. Comparative study of predictability of response surface methodology (RSM) and artificial neural network-particle swarm optimization (ANN-PSO) for total colour difference of pineapple fortified rasgulla processing. Int. J. Intell. Netw. 2020, 1, 17–31. [Google Scholar] [CrossRef]
- Sarkar, T.; Salauddin, M.; Hazra, S.K.; Chakraborty, R. Artificial neural network modelling approach of drying kinetics evolution for hot air oven, microwave, microwave convective and freeze dried pineapple. SN Appl. Sci. 2020, 2, 1621. [Google Scholar] [CrossRef]
- Riza, M.; Sentia, P.D.; Dewi, R. Modeling Biopolymer and Glucose as Carbon Source Using Artificial Neural Network. IOP Conf. Ser. Mater. Sci. Eng. 2019, 536, 12044. [Google Scholar] [CrossRef]
- Karimi, M.A.; Pourhakkak, P.; Adabi, M.; Firoozi, S.; Adabi, M.; Naghibzadeh, M. Using an artificial neural network for the evaluation of the parameters controlling PVA/chitosan electrospun nanofibers diameter. e-Polymers 2015, 15, 127–138. [Google Scholar] [CrossRef]
- Huertas, M.J.; Matilla, M.A. Training bacteria to produce environmentally friendly polymers of industrial and medical relevance. Microb. Biotechnol. 2020, 13, 14–16. [Google Scholar] [CrossRef]
- García, C.; Prieto, M.A. Bacterial cellulose as a potential bioleather substitute for the footwear industry. Microb. Biotechnol. 2019, 12, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef]
- Jacek, P.; Dourado, F.; Gama, M.; Bielecki, S. Molecular aspects of bacterial nanocellulose biosynthesis. Microb. Biotechnol. 2019, 12, 633–649. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Barud, H.; Farinas, C.S.; Vasconcellos, V.M.; Claro, A.M. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019, 3, 7. [Google Scholar] [CrossRef]
- Chen, G.; Wu, G.; Chen, L.; Wang, W.; Hong, F.F.; Jönsson, L.J. Comparison of productivity and quality of bacterial nanocellulose synthesized using culture media based on seven sugars from biomass. Microb. Biotechnol. 2019, 12, 677–687. [Google Scholar] [CrossRef]
- Pérez-Mendoza, D.; Sanjuán, J. Exploiting the commons: Cyclic diguanylate regulation of bacterial exopolysaccharide production. Curr. Opin. Microbiol. 2016, 30, 36–43. [Google Scholar] [CrossRef]
- Anderson, L.A.; Islam, M.A.; Prather, K.L.J. Synthetic biology strategies for improving microbial synthesis of “green” biopolymers. J. Biol. Chem. 2018, 293, 5053–5061. [Google Scholar] [CrossRef] [PubMed]
- Valentine, M.E.; Kirby, B.D.; Withers, T.R.; Johnson, S.L.; Long, T.E.; Hao, Y.; Lam, J.S.; Niles, R.M.; Yu, H.D. Generation of a highly attenuated strain of Pseudomonas aeruginosa for commercial production of alginate. Microb. Biotechnol. 2020, 13, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Eisinger, V.M.; Rowen, D.W.; Yu, H.D. Regulated proteolysis controls mucoid conversion in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2007, 104, 8107–8112. [Google Scholar] [CrossRef] [PubMed]
- Marín, P.; Martirani-Von Abercron, S.M.; Urbina, L.; Pacheco-Sánchez, D.; Castañeda-Cataña, M.A.; Retegi, A.; Eceiza, A.; Marqués, S. Bacterial nanocellulose production from naphthalene. Microb. Biotechnol. 2019, 12, 662–676. [Google Scholar] [CrossRef]
- Stabenfeldt, S.E.; García, A.J.; LaPlaca, M.C. Thermoreversible laminin-functionalized hydrogel for neural tissue engineering. J. Biomed. Mater. Res. A 2006, 77, 718–725. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Kayserilioğlu, B.Ş.; Bakir, U.; Yilmaz, L.; Akkaş, N. Use of xylan, an agricultural by-product, in wheat gluten based biodegradable films: Mechanical, solubility and water vapor transfer rate properties. Bioresour. Technol. 2003, 87, 239–246. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical Biopolymers, their Origin and Evolution in Biomedical Sciences: A Systematic Review. J. Clin. Diagn. Res. 2015, 9, ZE21–ZE25. [Google Scholar] [CrossRef]
- Richards, A.J.; Hagelstein, S.M.; Patel, G.K.; Ivins, N.M.; Sweetland, H.M.; Harding, K.G. Early use of negative pressure therapy in combination with silver dressings in a difficult breast abscess. Int. Wound J. 2011, 8, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Li, J.; Xu, Y.; Wang, X.; Chen, H. In situ injectable nano-composite hydrogel composed of curcumin, N,O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int. J. Pharm. 2012, 437, 110–119. [Google Scholar] [CrossRef]
- Hooper, S.J.; Percival, S.L.; Hill, K.E.; Thomas, D.W.; Hayes, A.J.; Williams, D.W. The visualisation and speed of kill of wound isolates on a silver alginate dressing. Int. Wound J. 2012, 9, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shen, X.; Chen, Y.; Guo, J.; Chen, Q.; Jiang, X. pH-Induced Self-Assembly and Capsules of Sodium Alginate. Biomacromolecules 2005, 6, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Abbah, S.-A.; Liu, J.; Lam, R.W.M.; Goh, J.C.H.; Wong, H.-K. In vivo bioactivity of rhBMP-2 delivered with novel polyelectrolyte complexation shells assembled on an alginate microbead core template. J. Control. Release 2012, 162, 364–372. [Google Scholar] [CrossRef]
- Michaelsen, T.E.; Gilje, A.; Samuelsen, A.B.; Høgåsen, K.; Paulsen, B.S. Interaction between human complement and a pectin type polysaccharide fraction, PMII, from the leaves of Plantago major L. Scand. J. Immunol. 2000, 52, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Mangiafico, S.; Spadaro, A. Methylprednisolone delivery by Hyalobend corneal shields and its effects on rabbit ocular inflammation. J. Ocul. Pharmacol. Ther. 1996, 12, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, A.; Berkland, C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013, 9, 7081–7092. [Google Scholar] [CrossRef]
- McDonald, C.C.; Kaye, S.B.; Figueiredo, F.C.; Macintosh, G.; Lockett, C. A randomised, crossover, multicentre study to compare the performance of 0.1% (w/v) sodium hyaluronate with 1.4% (w/v) polyvinyl alcohol in the alleviation of symptoms associated with dry eye syndrome. Eye 2002, 16, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Uthman, I.; Raynauld, J.-P.; Haraoui, B. Intra-articular therapy in osteoarthritis. Postgrad. Med. J. 2003, 79, 449–453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dechert, T.A.; Ducale, A.E.; Ward, S.I.; Yager, D.R. Hyaluronan in human acute and chronic dermal wounds. Wound Repair Regen. Off. Publ. Wound Health Soc. Eur. Tissue Repair Soc. 2006, 14, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Shaikh, S.; Sayyed, R. Microbial biopolymers in biomedical field. MOJ Cell Sci. Rep. 2016, 3, 65–67. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.-W.; Wu, Q.; Chen, G.-Q. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials 2004, 25, 669–675. [Google Scholar] [CrossRef]
- Seyednejad, H.; Gawlitta, D.; Dhert, W.J.A.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Preparation and characterization of a three-dimensional printed scaffold based on a functionalized polyester for bone tissue engineering applications. Acta Biomater. 2011, 7, 1999–2006. [Google Scholar] [CrossRef]
- Díaz, A.; Katsarava, R.; Puiggalí, J. Synthesis, properties and applications of biodegradable polymers derived from diols and dicarboxylic acids: From polyesters to poly(ester amide)s. Int. J. Mol. Sci. 2014, 15, 7064–7123. [Google Scholar] [CrossRef]
- Sodian, R.; Sperling, J.S.; Martin, D.P.; Egozy, A.; Stock, U.; Mayer, J.E.J.; Vacanti, J.P. Fabrication of a trileaflet heart valve scaffold from a polyhydroxyalkanoate biopolyester for use in tissue engineering. Tissue Eng. 2000, 6, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Brandl, H.; Gross, R.A.; Lenz, R.W.; Fuller, R.C. Pseudomonas oleovorans as a Source of Poly(beta-Hydroxyalkanoates) for Potential Applications as Biodegradable Polyesters. Appl. Environ. Microbiol. 1988, 54, 1977–1982. [Google Scholar] [CrossRef]
- Watanabe, M.; Maemura, K.; Kanbara, K.; Tamayama, T.; Hayasaki, H. GABA and GABA receptors in the central nervous system and other organs. Int. Rev. Cytol. 2002, 213, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarzadeh, A.; Alibakhshi, A.; Hejazi, M.; Omidi, Y.; Ezzati Nazhad Dolatabadi, J. Bacterial-derived biopolymers: Advanced natural nanomaterials for drug delivery and tissue engineering. TrAC Trends Anal. Chem. 2016, 82, 367–384. [Google Scholar] [CrossRef]
- Darroudi, M.; Ahmad, M.B.; Abdullah, A.H.; Ibrahim, N.A. Green synthesis and characterization of gelatin-based and sugar-reduced silver nanoparticles. Int. J. Nanomed. 2011, 6, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Mohy, E.M.; Soliman, E.; Hashem, A.; Tamer, T. Biopolymer Modifications for Biomedical Applications. In Infrared Spectroscopy—Life and Biomedical Sciences; Theophanides, T., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P.M. Edible Polymers: Challenges and Opportunities. J. Polym. 2014, 2014, 427259. [Google Scholar] [CrossRef]
- Chaudhary, D.; Dong, Y.; Kar, K.K. Hydrophilic plasticized biopolymers: Morphological influence on physical properties. Mater. Lett. 2010, 64, 872–875. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.; Siddhartha, P.; Ray, R. Microbiologically synthesized nanoparticles and their role in silencing the biofilm signaling cascade. Front. Microbiol. 2021, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Blanco, I.; Romani, S.; Tylewicz, U.; Rocculi, P.; Rosa, M.D. Poly(lactic acid)-modified films for food packaging application: Physical, mechanical, and barrier behavior. J. Appl. Polym. Sci. 2012, 125, E390–E401. [Google Scholar] [CrossRef]
- Majeed, K.; Jawaid, M.; Hassan, A.; Abu Bakar, A.; Abdul Khalil, H.P.S.; Salema, A.A.; Inuwa, I. Potential materials for food packaging from nanoclay/natural fibres filled hybrid composites. Mater. Des. 2013, 46, 391–410. [Google Scholar] [CrossRef]
- Soo-Min, H.; Ilhan, C.; Dong-Hwa, N.; Tae-Hyuk, K.; Balasingam, M. Improvement of Surface Erosion Resistance of Sand by Microbial Biopolymer Formation. J. Geotech. Geoenvironmental Eng. 2018, 144, 6018004. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Cho, G.-C. Introduction of Microbial Biopolymers in Soil Treatment for Future Environmentally-Friendly and Sustainable Geotechnical Engineering. Sustainability 2016, 8, 251. [Google Scholar] [CrossRef]
- Chang, I.; Jeon, M.; Cho, G.-C. Application of Microbial Biopolymers as an Alternative Construction Binder for Earth Buildings in Underdeveloped Countries. Int. J. Polym. Sci. 2015, 2015, 326745. [Google Scholar] [CrossRef] [PubMed]
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan Gum. Crit. Rev. Biotechnol. 2000, 20, 177–211. [Google Scholar] [CrossRef]
- Jeong, M.S.; Noh, D.-H.; Hong, E.; Lee, K.S.; Kwon, T.-H. Systematic Modeling Approach to Selective Plugging Using In Situ Bacterial Biopolymer Production and Its Potential for Microbial-enhanced Oil Recovery. Geomicrobiol. J. 2019, 36, 468–481. [Google Scholar] [CrossRef]


| Type of Polymer | Localization of the Polymer | Primary Structure | Major Component | Precursors | Enzyme for Polemerization and Operon | Producer | Industrial Application | Reference |
|---|---|---|---|---|---|---|---|---|
| Polysaccharides | ||||||||
| Hyaluronic acid | Produced extracellularly | β-(1,4) linkage | N-acetyl glucosamine and Glucuronate | UDP–N-acetyl glucosamine and UDP–d-glucuronate | Hyaluron synthase (HasA) has operon | Pasteurella Multocida and Streptococcus sp. | Drug delivery, cosmetic, Viscosupplementation, and repair of tissue | [34] |
| Cellulose | Produced extracellularly | β-(1,4) linkage | D-glucose | UDP-D-glucose | Cellulose synthetase (BcsA) bcs operon | Betaproteobacteria, Alphaproteobacteria, Gammanproteobacteria, and Gram-positive bacteria | Wound dressing and in food industry | [34] |
| K30 antigen | Capsular | β-(1,2) linkage | Glucuronate Mannose,= and galactose | UDP–D-glucuronate UDP–D-galactose, and UDP–D-glucose, | Polysaccharide polymerase (Wzy) | Escherichia coli | NA | [34] |
| Colanic acid | Extracellular | β-(1,4) linkage | Glucuronate, glucose fucose, and galactose | UDP–D-glucose, UDP–D-galactose, GDP–L-fucose, and UDP–D-glucuronate | Colanic acid polymerase (WcaD) | Shigella sp., E. coli, Enterobacter sp., and Salmonella sp | NA | [34] |
| Gellan | Produced extracellularly | β-(1,3) linkage | Glucuronate, rhamnoseand Glucose | dTDP–rhamnose, UDP–glucuronate, and UDP–glucose | Gellan synthase (Gel G) | Sphingomonas sp. | Food additive, culture media additive for encapsulation | [34] |
| Cudlan | Produced extracellularly | β-(1,3) linkage | Glucose | UDP-glucose | Curdlan synthase (Crd S) | Rhizobium sp., Cellulomonas spp, and Agrobacterium sp. | Food additives | [34] |
| Glycogen | Produced intracellularly | α-(1,6)-branched and α-(1,4)-linked polymer | Glucose | ADP-glucose | Glycogen synthase (GlgA) glg operon | Archea and Bacteria | NA | [34] |
| Alginate | Produced extracellularly | β-(1,4) linkage | Guluronic acid and Mannuronic acid | GDP–mannuronic acid | Glycosyl transferase (Alg 8) alg operon | Azotobacter sp. and Pseudomonas sp. | Development of biomaterials | [34] |
| GBS polysaccharides | Capsular | Galactose, Glucose, and N-acetylneuraminic acid; N-acetylglucosamine or rhamnose | Streptococcus agalactiae | Currently finding its application in the investigation of vaccines | [34] | |||
| Pel | Acetylated -(1,4) linkage | -- | N-acetylgalactosamine and N-acetylglucosamine | P. aeruginosa | [34] | |||
| Psl | L-rhamnose, D-mannose, and D-glucose | P. aeruginosa | MEDI3902d (IgG1 mAb) targets Psl | [34] | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, S.; Lahiri, D.; Nag, M.; Dey, A.; Sarkar, T.; Pathak, S.K.; Atan Edinur, H.; Pati, S.; Ray, R.R. Bacterial Biopolymer: Its Role in Pathogenesis to Effective Biomaterials. Polymers 2021, 13, 1242. https://doi.org/10.3390/polym13081242
Ghosh S, Lahiri D, Nag M, Dey A, Sarkar T, Pathak SK, Atan Edinur H, Pati S, Ray RR. Bacterial Biopolymer: Its Role in Pathogenesis to Effective Biomaterials. Polymers. 2021; 13(8):1242. https://doi.org/10.3390/polym13081242
Chicago/Turabian StyleGhosh, Sreejita, Dibyajit Lahiri, Moupriya Nag, Ankita Dey, Tanmay Sarkar, Sushil Kumar Pathak, Hisham Atan Edinur, Siddhartha Pati, and Rina Rani Ray. 2021. "Bacterial Biopolymer: Its Role in Pathogenesis to Effective Biomaterials" Polymers 13, no. 8: 1242. https://doi.org/10.3390/polym13081242
APA StyleGhosh, S., Lahiri, D., Nag, M., Dey, A., Sarkar, T., Pathak, S. K., Atan Edinur, H., Pati, S., & Ray, R. R. (2021). Bacterial Biopolymer: Its Role in Pathogenesis to Effective Biomaterials. Polymers, 13(8), 1242. https://doi.org/10.3390/polym13081242