PHB/CHIT Scaffold as a Promising Biopolymer in the Treatment of Osteochondral Defects—An Experimental Animal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Composite Scaffold
2.2. Animals
2.3. Surgical Manipulation with Biopolymer Composites
2.4. Monitoring Phase
2.5. X-ray Examination
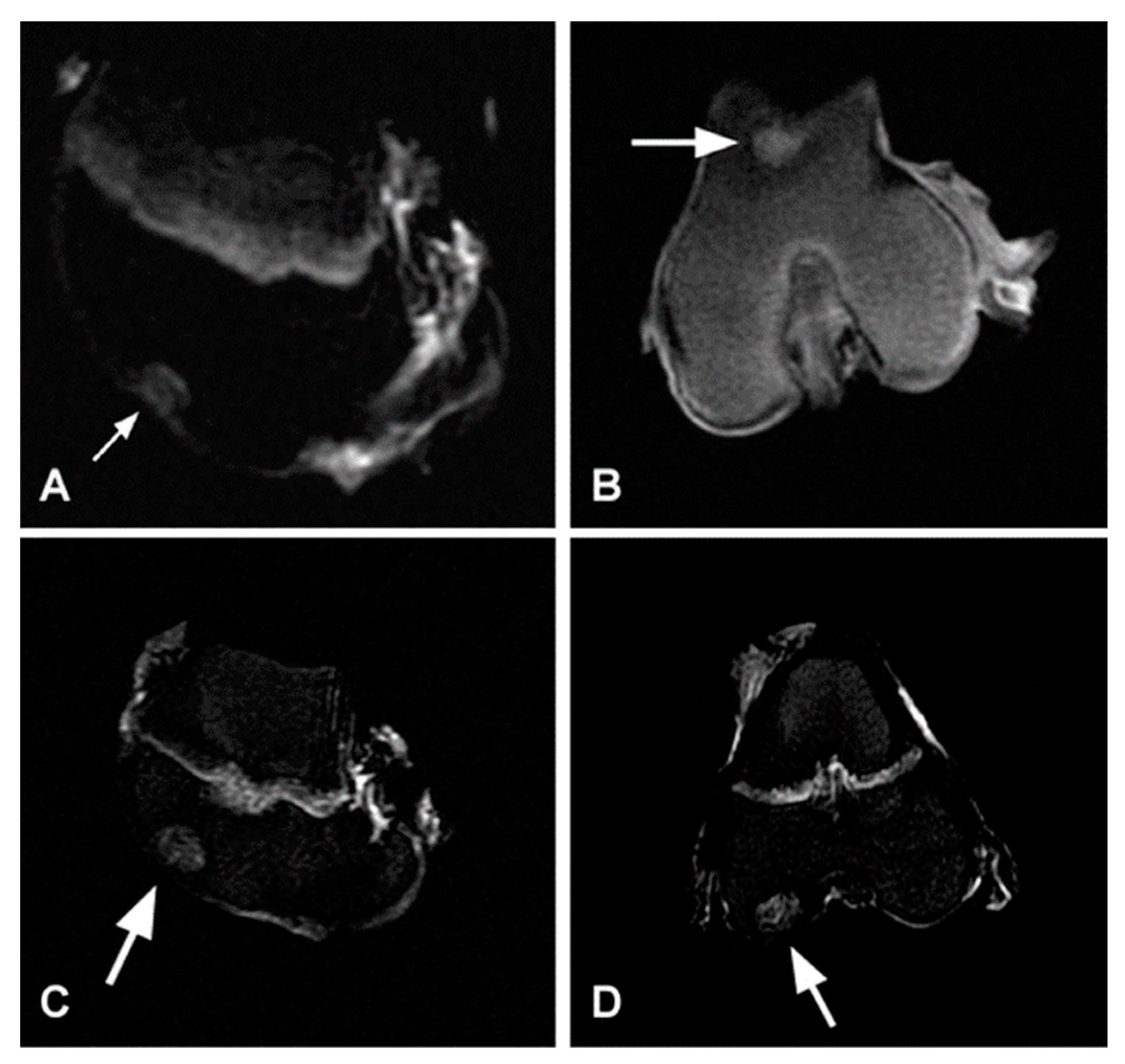
2.6. CT Examination
2.7. MRI (Magnetic Resonance Imaging) Examination
2.8. Histology
2.8.1. Hematoxylin-Eosin Staining
2.8.2. Alcian Blue Staining
2.8.3. Safranin-O Staining
2.8.4. Picrosirius Red Staining
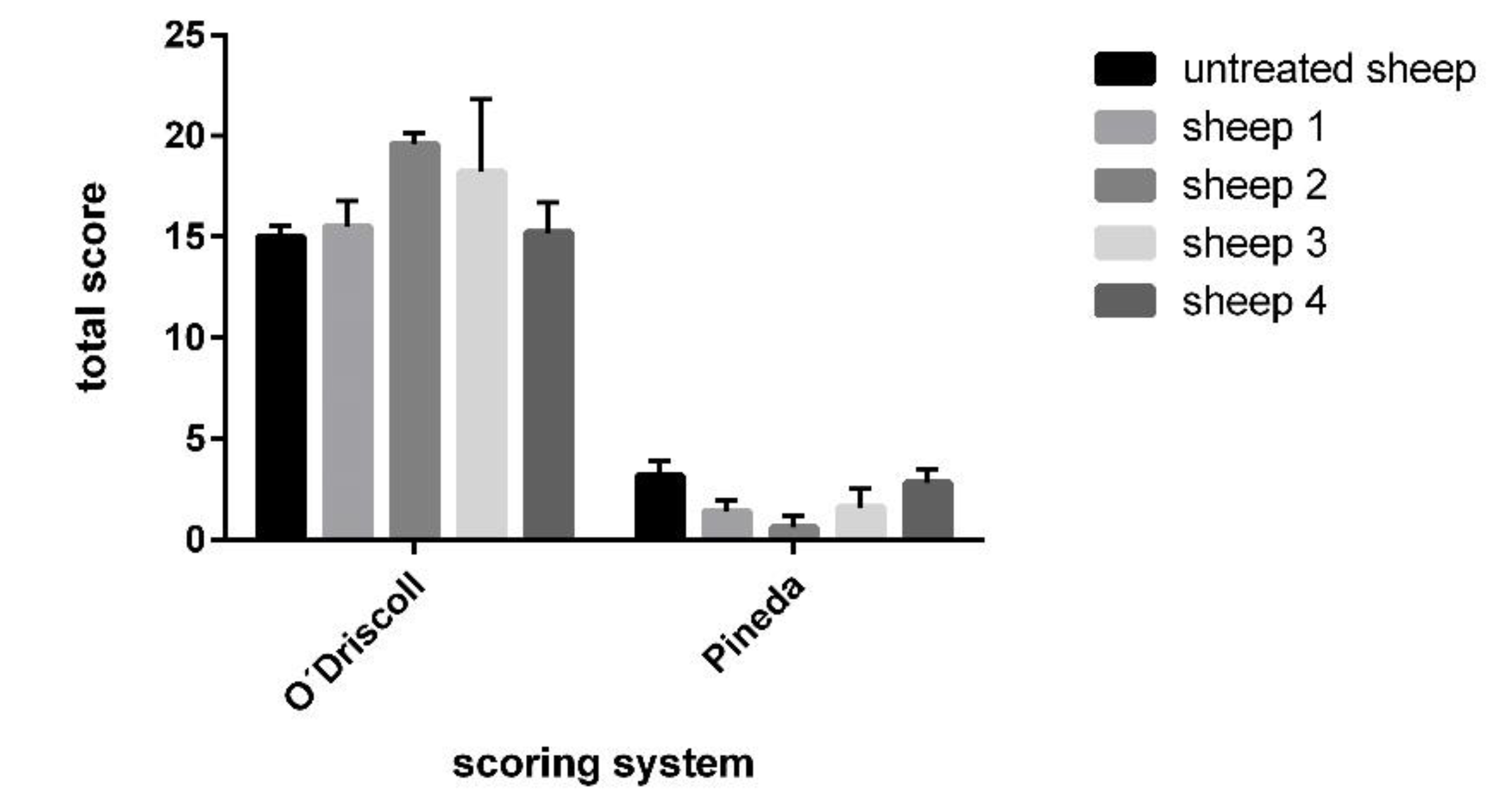
2.8.5. Histological Scoring Systems
Pineda Scoring System
O’Driscoll Scoring System
Polarized Microscopy
2.9. Statistical Analyses
3. Results
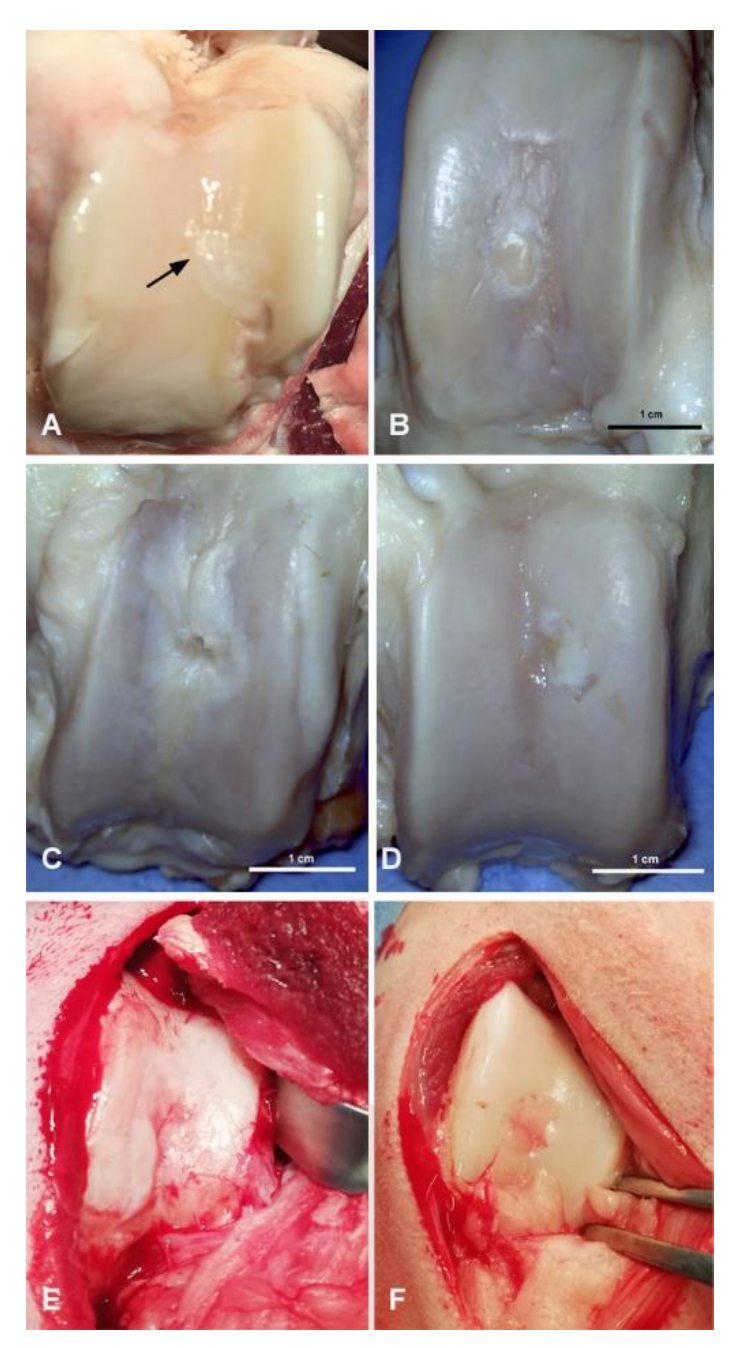
3.1. Macroscopic Evaluation of Repaired Cartilage Defects
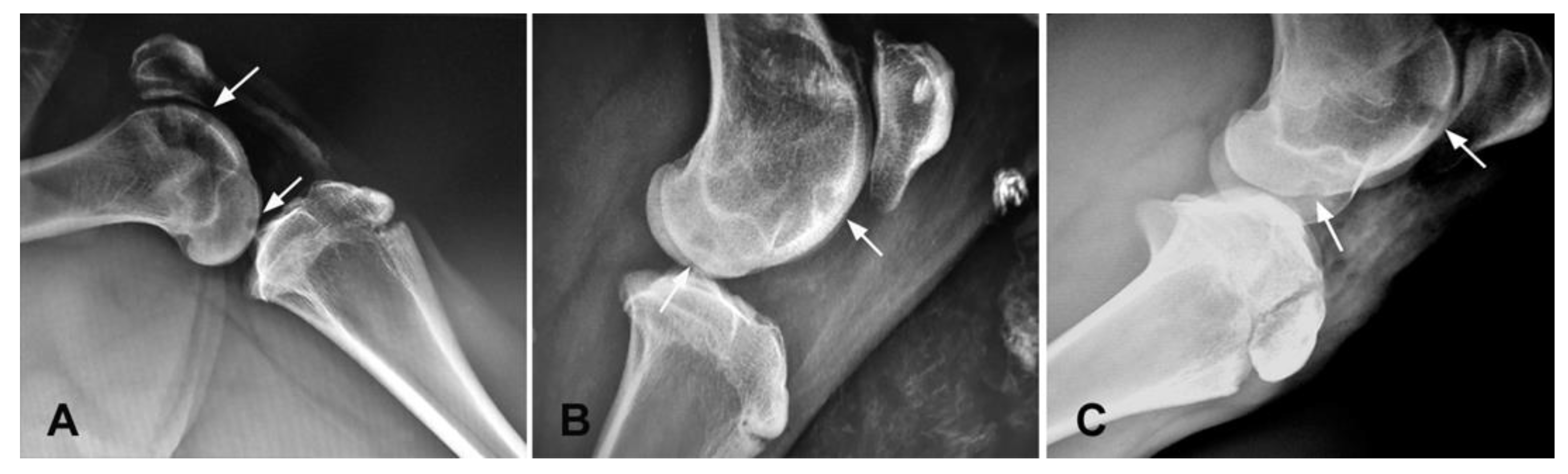
3.2. X-ray Evaluation
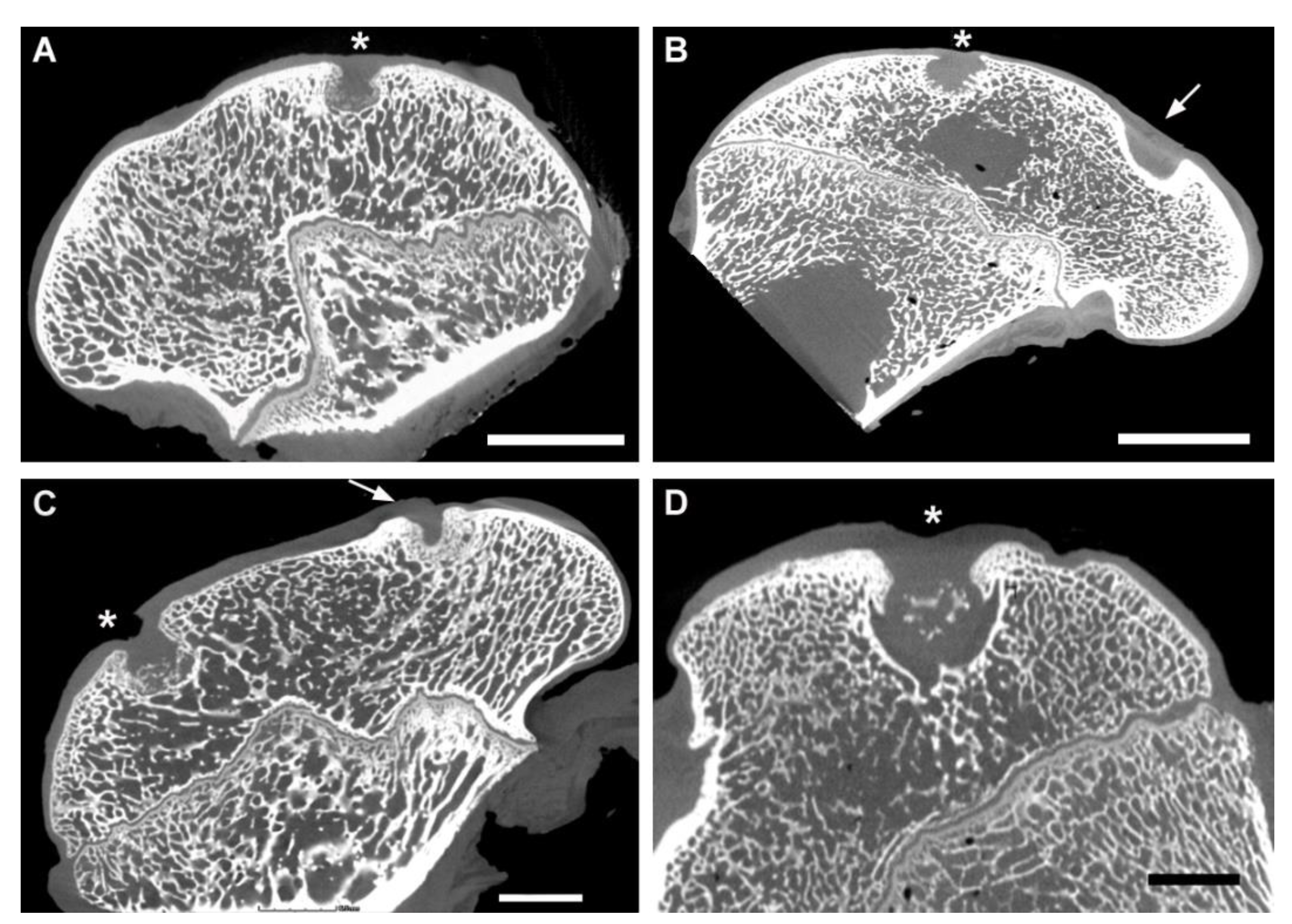
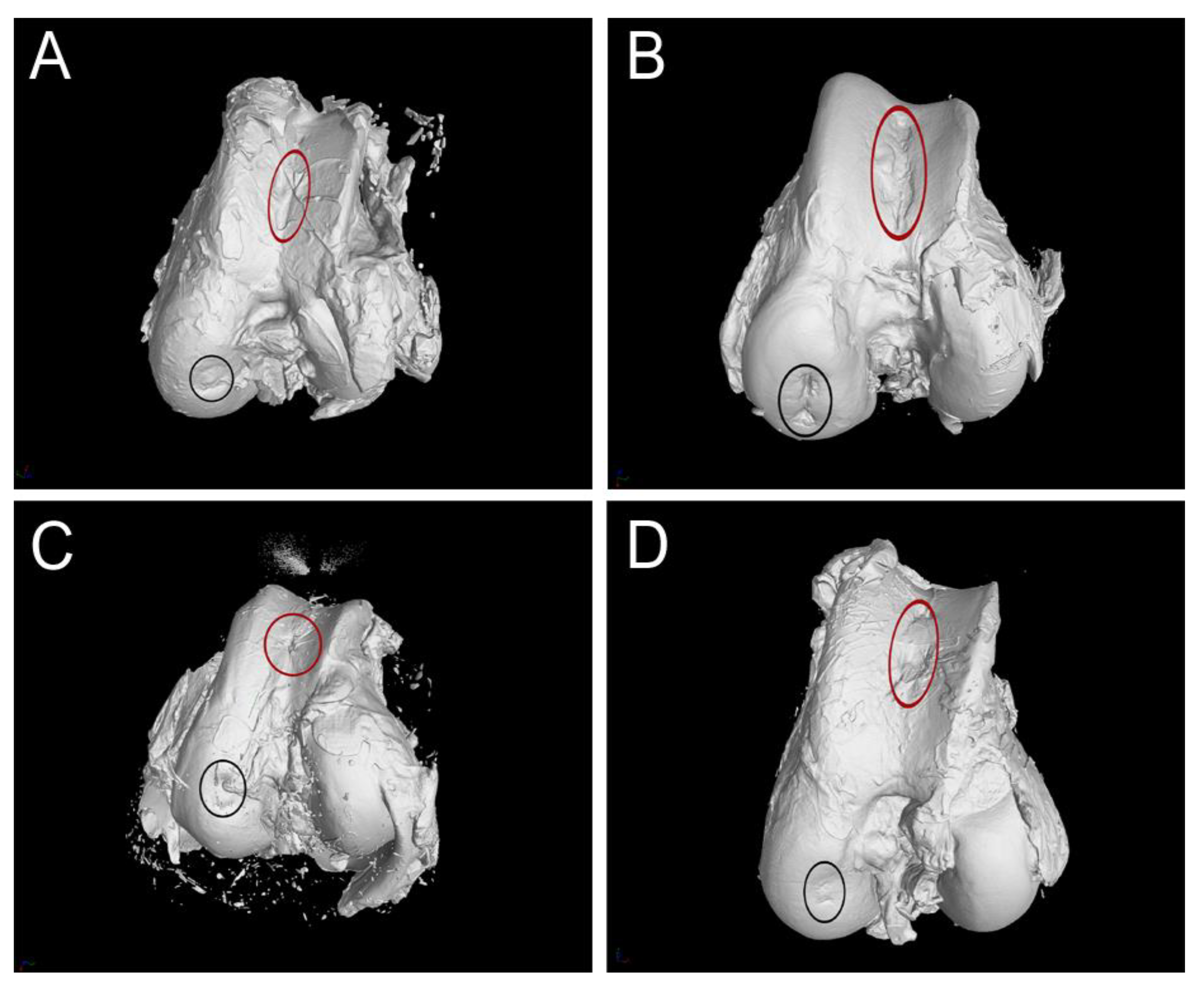
3.3. CT Evaluation
3.4. MRI Evaluation
3.5. Histological Evaluation of Repaired Cartilage Defects
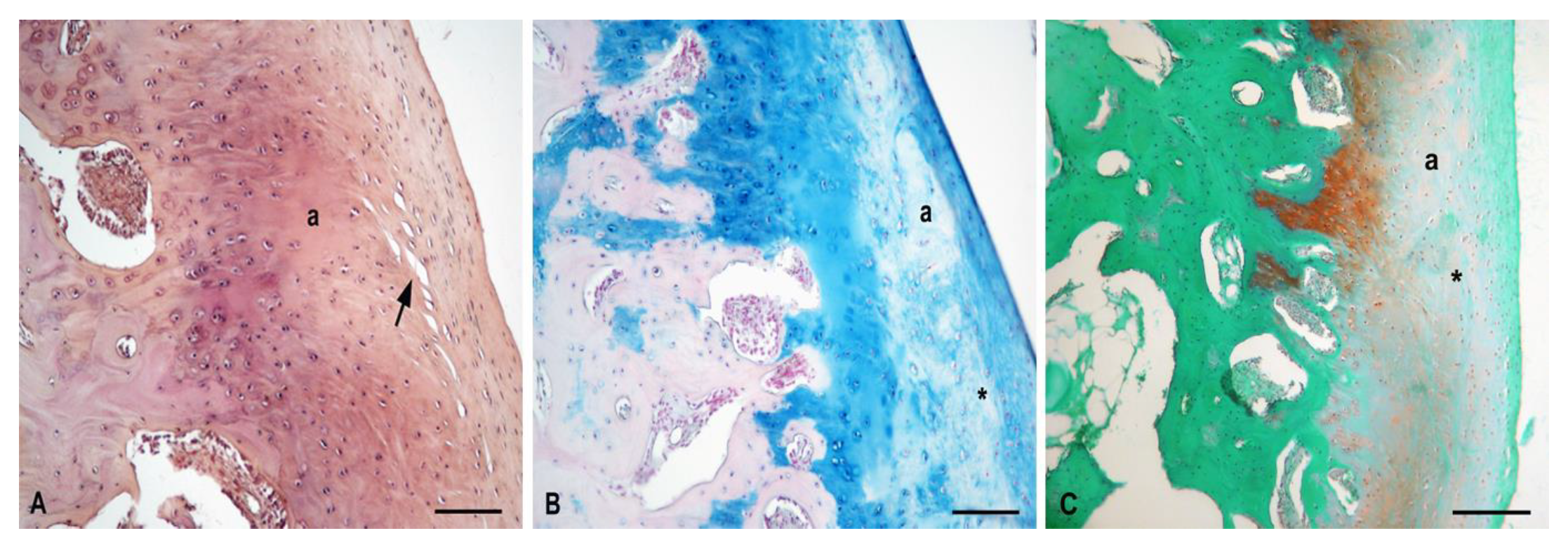
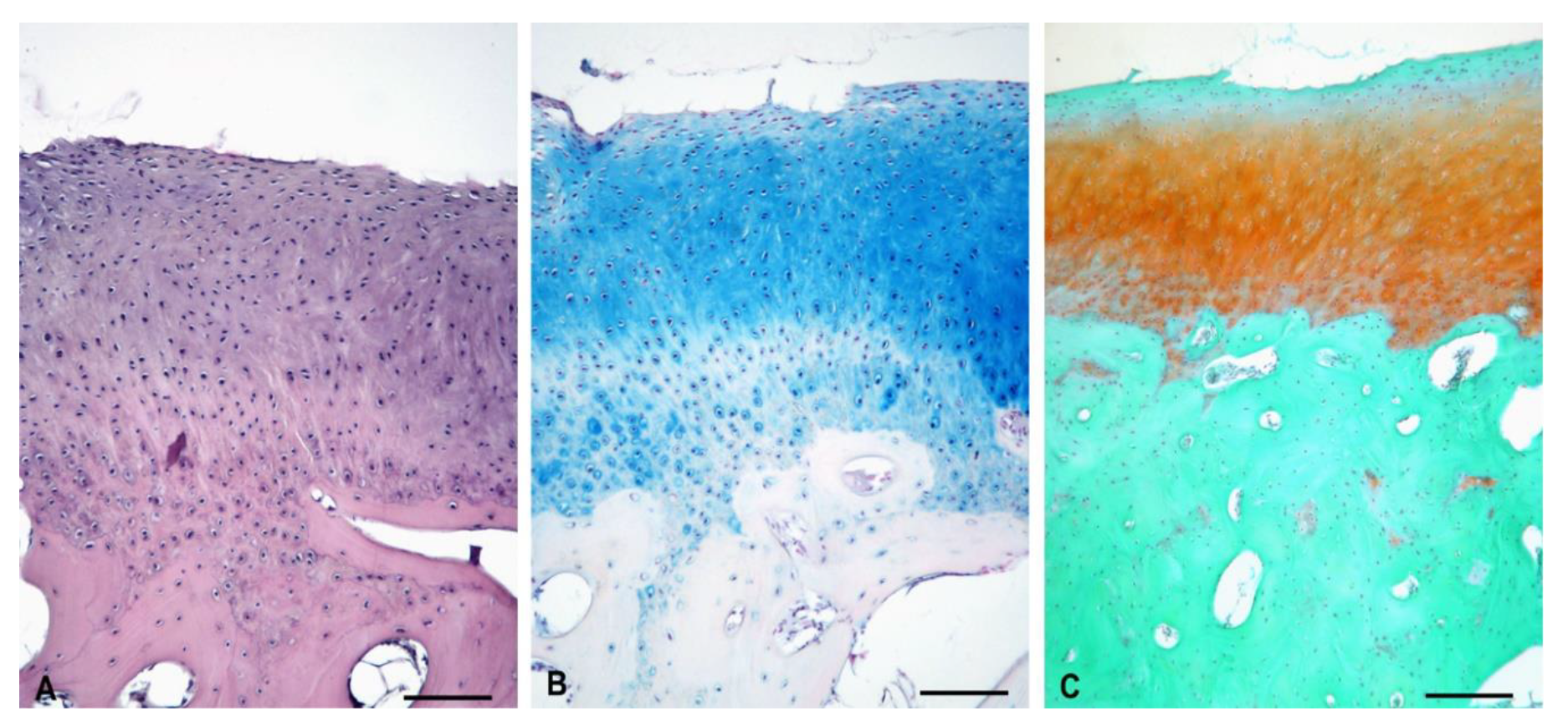
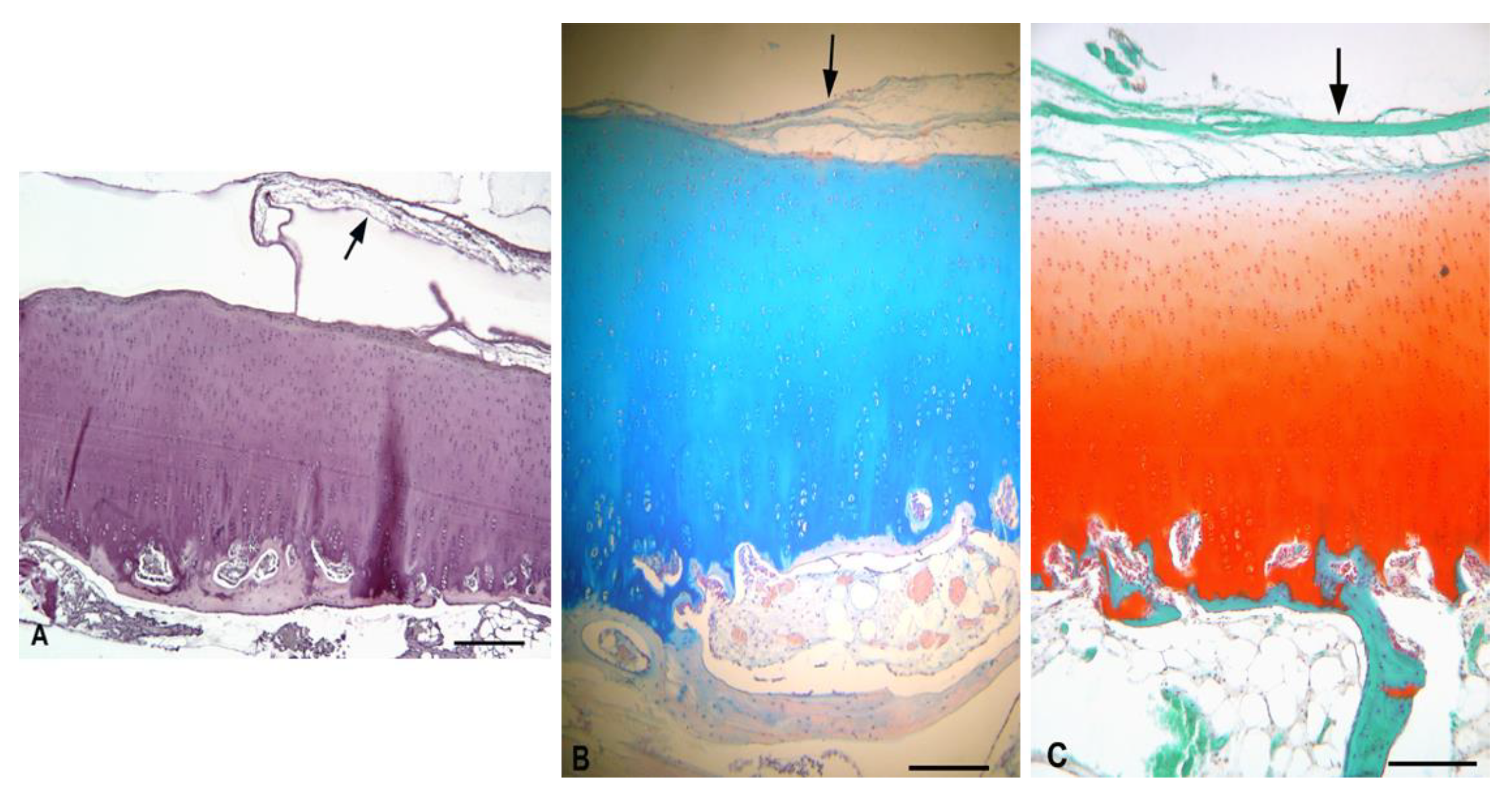
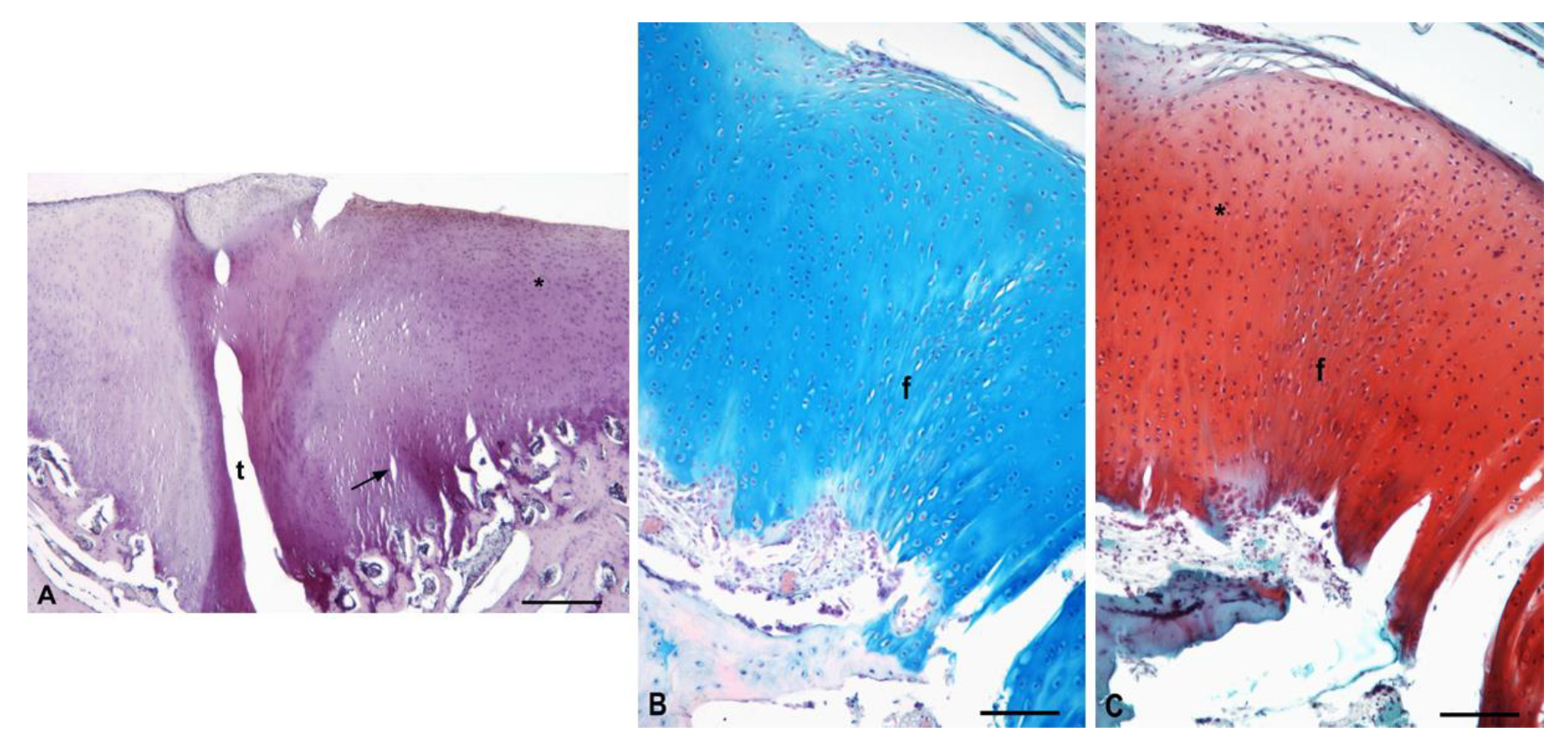
3.5.1. Experimental Sheep 1
3.5.2. Experimental Sheep 2
3.5.3. Experimental Sheep 3
3.5.4. Experimental Sheep 4
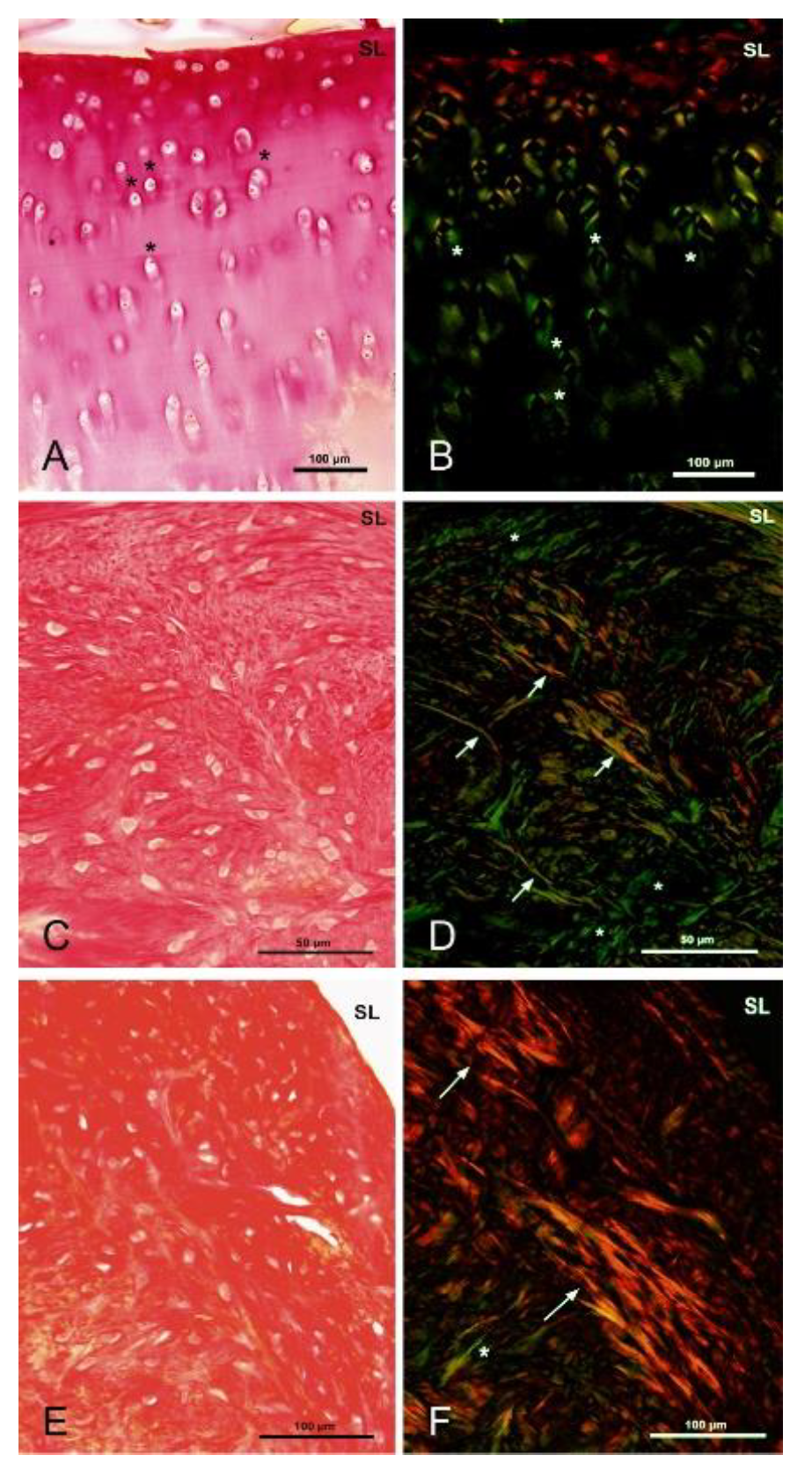
3.5.5. Polarized Microscopy Evaluation of Collagen
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, M.H.; Pawlina, W. Histology: A Text and Atlas, 6th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Baltimore, MA, USA, 2011; pp. 198–207. [Google Scholar]
- Karuppal, R. Current concepts in the articular cartilage repair and regeneration. J. Orthop. 2017, 14, A1–A3. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G. New perspectives for articular cartilage repair treatment through tissue engineering: A contemporary review. World J. Orthop. 2014, 5, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Poulet, B. Models to define the stages of articular cartilage degradation in osteoarthritis development. Int. J. Exp. Pathol. 2017, 98, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Music, E.; Futrega, K.; Doran, M. Sheep as a model for evaluating mesenchymal stem/stromal cell (MSC)-based chondral defect repair. Osteoarthr. Cartil. 2018, 26, 730–740. [Google Scholar] [CrossRef]
- Neidlin, M.; Chantzi, E.; Macheras, G.; Gustafsson, M.G.; Alexopoulos, L.G. An ex vivo tissue model of cartilage degradation suggests that cartilage state can be determined from secreted key protein patterns. PLoS ONE 2019, 14, e0224231. [Google Scholar] [CrossRef]
- Berenbaum, F.; Wallace, I.J.; Lieberman, D.E.; Felson, D.T. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2018, 14, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, A.; Furini, F.; Scirè, C.A. Osteoarthritis and its management—Epidemiology, nutritional aspects and environmental factors. Autoimmun. Rev. 2018, 17, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef]
- Redman, S.N.; Oldfield, S.F.; Archer, C.W. Current strategies for articular cartilage repair. Eur. Cells Mater. 2005, 9, 23–32. [Google Scholar] [CrossRef]
- Chu, C.R.; Szczodry, M.; Bruno, S. Animal Models for Cartilage Regeneration and Repair. Tissue Eng. Part B Rev. 2010, 16, 105–115. [Google Scholar] [CrossRef]
- Dias, I.R.; Viegas, C.A.; Carvalho, P.P. Large Animal Models for Osteochondral Regeneration. Tissue Eng. 2018, 1059, 441–501. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Hurtig, M.; Rossomacha, E.; Sun, J.; Chevrier, A.; Shive, M.S.; Buschmann, M.D. Chitosan-Glycerol Phosphate/Blood Implants Improve Hyaline Cartilage Repair in Ovine Microfracture Defects. J. Bone Jt. Surg. Am. Vol. 2005, 87, 2671–2686. [Google Scholar] [CrossRef]
- Hao, T.; Wen, N.; Cao, J.-K.; Wang, H.-B.; Lü, S.-H.; Liu, T.; Lin, Q.-X.; Duan, C.-M.; Wang, C.-Y. The support of matrix accumulation and the promotion of sheep articular cartilage defects repair in vivo by chitosan hydrogels. Osteoarthr. Cartil. 2010, 18, 257–265. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, Q.; Wang, F.; Zhang, X.; Chen, F.; Liu, X.; Yang, Q.; Zhu, L. Animal Models Used for Testing Hydrogels in Cartilage Regeneration. Curr. Stem Cell Res. Ther. 2018, 13, 517–525. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Rogina, A.; Pušić, M.; Štefan, L.; Ivković, A.; Urlić, I.; Ivanković, M.; Ivanković, H. Characterization of Chitosan-Based Scaffolds Seeded with Sheep Nasal Chondrocytes for Cartilage Tissue Engineering. Ann. Biomed. Eng. 2021, 1–15. [Google Scholar] [CrossRef]
- Victor, R.D.S.; Santos, A.M.D.C.; De Sousa, B.V.; Neves, G.D.A.; Santana, L.N.D.L.; Menezes, R.R. A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef]
- Concha, M.; Vidal, A.; Giacaman, A.; Ojeda, J.; Pavicic, F.; Oyarzun-Ampuero, F.A.; Torres, C.; Cabrera, M.; Moreno-Villoslada, I.; Orellana, S.L. Aerogels made of chitosan and chondroitin sulfate at high degree of neutralization: Biological properties toward wound healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2464–2471. [Google Scholar] [CrossRef]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104, 1003–1011. [Google Scholar] [CrossRef]
- Oprenyeszk, F.; Sanchez, C.; Dubuc, J.-É.; Maquet, V.; Henrist, C.; Compère, P.; Henrotin, Y. Chitosan Enriched Three-Dimensional Matrix Reduces Inflammatory and Catabolic Mediators Production by Human Chondrocytes. PLoS ONE 2015, 10, e0128362. [Google Scholar] [CrossRef]
- Comblain, F.; Rocasalbas, G.; Gauthier, S.; Henrotin, Y. Chitosan: A promising polymer for cartilage repair and viscosupplementation. Bio Med. Mater. Eng. 2017, 28, S209–S215. [Google Scholar] [CrossRef]
- Islam, M.; Shahruzzaman, M.; Biswas, S.; Sakib, N.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Hill, R.G. Biomedical polymers. In Biomaterials, Artificial Organs and Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2005; pp. 97–106. [Google Scholar]
- Dariš, B.; Knez, Ž. Poly(3-hydroxybutyrate): Promising biomaterial for bone tissue engineering. Acta Pharm. 2020, 70, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Giretova, M.; Medvecky, L.; Petrovova, E.; Cizkova, D.; Danko, J.; Mudronova, D.; Slovinska, L.; Bures, R. Polyhydroxybutyrate/Chitosan 3D Scaffolds Promote In Vitro and In Vivo Chondrogenesis. Appl. Biochem. Biotechnol. 2019, 189, 556–575. [Google Scholar] [CrossRef] [PubMed]
- Kok, A.C.; van Bergen, C.J.A.; Tuijthof, G.J.M.; Klinkenbijl, M.N.; van Noorden, C.J.F.; van Dijk, C.N.; Kerkhoffs, G.M.M.J. Macroscopic ICRS Poorly Correlates with O’Driscoll Histological Cartilage Repair Assessment in a Goat Model. Clin. Res. Foot Ankle 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Moojen, D.; Saris, D.; Yang, K.A.; Dhert, W.; Verbout, A. The Correlation and Reproducibility of Histological Scoring Systems in Cartilage Repair. Tissue Eng. 2002, 8, 627–634. [Google Scholar] [CrossRef]
- Orth, P.; Madry, H. Complex and elementary histological scoring systems for articular cartilage repair. Histol. Histopathol. 2015, 30, 911–919. [Google Scholar]
- Medvecky, L.; Giretova, M.; Stulajterova, R. Properties and in vitro characterization of polyhydroxybutyrate–chitosan scaffolds prepared by modified precipitation method. J. Mater. Sci. Mater. Electron. 2014, 25, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Giretova, M.; Medvecky, L.; Stulajterova, R.; Sopcak, T.; Briancin, J.; Tatarkova, M. Effect of enzymatic degradation of chitosan in polyhydroxybutyrate/chitosan/calcium phosphate composites on in vitro osteoblast response. J. Mater. Sci. Mater. Electron. 2016, 27, 181. [Google Scholar] [CrossRef] [PubMed]
- Tothova, C.; Mihajlovicova, X.; Novotny, J.; Nagy, O.; Giretova, M.; Kresakova, L.; Tomco, M.; Zert, Z.; Vilhanova, Z.; Varga, M.; et al. The Serum Protein Profile and Acute Phase Proteins in the Postoperative Period in Sheep after Induced Articular Cartilage Defect. Materials 2019, 12, 142. [Google Scholar] [CrossRef]
- Junqueira, L.C.U.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. J. Mol. Histol. 1979, 11, 447–455. [Google Scholar] [CrossRef]
- Rutgers, M.; van Pelt, M.; Dhert, W.; Creemers, L.; Saris, D. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthr. Cartil. 2010, 18, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Houlton, J.E.F.; Adams, S.B.; Rushton, N. The Surgical Anatomy of the Stifle Joint in Sheep. Veter Surg. 1998, 27, 596–605. [Google Scholar] [CrossRef] [PubMed]
- McIlwrith, C.W.; Frisbie, D.D.; Kawcak, C.E.; van Weeren, R. Joint Disease. In The Horse, 2nd ed.; Elsevier: St. Louis, MO, USA, 2016; pp. 2–3. [Google Scholar]
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular fibrocartilage—Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2019, 146, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, R.; Hansen, U.; Raiteri, R.; Loparic, M.; Düggelin, M.; Mathys, D.; Friederich, N.F.; Bruckner, P.; Stolz, M. Supramolecular Organization of Collagen Fibrils in Healthy and Osteoarthritic Human Knee and Hip Joint Cartilage. PLoS ONE 2016, 11, e0163552. [Google Scholar] [CrossRef]
- Guilak, F.; Nims, R.J.; Dicks, A.; Wu, C.-L.; Meulenbelt, I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018, 71–72, 40–50. [Google Scholar] [CrossRef]
- Ravanetti, F.; Galli, C.; Manfredi, E.; Cantoni, A.M.; Scarpa, E.; Macaluso, G.M.; Cacchioli, A. Chitosan-based scaffold modified with D-(+) raffinose for cartilage repair: An in vivo study. J. Negat. Results Biomed. 2015, 14, 2. [Google Scholar] [CrossRef]
- Roffi, A.; Kon, E.; Perdisa, F.; Fini, M.; Di Martino, A.; Parrilli, A.; Salamanna, F.; Sandri, M.; Sartori, M.; Sprio, S.; et al. A Composite Chitosan-Reinforced Scaffold Fails to Provide Osteochondral Regeneration. Int. J. Mol. Sci. 2019, 20, 2227. [Google Scholar] [CrossRef] [PubMed]
- Hoemann, C.D.; Guzmán-Morales, J.; Picard, G.; Chen, G.; Veilleux, D.; Chevrier, A.; Sim, S.; Garon, M.; Quenneville, E.; Lafantaisie-Favreau, C.-H.; et al. Guided bone marrow stimulation for articular cartilage repair through a freeze-dried chitosan microparticle approach. Materials 2020, 9, 100609. [Google Scholar] [CrossRef]
- Gupta, A.; Bhat, S.; Jagdale, P.R.; Chaudhari, B.P.; Lidgren, L.; Gupta, K.C.; Kumar, A. Evaluation of Three-Dimensional Chitosan-Agarose-Gelatin Cryogel Scaffold for the Repair of Subchondral Cartilage Defects: AnIn VivoStudy in a Rabbit Model. Tissue Eng. Part A 2014, 20, 3101–3111. [Google Scholar] [CrossRef]
- Petrovova, E.; Giretova, M.; Kvasilova, A.; Benada, O.; Danko, J.; Medvecky, L.; Sedmera, D. Preclinical alternative model for analysis of porous scaffold biocompatibility in bone tissue engineering. ALTEX 2019, 36, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.D.; Hurtig, M.B.; Quenneville, E.; Rivard, G.-É.; Hoemann, C.D. Effect of a Rapidly Degrading Presolidified 10 kDa Chitosan/Blood Implant and Subchondral Marrow Stimulation Surgical Approach on Cartilage Resurfacing in a Sheep Model. Cartilage 2017, 8, 417–431. [Google Scholar] [CrossRef]
- Ruediger, T.; Horbert, V.; Reuther, A.; Kalla, P.K.; Burgkart, R.H.; Walther, M.; Kinne, R.W.; Mika, J. Thickness of the Stifle Joint Articular Cartilage in Different Large Animal Models of Cartilage Repair and Regeneration. Cartilage 2020, 7, 126–147. [Google Scholar] [CrossRef]
- Ahern, B.; Parvizi, J.; Boston, R.; Schaer, T. Preclinical animal models in single site cartilage defect testing: A systematic review. Osteoarthr. Cartil. 2009, 17, 705–713. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The Role of Tissue Engineering in Articular Cartilage Repair and Regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Vyas, C.; Mishbak, H.; Cooper, G.; Peach, C.; Pereira, R.F.; Bartolo, P. Biological perspectives and current biofabrication strategies in osteochondral tissue engineering. Biomanuf. Rev. 2020, 5, 1–24. [Google Scholar] [CrossRef]
- Matsiko, A.; Levingstone, T.J.; O’Brien, F.J. Advanced Strategies for Articular Cartilage Defect Repair. Materials 2013, 6, 637–668. [Google Scholar] [CrossRef] [PubMed]
- Eldracher, M.; Orth, P.; Cucchiarini, M.; Pape, D.; Madry, H. Small Subchondral Drill Holes Improve Marrow Stimulation of Articular Cartilage Defects. Am. J. Sports Med. 2014, 42, 2741–2750. [Google Scholar] [CrossRef]
- Pot, M.W.; Gonzales, V.K.; Buma, P.; IntHout, J.; Van Kuppevelt, T.H.; De Vries, R.B.; Daamen, W.F. Improved cartilage regeneration by implantation of acellular biomaterials after bone marrow stimulation: A systematic review and meta-analysis of animal studies. PeerJ 2016, 4, e2243. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health A Multidiscip. Approach 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Mohan, N.; Mohanan, P.; Sabareeswaran, A.; Nair, P. Chitosan-hyaluronic acid hydrogel for cartilage repair. Int. J. Biol. Macromol. 2017, 104, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
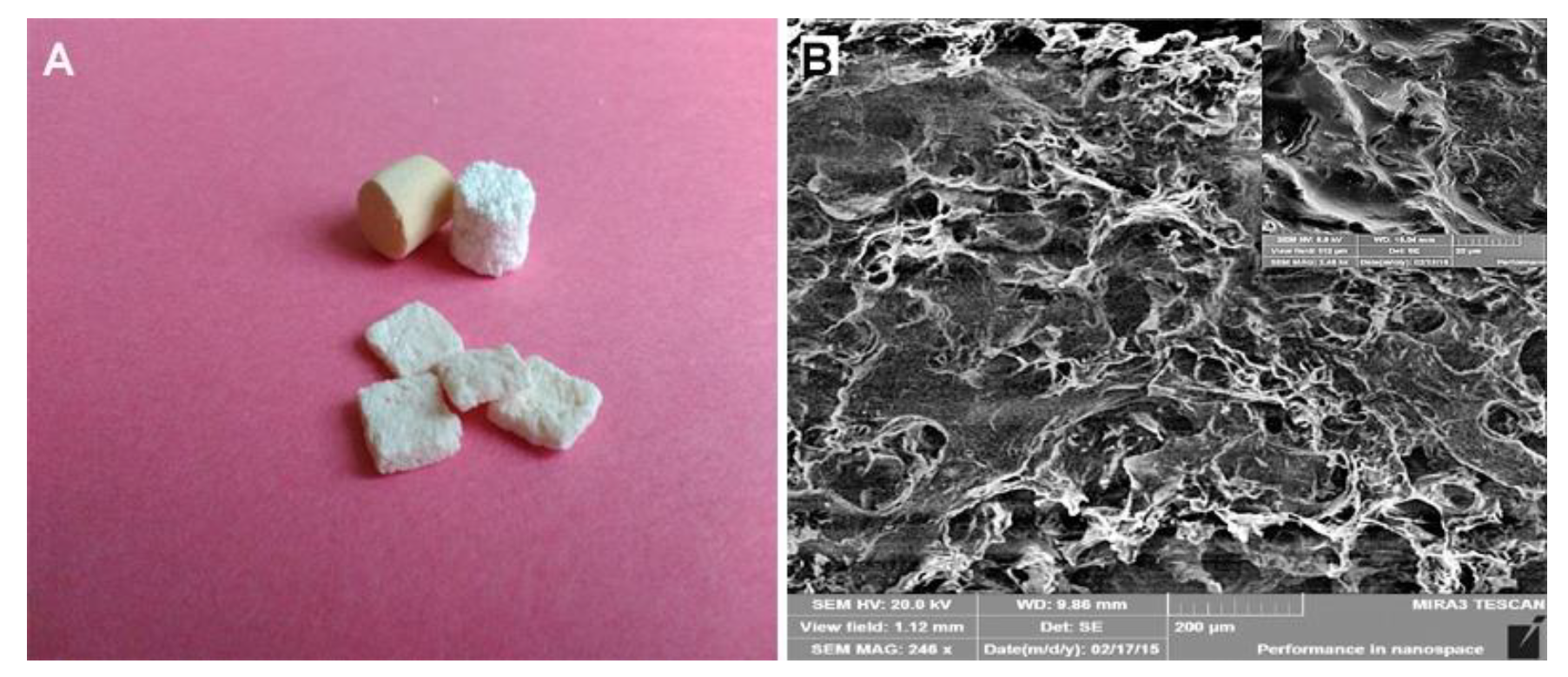
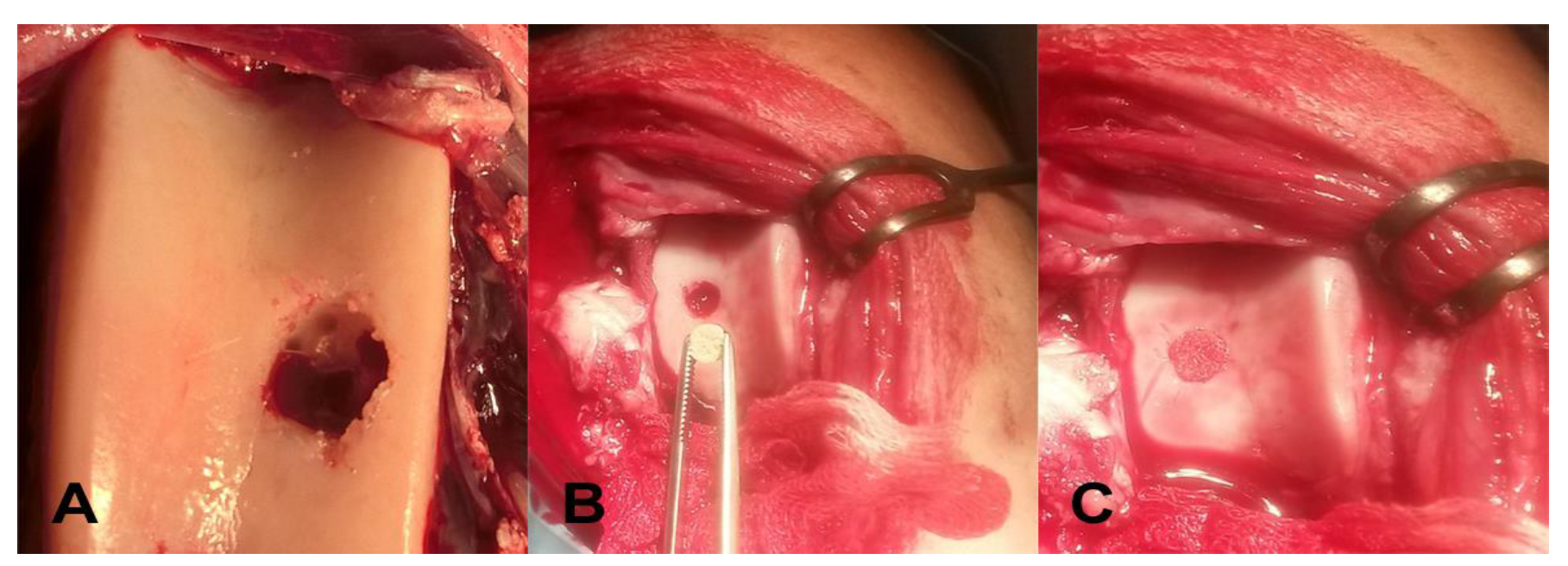
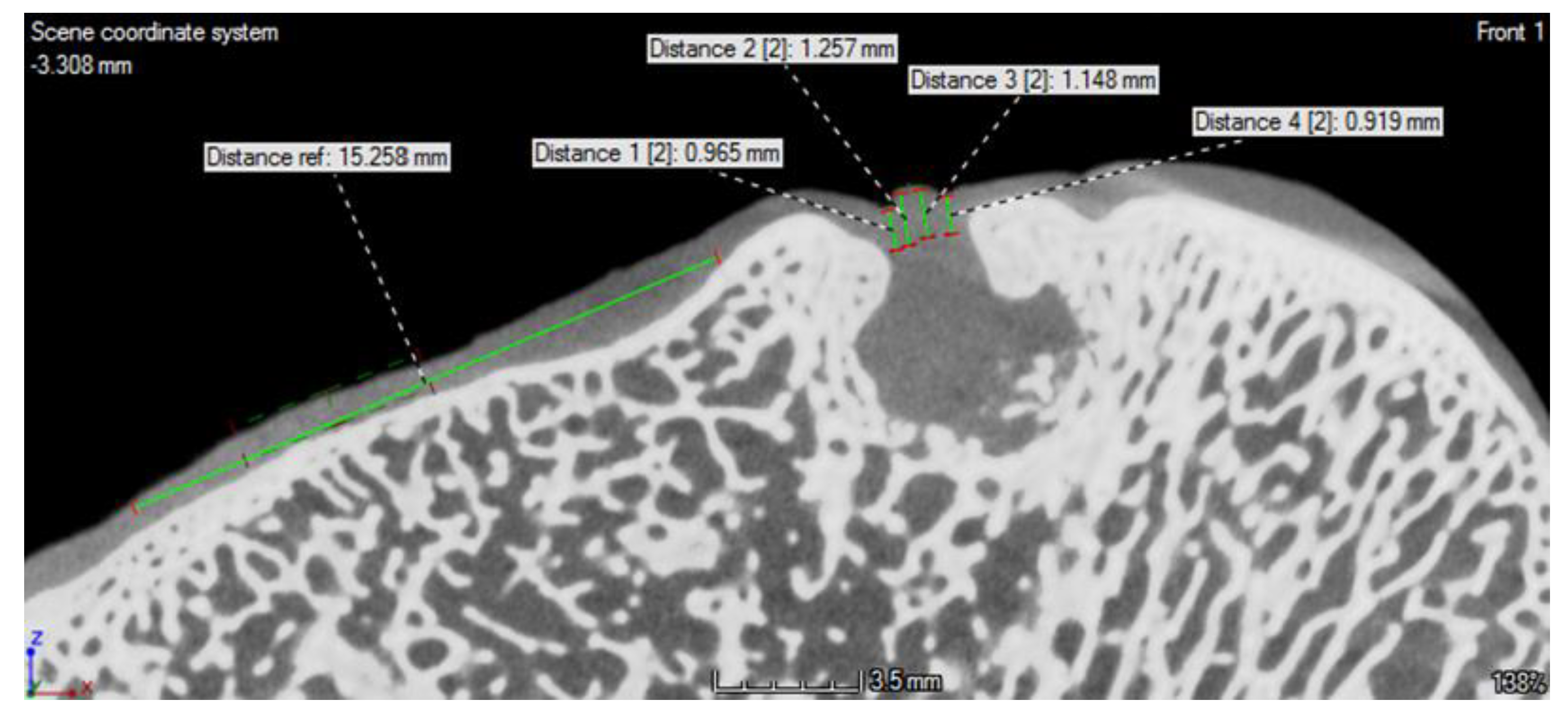
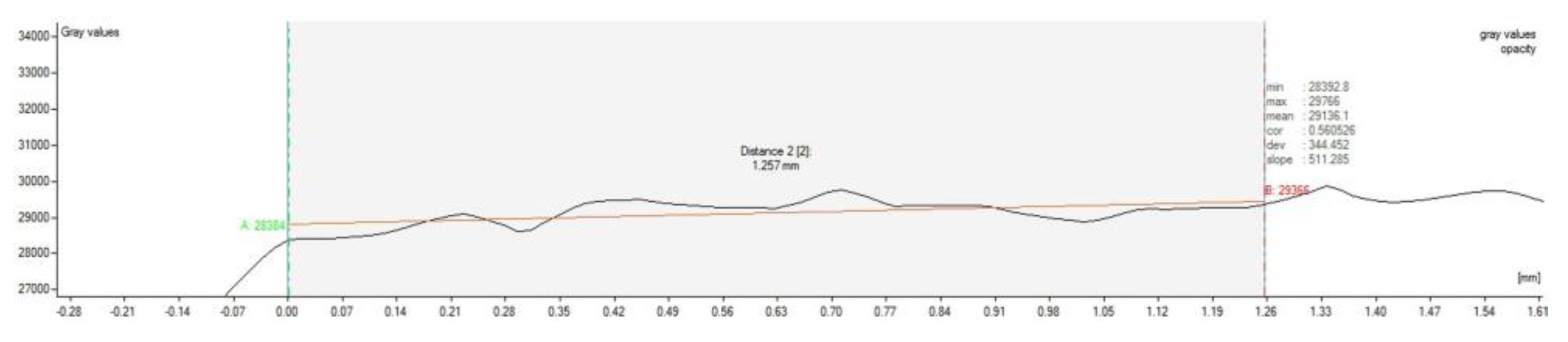


| Animals | Defects Location | Scaffold Implantation |
|---|---|---|
| Experimental sheep 1 | left trochlea | + |
| left condyle | + | |
| Experimental sheep 2 | left trochlea | + |
| left condyle | + | |
| Experimental sheep 3 | left trochlea | + |
| left condyle | + | |
| Experimental sheep 4 | left trochlea | + |
| left condyle | + | |
| Experimental sheep 5 | left trochlea | - |
| left condyle | - | |
| Experimental sheep 6 | left trochlea | - |
| left condyle | - |
| Sample | Average T | Reference Average | Percentage Difference (%) |
|---|---|---|---|
| Experimental sheep 1 | 28,559.66 | 28,659.53 | 0.35 |
| Experimental sheep 2 | 38,320.75 | 39,369.86 | 2.67 |
| Experimental sheep 3 | 29,388.88 | 29,407.09 | 0.06 |
| Experimental sheep 4 | 34,143.07 | 35,803.56 | 4.64 |
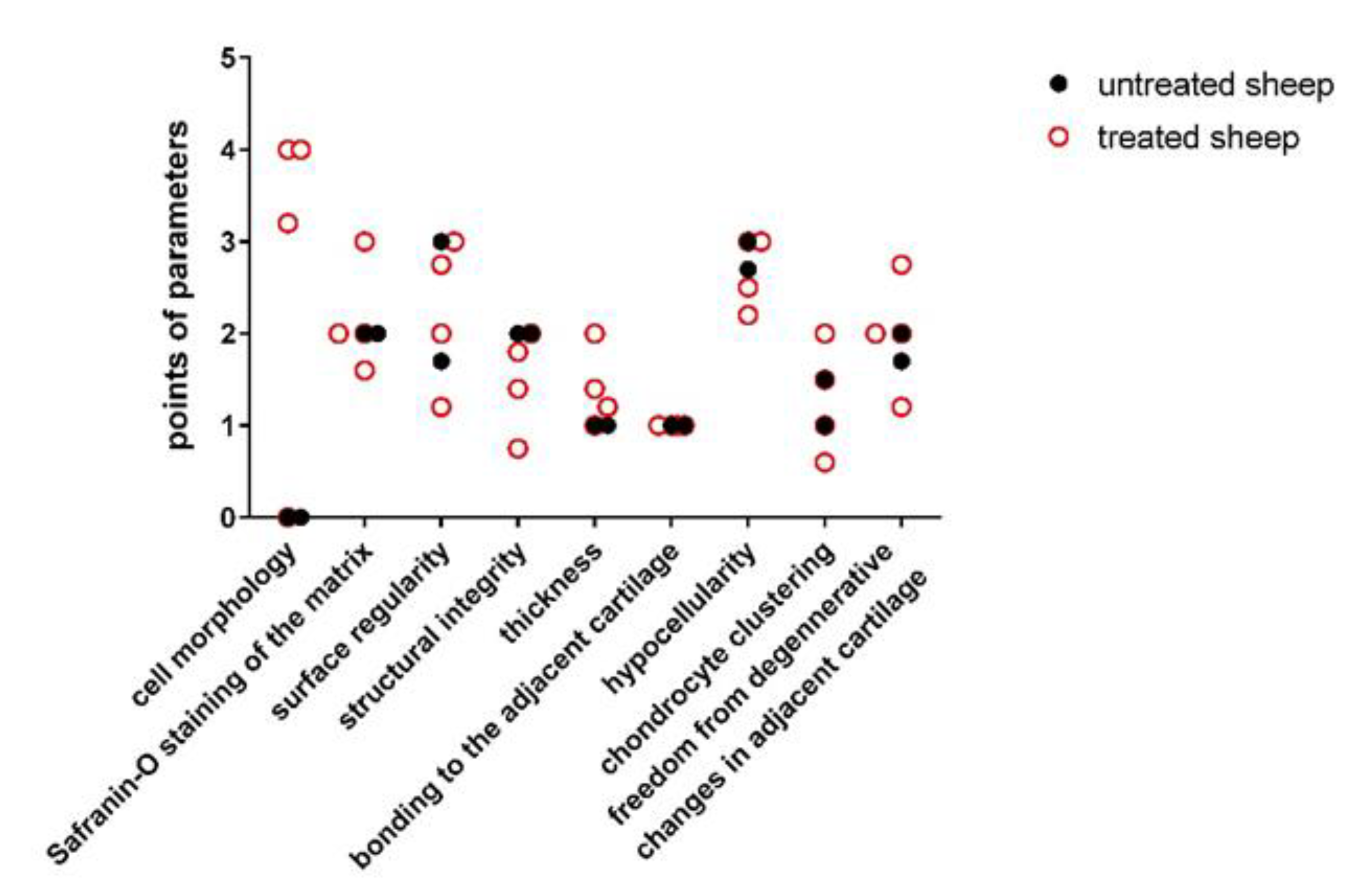
| Parameters | Untreated Sheep 1 | Untreated Sheep 2 | Experimental Sheep 1 | Experimental Sheep 2 | Experimental Sheep 3 | Experimental Sheep 4 |
|---|---|---|---|---|---|---|
| Degenerative changes in adjacent cartilage | 1.67 ± 0.58 | 2.00 ± 0.00 | 1.20 ± 0.45 | 2.00 ± 0.00 | 2.00 ± 0.71 | 2.75 ± 0.50 |
| Chondrocyte clustering | 1.00 ± 0.00 | 1.50 ± 0.58 | 0.60 ± 0.55 | 1.00 ± 0.00 | 2.00 ± 0.00 | 1.50 ± 0.60 |
| Hypocellularity | 2.67 ± 0.58 | 3.00 ± 0.00 | 2.20 ± 0.45 | 3.00 ± 0.00 | 3.00 ± 0.00 | 2.50 ± 0.60 |
| Bonding to the adjacent cartilage | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| Thickness of cartilage | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.20 ± 0.45 | 2.00 ± 0.00 | 1.40 ± 0.55 | 1.00 ± 0.00 |
| Structural integrity | 2.00 ± 0.00 | 2.00 ± 0.00 | 1.80 ± 0.45 | 2.00 ± 0.00 | 1.40 ± 0.89 | 0.75 ± 0.96 |
| Surface regularity | 1.67 ± 1.15 | 3.00 ± 0.00 | 1.20 ± 0.45 | 3.00 ± 0.00 | 2.00 ± 0.70 | 2.75 ± 0.50 |
| Safranin-O staining of the matrix | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 1.60 ± 0.55 | 2.00 ± 0.00 | 3.00 ± 0.00 |
| Cell morphology | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.00 ± 0.00 | 4.00 ± 0.00 | 3.20 ± 1.79 | 0.00 ± 0.00 |
| Total average score | 13.00 ± 2.00 | 15.50 ± 0.58 | 15.20 ± 1.48 | 19.60 ± 0.55 | 18.20 ± 3.63 | 15.50 ± 1.29 |
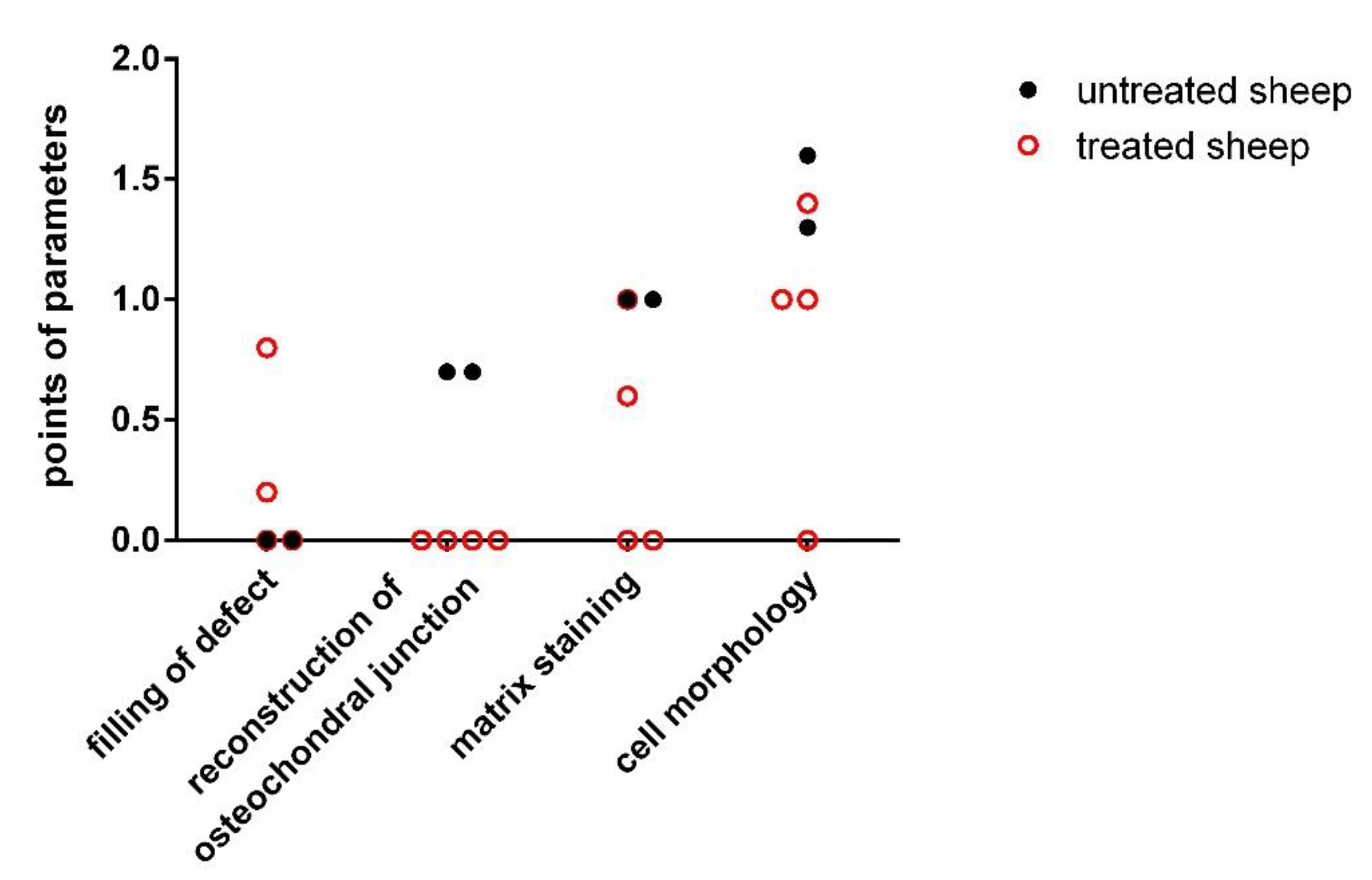
| Parameters | Untreated Sheep 1 | Untreated Sheep 2 | Experimental Sheep 1 | Experimental Sheep 2 | Experimental Sheep 3 | Experimental Sheep 4 |
|---|---|---|---|---|---|---|
| Cell morphology | 1.33 ± 0.52 | 1.80 ± 0.49 | 1.00 ± 0.00 | 0.00 ± 0.00 | 1.00 ± 0.00 | 1.40 ± 0.55 |
| Staining of the matrix | 1.17 ± 0.41 | 1.00 ± 0.00 | 1.00 ± 0.00 | 0.6 ± 0.55 | 0.20 ± 0.45 | 0.00 ± 0.00 |
| Reconstitution of the osteochondral junction | 0.83 ± 0.41 | 0.29 ± 0.49 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Filling of the defect | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.80 ± 0.84 | 0.00 ± 0.00 | 0.40 ± 0.55 | 0.00 ± 0.00 |
| Total average score | 3.33 ± 0.82 | 3.00 ± 0.58 | 2.80 ± 0.75 | 0.60 ± 0.55 | 1.20 ± 0.45 | 1.40 ± 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovova, E.; Tomco, M.; Holovska, K.; Danko, J.; Kresakova, L.; Vdoviakova, K.; Simaiova, V.; Kolvek, F.; Hornakova, P.; Toth, T.; et al. PHB/CHIT Scaffold as a Promising Biopolymer in the Treatment of Osteochondral Defects—An Experimental Animal Study. Polymers 2021, 13, 1232. https://doi.org/10.3390/polym13081232
Petrovova E, Tomco M, Holovska K, Danko J, Kresakova L, Vdoviakova K, Simaiova V, Kolvek F, Hornakova P, Toth T, et al. PHB/CHIT Scaffold as a Promising Biopolymer in the Treatment of Osteochondral Defects—An Experimental Animal Study. Polymers. 2021; 13(8):1232. https://doi.org/10.3390/polym13081232
Chicago/Turabian StylePetrovova, Eva, Marek Tomco, Katarina Holovska, Jan Danko, Lenka Kresakova, Katarina Vdoviakova, Veronika Simaiova, Filip Kolvek, Petra Hornakova, Teodor Toth, and et al. 2021. "PHB/CHIT Scaffold as a Promising Biopolymer in the Treatment of Osteochondral Defects—An Experimental Animal Study" Polymers 13, no. 8: 1232. https://doi.org/10.3390/polym13081232
APA StylePetrovova, E., Tomco, M., Holovska, K., Danko, J., Kresakova, L., Vdoviakova, K., Simaiova, V., Kolvek, F., Hornakova, P., Toth, T., Zivcak, J., Gal, P., Sedmera, D., Luptakova, L., & Medvecky, L. (2021). PHB/CHIT Scaffold as a Promising Biopolymer in the Treatment of Osteochondral Defects—An Experimental Animal Study. Polymers, 13(8), 1232. https://doi.org/10.3390/polym13081232








