pH/Thermo-Responsive Grafted Alginate-Based SiO2 Hybrid Nanocarrier/Hydrogel Drug Delivery Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel Preparation and Rheology Study
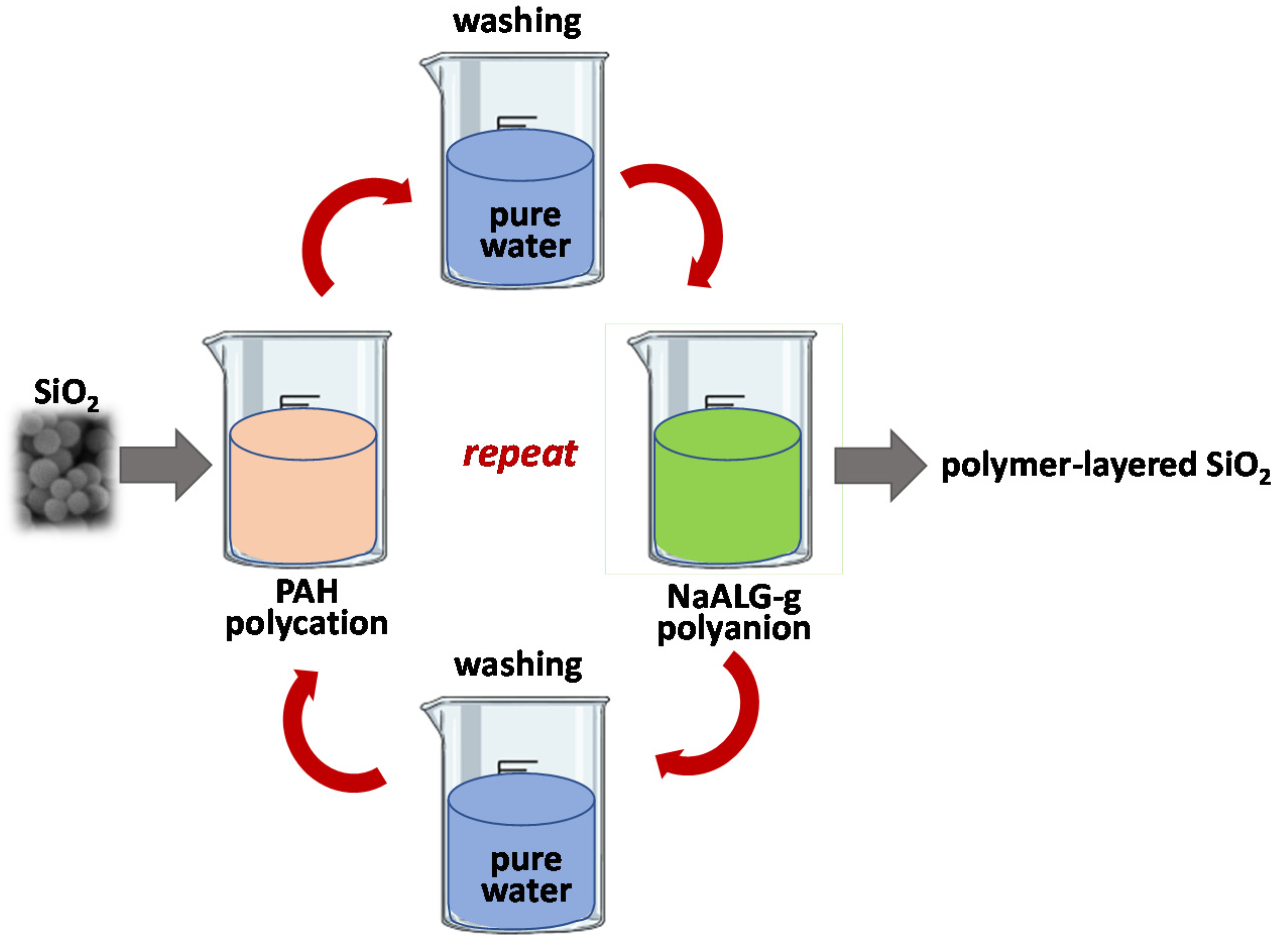
2.3. Preparation of LBL SiO2 Particles
2.4. Loading and Release of RB from the LBL SiO2 Particles
2.5. Particle/Hydrogel Composite System
2.6. Techniques
3. Results
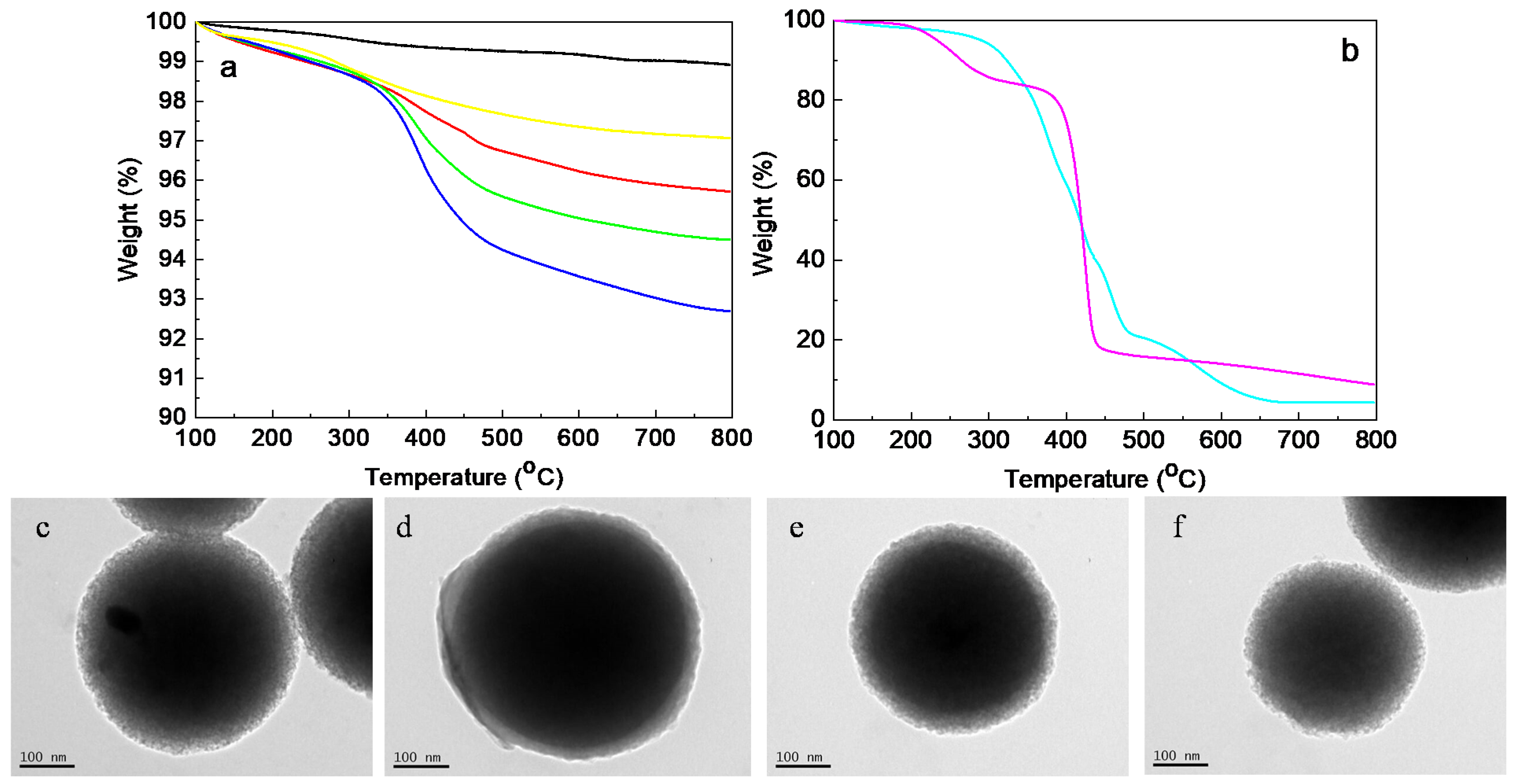
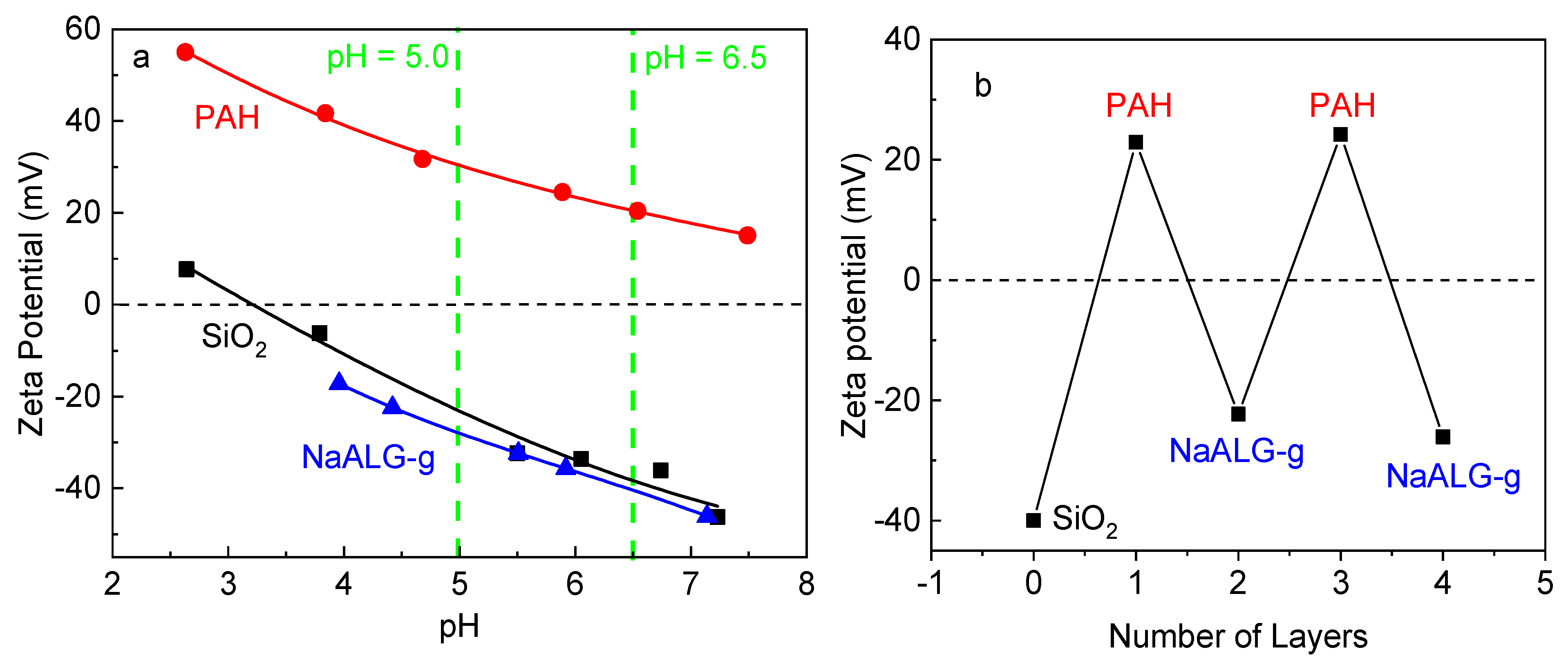
3.1. Nanoparticle Fabrication by LbL and Characterization
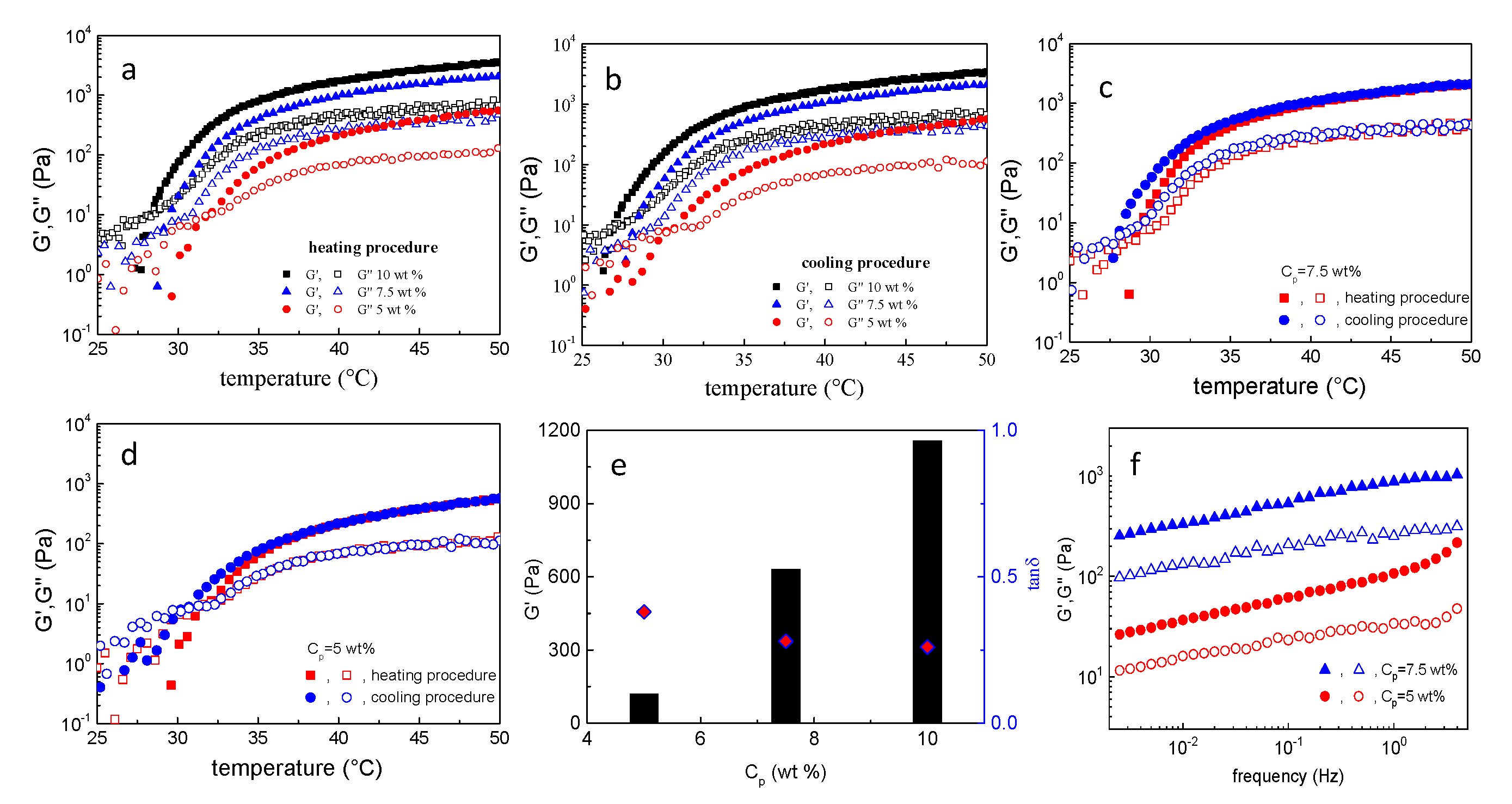
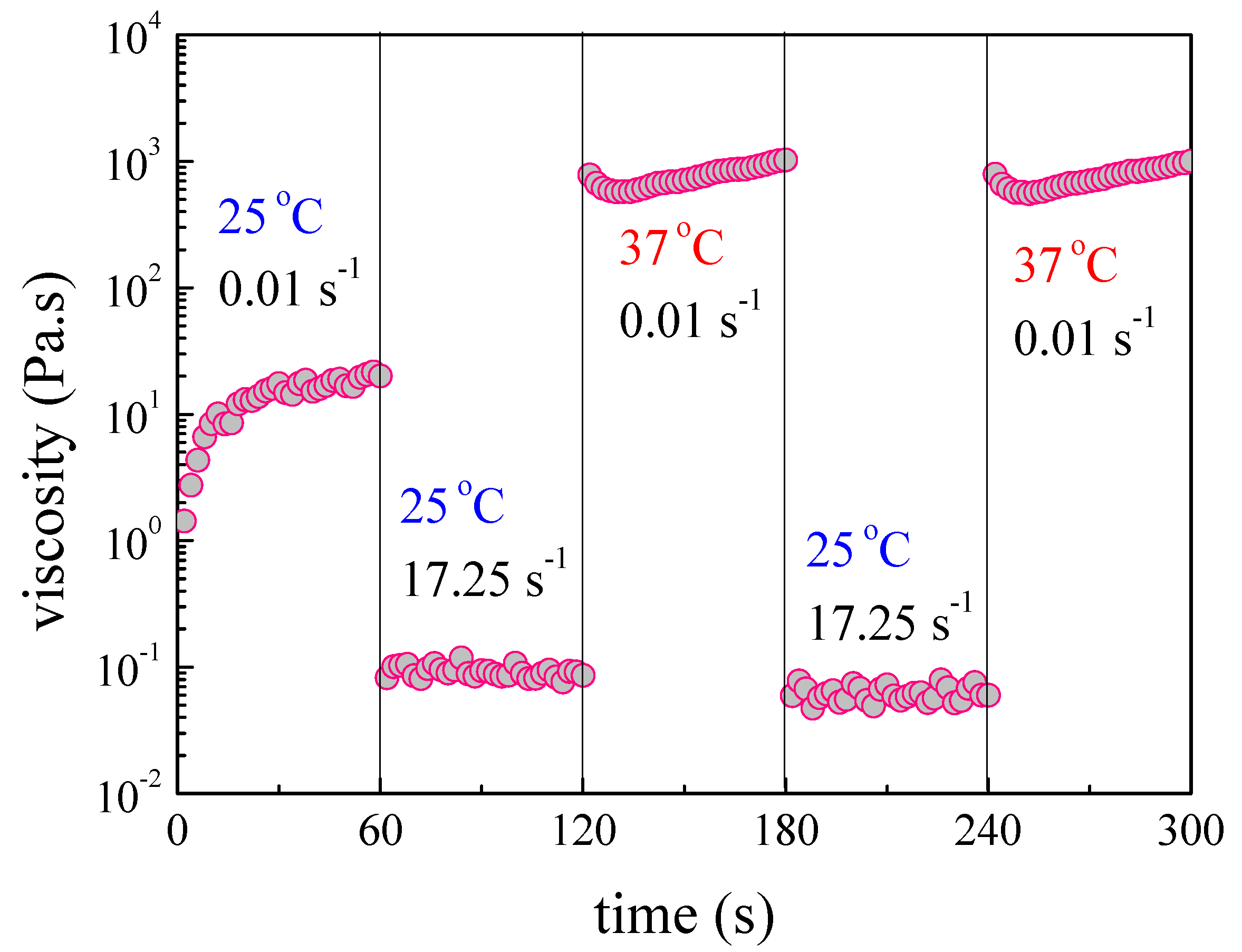
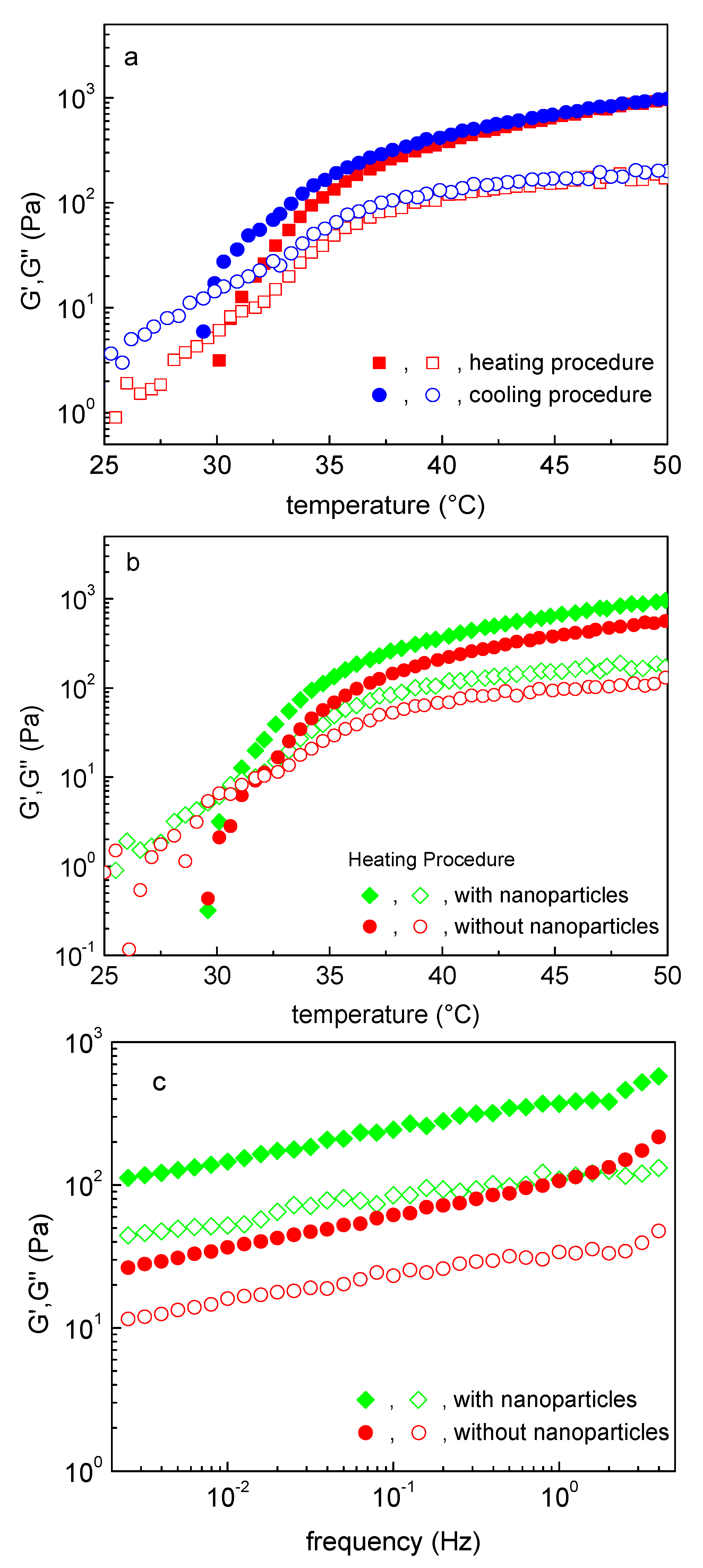
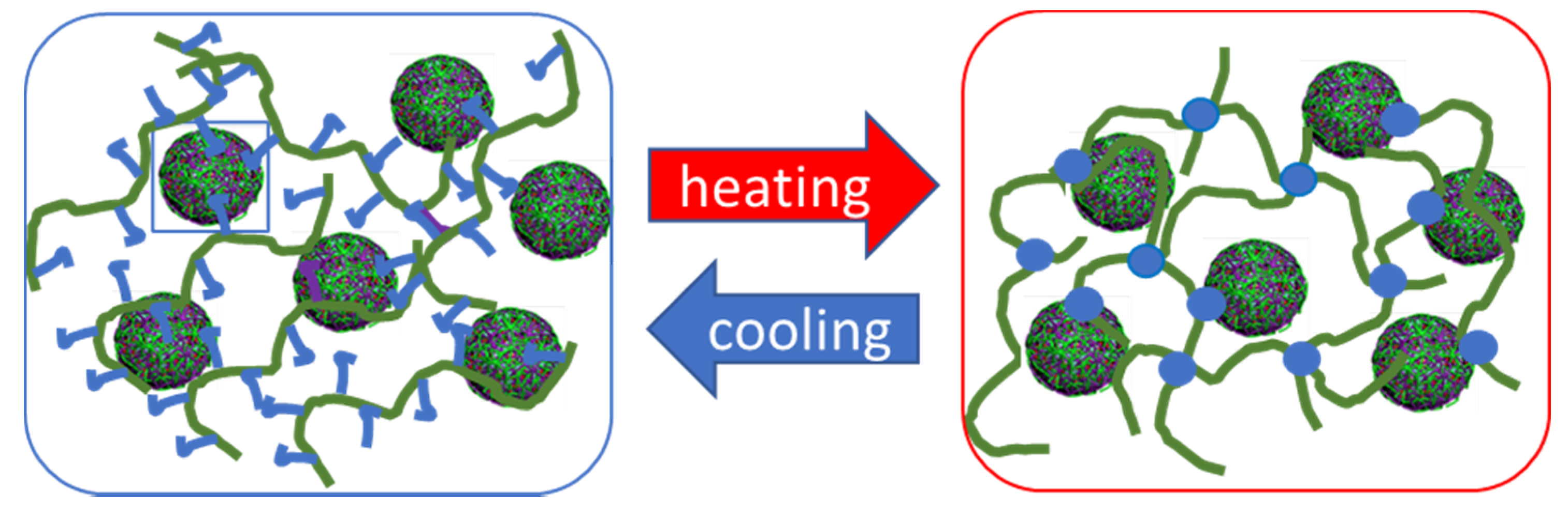
3.2. NaALG-g Rheological Properties: Thermo-Induced Gelation, Injectability
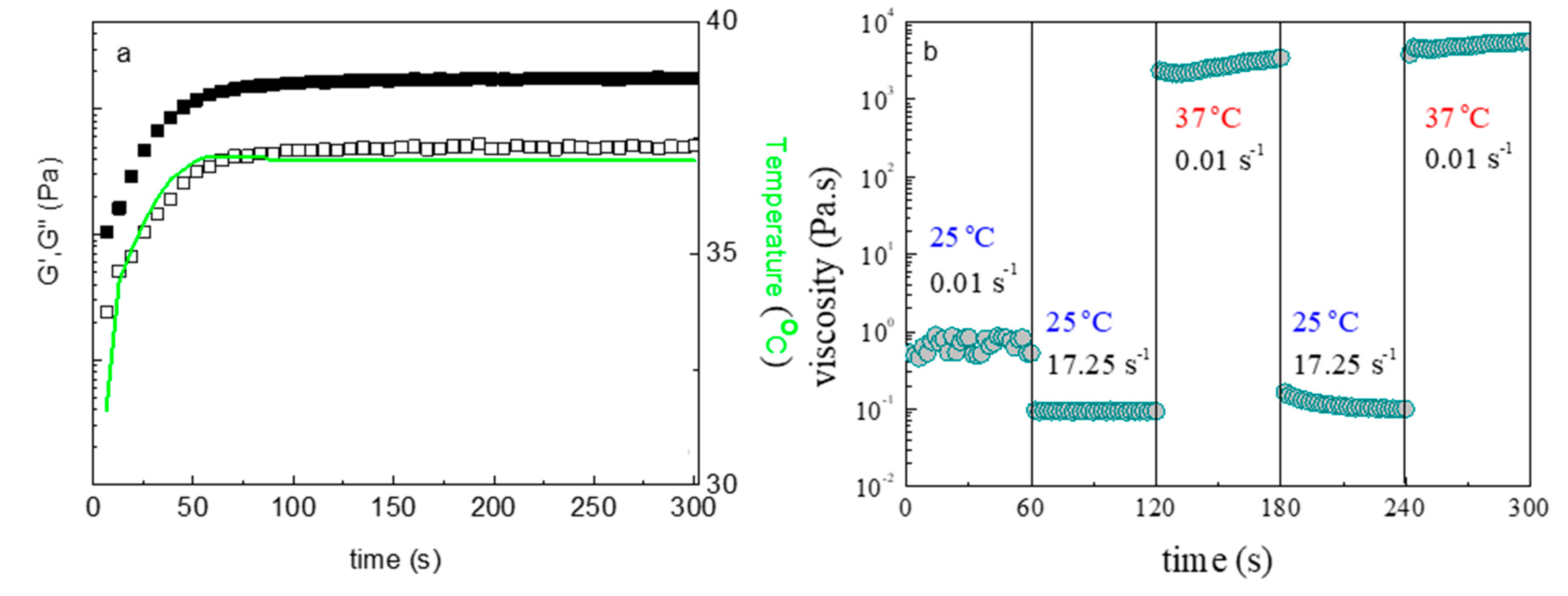
3.3. Nanoparticle/Hydrogel Composite
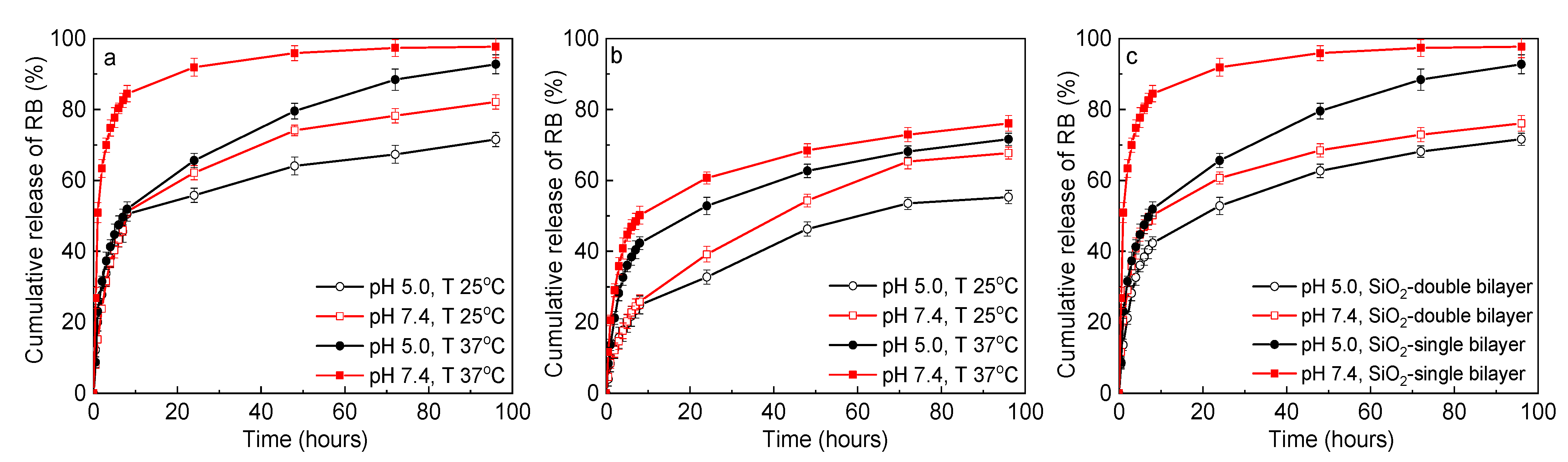
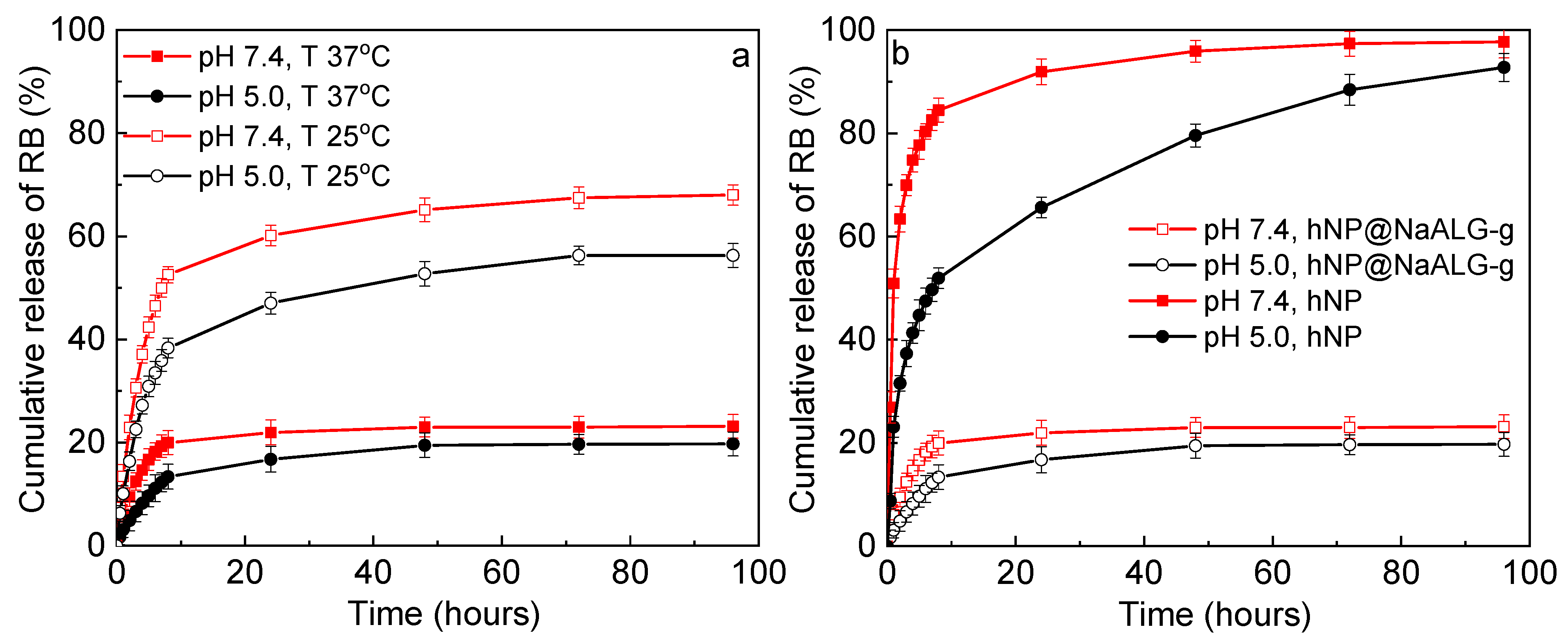
3.4. Controlled RB Release In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, Biodistribution, and Drug-Delivery Effciency of Mesoporous Silica Nanoparticles for Cancer Therapy in Animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Hung, Y.; Mou, C.-Y. Mesoporous silica nanoparticles as nanocarriers. Chem. Commun. 2011, 47, 9972–9985. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, J. Mesoporous silica nanoparticle based nano drug delivery systems: Synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem. 2011, 21, 5845–5855. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.T.; Cheng, S.H.; Souris, J.S.; Chen, C.T.; Mou, C.Y.; Lo, L.W. Theranostic Applications of Mesoporous Silica Nanoparticles and Their Organic/Inorganic Hybrids. J. Mater. Chem. B 2013, 1, 3128–3135. [Google Scholar] [CrossRef]
- Colilla, M.; Gonzáleza, B.; Vallet-Regí, M. Mesoporous silica nanoparticles for the design of smart delivery nanodevices. Biomater. Sci. 2013, 1, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef]
- Carvalho, G.C.; Sábio, R.M.; Ribeiro, T.d.C.; Monteiro, A.S.; Pereira, D.V.; Ribeiro, S.J.L.; Chorilli, M. Highlights in Mesoporous Silica Nanoparticles as a Multifunctional Controlled Drug Delivery Nanoplatform for Infectious Diseases Treatment. Pharm. Res. 2020, 37, 191. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, H.; Khavandi, A.; Alamolhoda, S.; Naimi-Jamal, M.R. pH-Sensitive magnetite mesoporous silica nanocomposites for controlled drug delivery and hyperthermia. RSC Adv. 2020, 10, 39008–39016. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Li, T.; Shi, S.; Goel, S.; Shen, X.; Xie, X.; Chen, Z.; Zhang, H.; Li, S.; Qin, X.; Yang, H.; et al. Recent advancements in mesoporous silica nanoparticles towards therapeutic applications for cancer. Acta Biomater. 2019, 89, 1–13. [Google Scholar] [CrossRef]
- Iatridi, Z.; Evangelatou, K.; Theodorakis, N.; Angelopoulou, A.; Avgoustakis, K.; Tsitsilianis, C. Multicompartmental Mesoporous Silica/Polymer Nanostructured Hybrids: Design Capabilities by Integrating Linear and Star-shaped Block Copolymers. Polymers 2020, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Nik, A.B.; Zare, H.; Razavi, S.; Mohammadi, H.; Ahmadi, P.T.; Yazdani, N.; Bayandori, M.; Rabiee, N.; Mobarake, J.I. Smart drug delivery: Capping strategies for mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2020, 299, 110115. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Cattelan, G.; Gerbolés, A.G.; Foresti, R.; Pramstaller, P.P.; Rossini, A.; Miragoli, M.; Malvezzi, C.C. Alginate Formulations: Current Developments in the Race for Hydrogel-Based Cardiac Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zhang, B.; Nie, X.; Cheng, Y.; Hu, Z.; Liao, M.; Li, S. A sodium alginate-based sustained-release IPN hydrogel and its applications. RSC Adv. 2020, 10, 39722–39730. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C.G.; Huang, Z.H.; Xue, F.F. Preparation and characterization of nanoparticles based on hydrophobic alginate derivative as carriers for sustained release of vitamin D3. J. Agric. Food Chem. 2011, 59, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Song, X.; Wen, Y.; Zhu, J.-L.; Li, J. Injectable Thermoresponsive Hydrogel Formed by Alginate-g-Poly(Nisopropylacrylamide) Releasing Doxorubicin-Encapsulated Micelles as Smart Drug Delivery System. ACS Appl. Mater. Interfaces 2017, 9, 35673–35682. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.S.; Nayak, A.M. Alginates: Versatile Polymers in Biomedical Applications and Therapeutics, 1st ed.; Taylor and Francis: Boca Raton, FL, USA, 2019; pp. 1–646. [Google Scholar]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-based composites for environmental applications: A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 318–356. [Google Scholar] [CrossRef]
- Lin, X.; Ma, Q.; Su, J.; Wang, C.; Kankala, R.K.; Zeng, M.; Lin, H.; Zhou, S.-F. Dual-Responsive Alginate Hydrogels for Controlled Release of Therapeutics. Molecules 2019, 24, 2089. [Google Scholar] [CrossRef] [PubMed]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I.; Desbrieres, J.; Riess, G. Micellization of synthetic and polysaccharides-based graft copolymers in aqueous media. Prog. Polym. Sci. 2017, 73, 32–60. [Google Scholar]
- Atanase, L.I. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, S.; Liu, Y.; Geng, Q.; Ding, G.; Guo, M.; Deng, Y.; Zhu, J.; Li, J.; Cao, Y. Preparation and Characterization of Novel Functionalized Prochloraz Microcapsules Using Silica−Alginate−Elements as Controlled Release Carrier Materials. ACS Appl. Mater. Interfaces 2014, 6, 11783–11790. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yu, G.; He, F.; Zhou, Q.; Xiao, D.; Li, J.; Feng, Y. A pH-responsive emulsion stabilized by alginate-grafted anisotropic silica and its application in the controlled release of λ-cyhalothrin. Carbohydr. Polym. 2017, 176, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.-N.; Li, S.-J.; Li, G.-Q. Sodium alginate coated mesoporous silica for dual bio-responsive controlled drug Delivery. J. Drug Deliv. Sci. Technol. 2018, 46, 348–353. [Google Scholar] [CrossRef]
- de Lima, H.H.C.; Kupfer, V.L.; Moisés, M.P.; Guilherme, M.R.; Rinald, J.; Felisbino, S.L.; Rubira, A.F.; Rinaldi, A.W. Bionanocomposites based on mesoporous silica and alginate for enhanced drug delivery. Carbohydr. Polym. 2018, 196, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-T.; Liu, C.-H.; Yu, J.; Wu, K.C.-W. Liver cancer cells: Targeting and prolonged-release drug carriers consisting of mesoporous silica nanoparticles and alginate microspheres. Int. J. Nanomed. 2014, 9, 2767–2778. [Google Scholar]
- Feng, W.; Nie, W.; He, C.; Zhou, X.; Chen, L.; Qiu, K.; Wang, W.; Yin, Z. Effect of pH-Responsive Alginate/Chitosan Multilayers Coating on Delivery Efficiency, Cellular Uptake and Biodistribution of Mesoporous Silica Nanoparticles Based Nanocarriers. ACS Appl. Mater. Interfaces 2014, 6, 8447–8460. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Leng, F.; Zheng, L.; Yang, J.; Wang, W.; Huang, C.Z. Autofluorescent and pH-responsive mesoporous silica for cancer-targeted and controlled drug release. Microporous Mesoporous Mater. 2014, 186, 187–193. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Chen, Z.; Geng, Y.; Xie, X.; Shen, X.; Li, T.; Li, S.; Wu, C.; Liu, Y. Chemo-photodynamic combined gene therapy and dual-modal cancer imaging achieved by pH responsive alginate/chitosan multilayer-modified magnetic mesoporous silica nanocomposites. Biomater. Sci. 2017, 5, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Iatridi, Z.; Saravanou, S.F.; Tsitsilianis, C. Injectable self-assembling hydrogel from alginate grafted by P(N-isopropylacrylamide-co-N-tert-butylacrylamide) random copolymers. Carbohydr. Polym. 2019, 219, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, G.; Tsitsilianis, C. LCST polymers: Thermoresponsive nanostructured assemblies towards bioapplications. Polymer 2020, 211, 123146. [Google Scholar] [CrossRef]
- Pertici, V.; Trimaille, T.; Gigmes, D. Inputs of Macromolecular Engineering in the Design of Injectable Hydrogels Based on Synthetic Thermoresponsive Polymers. Macromolecules 2020, 53, 682–692. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release I: Fickian and Non-Fickian Release from Non-Swellable Devices in the Form of Slabs, Spheres, Cylinders or Discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release II: Fickian and Anomalous Release from Swellable Devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release III: Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Lencina, M.M.S.; Iatridi, Z.; Villar, M.A.; Tsitsilianis, C. Thermoresponsive hydrogels from alginate-based graft copolymers. Eur. Polym. J. 2014, 61, 33–44. [Google Scholar] [CrossRef]
- Popescu, M.-T.; Mourtas, S.; Pampalakis, G.; Antimisiaris, S.G.; Tsitsilianis, C. pH-Responsive Hydrogel/Liposome Soft Nanocomposites For Tuning Drug Release. Biomacromolecules 2011, 12, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.A.; Tibbitt, M.W.; Webber, M.J.; Mattix, B.A.; Veiseh, O.; Langer, R. Self-Assembled Hydrogels Utilizing Polymer-Nanoparticle Interactions. Nat. Commun. 2015, 6, 6259. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.-T.; Liontos, G.; Avgeropoulos, A.; Voulgari, E.; Avgoustakis, K.; Tsitsilianis, C. Injectable hydrogel: Amplifying the pH sensitivity of a triblock copolypeptide by conjugating the N-termini via dynamic covalent bonding. ACS Appl. Mater. Interfaces 2016, 8, 17539–17548. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, P.; Wang, W.; Feng, Z.; Zhang, J.; Deng, L.; Dong, A. An injectable nanocomposite hydrogel co-constructed with gold nanorods and paclitaxel-loaded nanoparticles for local chemo-photothermal synergetic cancer therapy. J. Mater. Chem. B 2019, 7, 2667–2677. [Google Scholar] [CrossRef] [PubMed]
- Fenton, O.S.; Tibbitt, M.W.; Appel, E.A.; Jhunjhunwala, S.; Webber, M.J.; Langer, R. Injectable Polymer−Nanoparticle Hydrogels for Local Immune Cell Recruitment. Biomacromolecules 2019, 20, 4430–4436. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, J.; Huang, W.; Yan, B.; Peng, Q.; Liu, J.; Chen, L.; Zeng, H. Injectable and Self-Healing Nanocomposite Hydrogels with Ultrasensitive pH-Responsiveness and Tunable Mechanical Properties: Implications for Controlled Drug Delivery. Biomacromolecules 2020, 21, 2409–2420. [Google Scholar] [CrossRef]
- Nutan, B.; Singh Chandel, A.K.; Biswas, A.; Kumar, A.; Yadav, A.; Maiti, P.; Jewrajka, S.K. Gold Nanoparticle Promoted Formation and Biological Properties of Injectable Hydrogels. Biomacromolecules 2020, 21, 3782–3794. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; He, X.; Shi, K.; Yuan, L.; Yang, Y.; Liu, Q.; Ming, Y.; Yi, C.; Qian, Z. Injectable Thermosensitive Hydrogel Containing Erlotinib-Loaded Hollow Mesoporous Silica Nanoparticles as a Localized Drug Delivery System for NSCLC Therapy. Adv. Sci. 2020, 7, 2001442. [Google Scholar] [CrossRef]
- Ye, Y.N.; Cui, K.; Indei, T.; Nakajima, T.; Hourdet, D.; Kurokawa, T.; Gong, J.P. Relaxation Dynamics and Underlying Mechanism of a Thermally Reversible Gel from Symmetric Triblock Copolymer. Macromolecules 2019, 52, 8651–8661. [Google Scholar] [CrossRef]
- Jung, H.; Gang, S.-E.; Kim, J.-M.; Heo, T.-Y.; Lee, S.; Shin, E.; Kim, B.-S.; Choi, S.-H. Regulating Dynamics of Polyether-Based Triblock Copolymer Hydrogels by End-Block Hydrophobicity. Macromolecules 2020, 53, 10339–10348. [Google Scholar] [CrossRef]
- Tanaka, F.; Edwards, S.F. Viscoelastic Properties of Physically Crosslinked Networks. 1. Transient Network Theory. Macromolecules 1992, 25, 1516–1523. [Google Scholar] [CrossRef]
- Zengin, A.; Castro, J.P.O.; Habibovic, P.; van Rijt, S.H. Injectable, self-healing mesoporous silica nanocomposite hydrogels with improved mechanical properties. Nanoscale 2021, 13, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Peppas, N.A. Effect of the Morphology of the hydrophilic Polymeric Matrices on the Diffusion and Release of water Soluble Drugs. J. Membr. Sci. 1981, 9, 211–227. [Google Scholar] [CrossRef]
- Noda, T.; Okuda, T.; Mizuno, R.; Ozeki, T.; OkamotoBiol, H. Two-Step Sustained-Release PLGA/Hyaluronic Acid Gel Formulation for Intra-articular Administration. Pharm. Bull. 2018, 41, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Serra, L.; Domenechc, J.; Peppas, N.A. Drug transport mechanisms and release kinetics from molecularly designed poly(acrylic acid-g-ethylene glycol) hydrogels. Biomaterials 2006, 27, 5440–5451. [Google Scholar] [CrossRef] [PubMed]


| Sample | Analysis in the Time Range: 0–8 h | ||
|---|---|---|---|
| k1 (hr-n) | n | R2 | |
| pH 5.0, RBhNP (SiO2-single bilayer) | 0.238 | 0.386 | 0.994 |
| pH 7.4, RBhNP (SiO2-single bilayer) | 0.526 | 0.239 | 0.981 |
| pH 5.0, RB/SiO2-double bilayer | 0.029 | 0.547 | 0.983 |
| pH 7.4, RB/SiO2-double bilayer | 0.029 | 0.435 | 0.985 |
| pH 5.0, RBhNP@NaALG-g | 0.029 | 0.738 | 0.999 |
| pH 7.4, RBhNP@NaALG-g | 0.056 | 0.656 | 0.984 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodorakis, N.; Saravanou, S.-F.; Kouli, N.-P.; Iatridi, Z.; Tsitsilianis, C. pH/Thermo-Responsive Grafted Alginate-Based SiO2 Hybrid Nanocarrier/Hydrogel Drug Delivery Systems. Polymers 2021, 13, 1228. https://doi.org/10.3390/polym13081228
Theodorakis N, Saravanou S-F, Kouli N-P, Iatridi Z, Tsitsilianis C. pH/Thermo-Responsive Grafted Alginate-Based SiO2 Hybrid Nanocarrier/Hydrogel Drug Delivery Systems. Polymers. 2021; 13(8):1228. https://doi.org/10.3390/polym13081228
Chicago/Turabian StyleTheodorakis, Nikolaos, Sofia-Falia Saravanou, Nikoleta-Paraskevi Kouli, Zacharoula Iatridi, and Constantinos Tsitsilianis. 2021. "pH/Thermo-Responsive Grafted Alginate-Based SiO2 Hybrid Nanocarrier/Hydrogel Drug Delivery Systems" Polymers 13, no. 8: 1228. https://doi.org/10.3390/polym13081228
APA StyleTheodorakis, N., Saravanou, S.-F., Kouli, N.-P., Iatridi, Z., & Tsitsilianis, C. (2021). pH/Thermo-Responsive Grafted Alginate-Based SiO2 Hybrid Nanocarrier/Hydrogel Drug Delivery Systems. Polymers, 13(8), 1228. https://doi.org/10.3390/polym13081228









