All-Solid-State Potentiometric Ion-Sensors Based on Tailored Imprinted Polymers for Pholcodine Determination
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
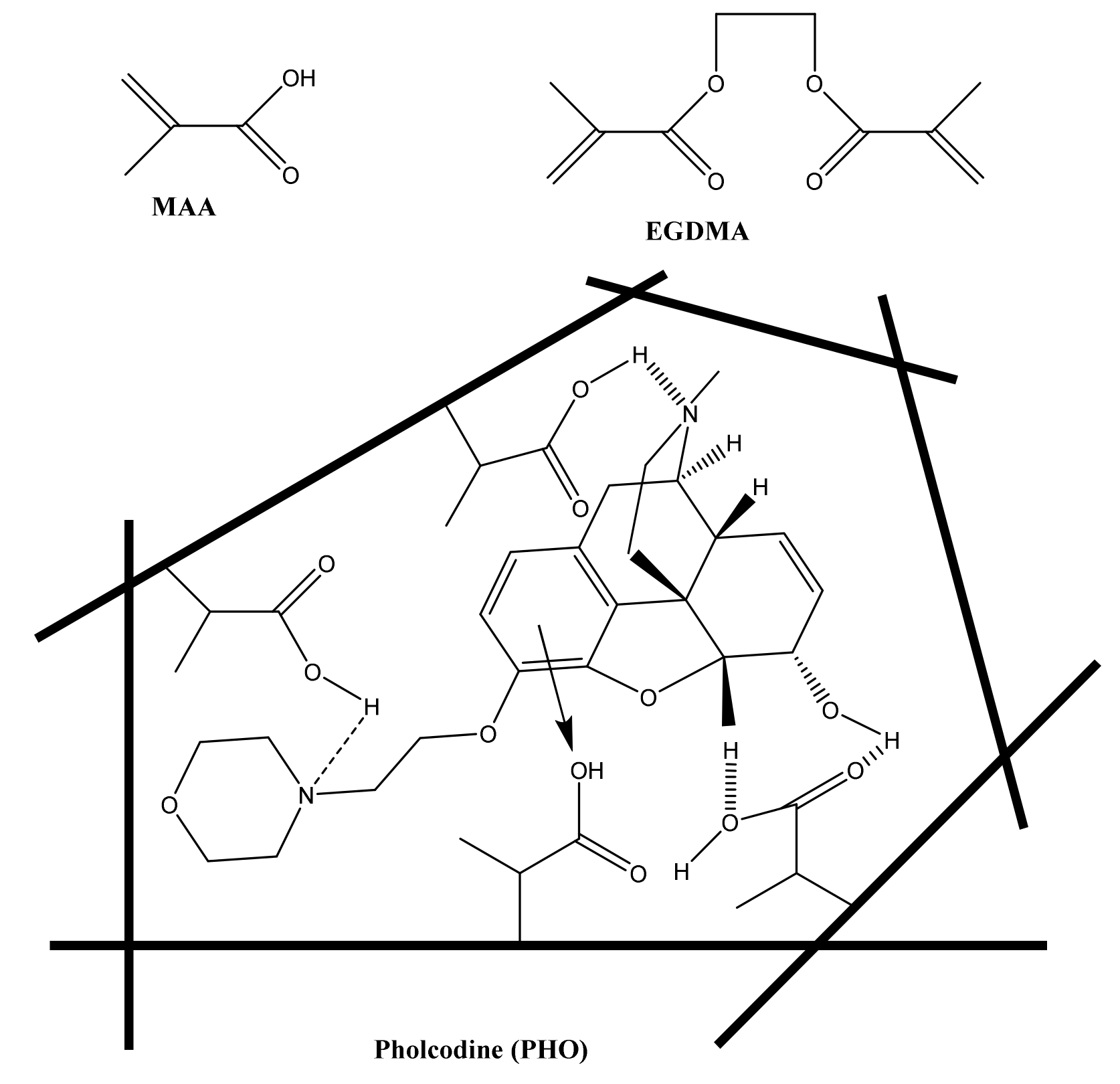
2.2. Reagents and Chemicals
2.3. Sensor Construction
3. Results and Discussion
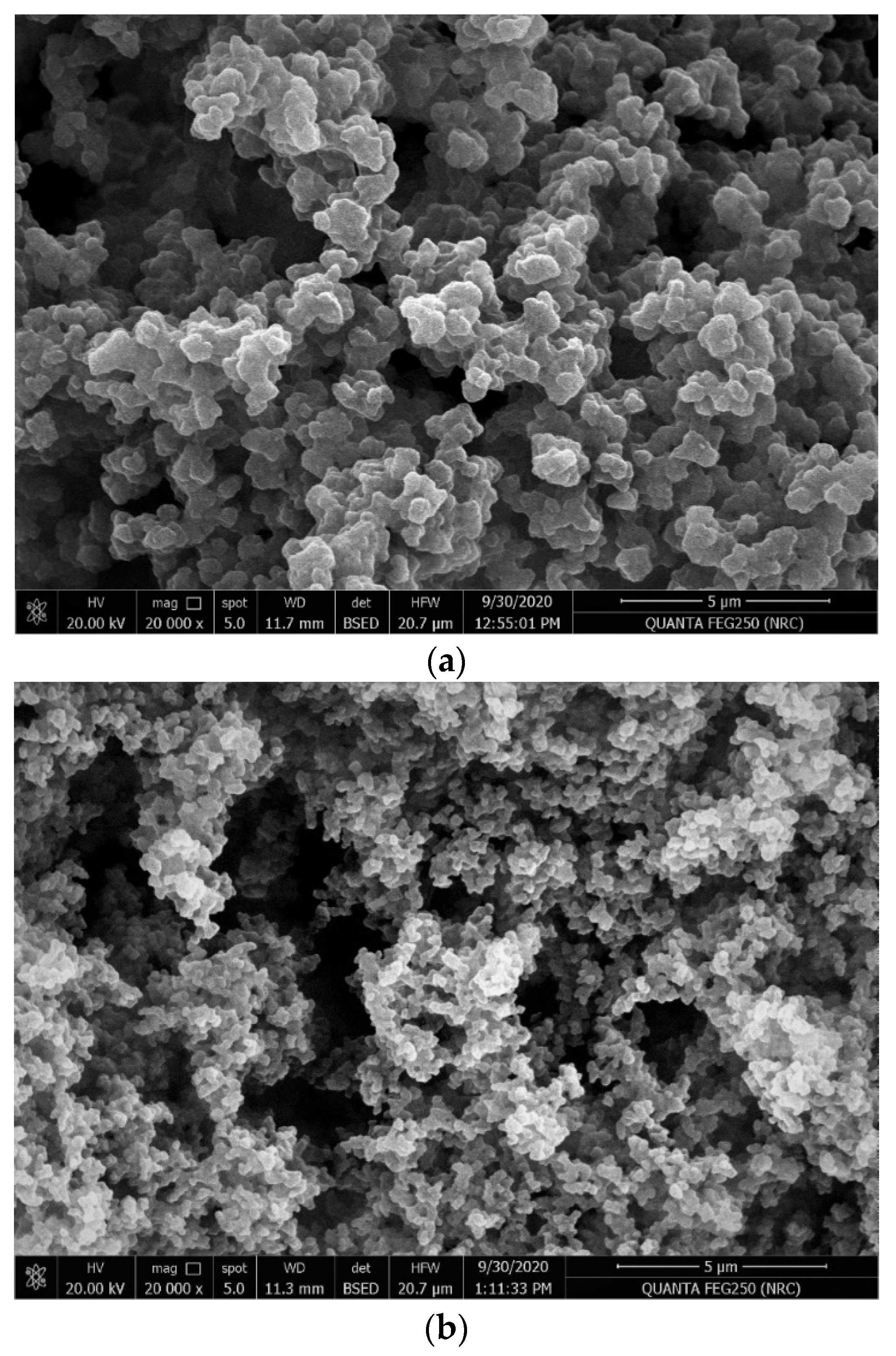
3.1. MIP Synthesis and Characterization
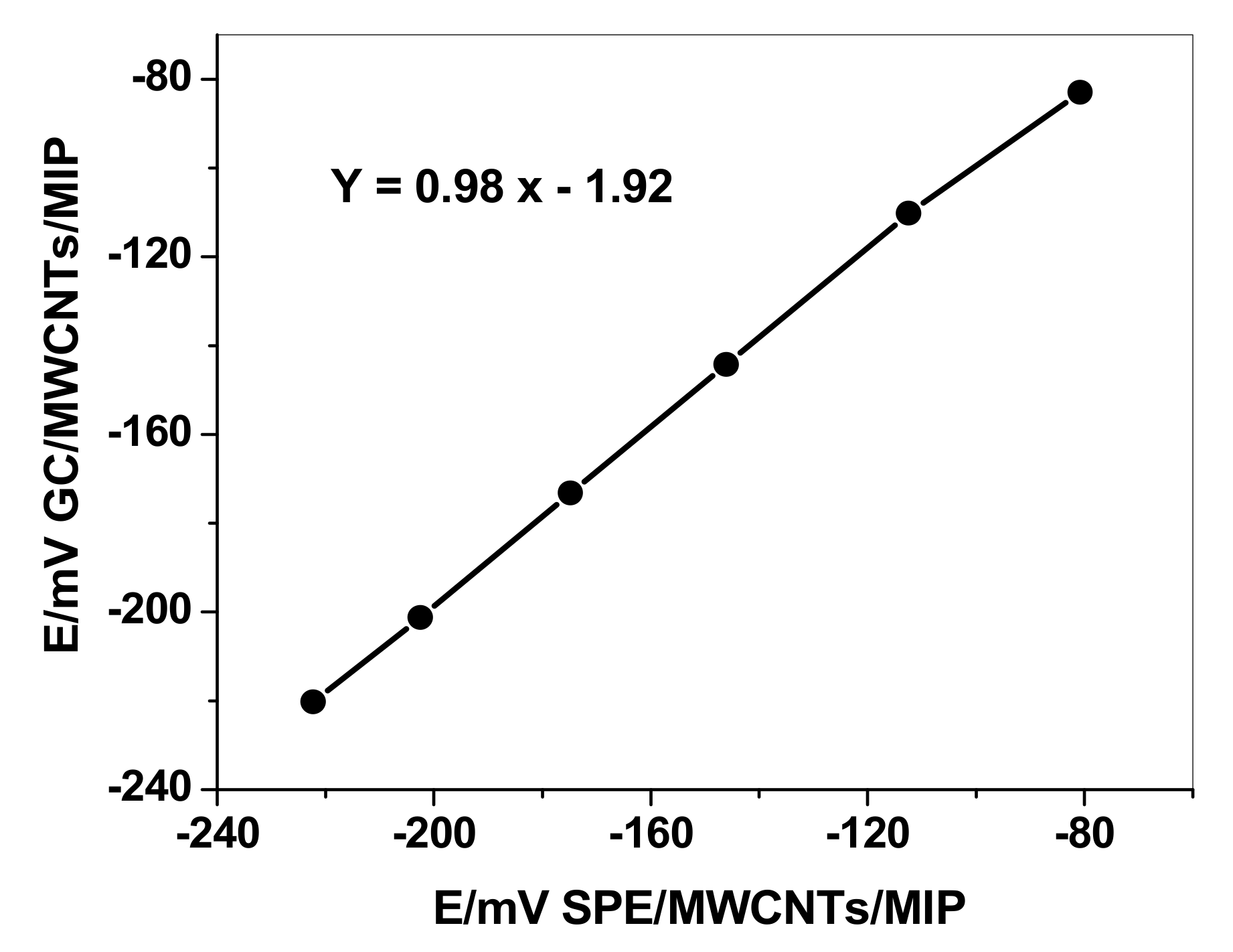
3.2. Potentiometric Characteristics of the Sensors
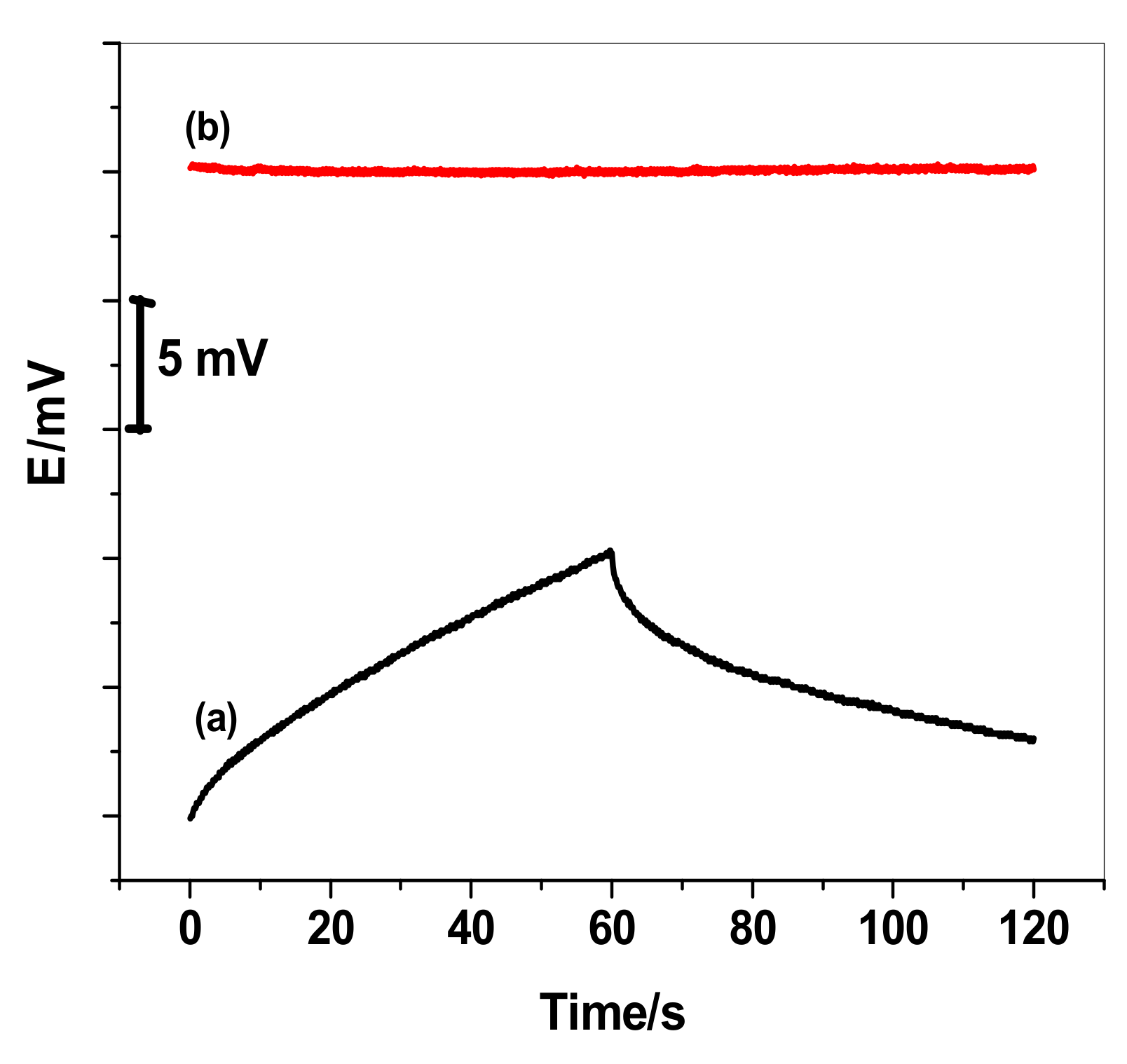
3.3. Potential Stability
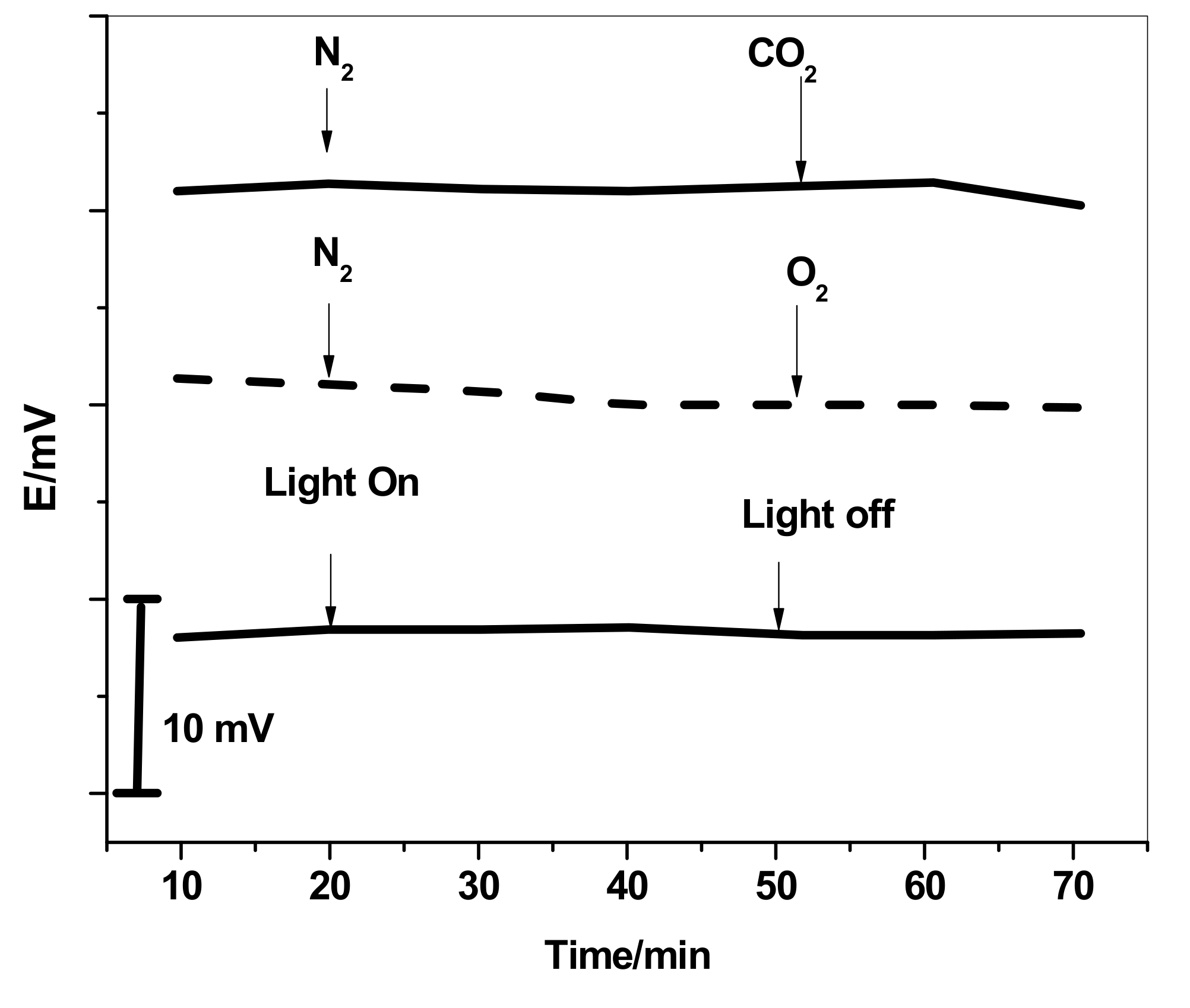
3.4. Effects of Light, O2, and CO2
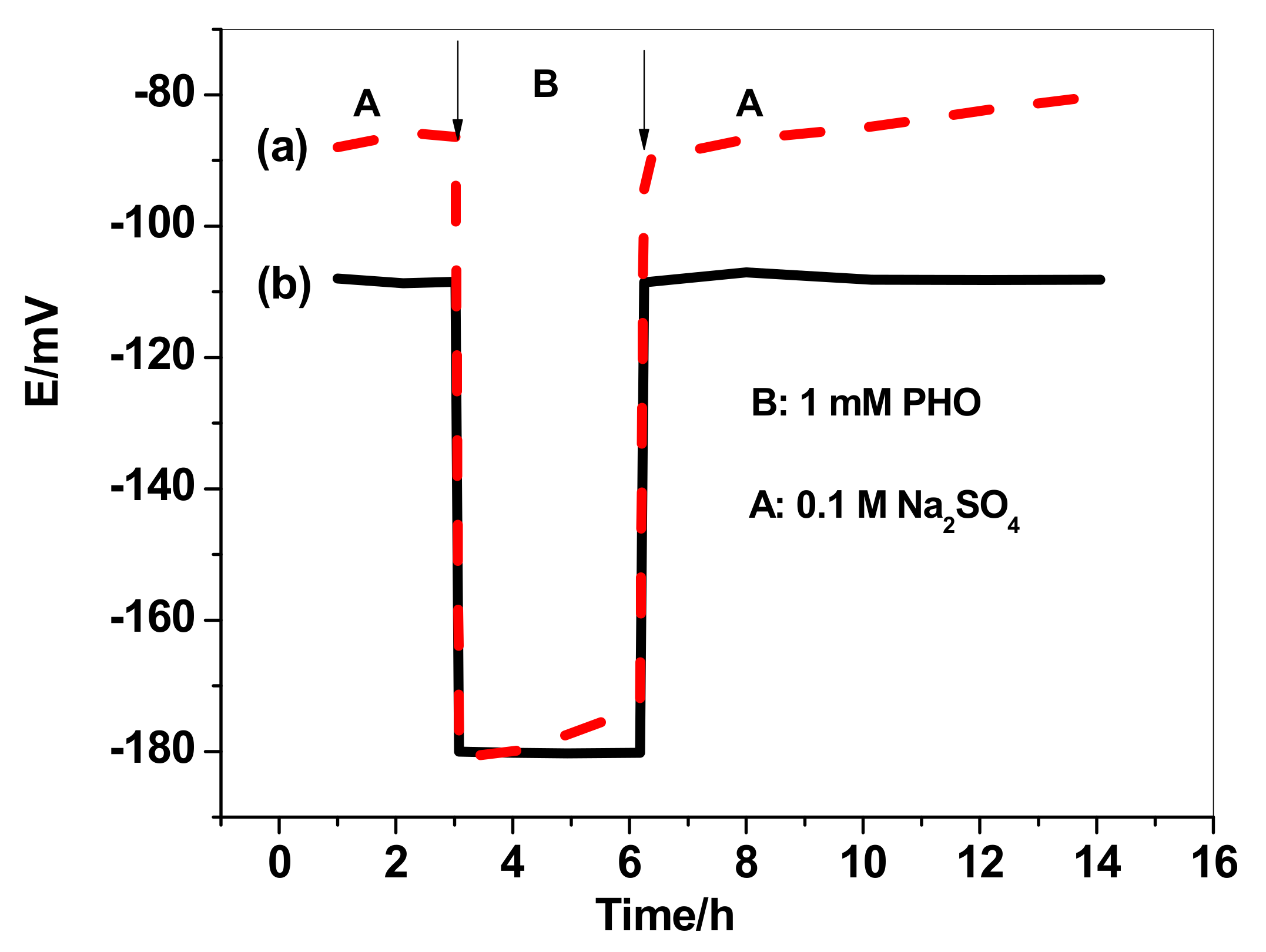
3.5. Water-Layer Test
3.6. Analytical Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Z.R.; Bochner, F.; Somogyi, A. Pharmacokinetics of pholcodine in healthy volunteers: Single and chronic dosing studies. Br. J. Clin. Pharmacol. 1988, 26, 445–453. [Google Scholar] [CrossRef]
- Florvaag, E.; Johansson, S.G.O. The pholcodine story. Immunol. Allergy Clinics N. Am. 2009, 29, 419–427. [Google Scholar] [CrossRef]
- Florvaag, E.; Johansson, S.G. The pholcodine case: Cough medicines, IgE sensitization, and anaphylaxis: A devious connection. World Allergy Organ. J. 2012, 5, 73–78. [Google Scholar] [CrossRef]
- Shi, B.; Min, H. Comparative study on determination of pholcodine by non-aqueous titration method and potentiometric titration. Chin. Pharm. Aff. 2011, 4, 23–29. [Google Scholar]
- Delouei, N.J.; Mokhtari, A.; Jamali, M.R. Determination of pholcodine in syrups and human plasma using the chemiluminescence system of tris (1,10 phenanthroline) ruthenium (II) and acidic Ce(IV). Luminescence 2017, 32, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Wen-Bing, S. Flow-injection chemiluminescence analysis of pholcodine by microemulsions. Chin. J. Anal. Lab. 2008, 6, 12. [Google Scholar]
- Moustafa, A.A.; Hegazy, M.A.; Mohamed, D.; Ali, O. Novel approach for the simultaneous determination of carbinoxamine maleate, pholcodine, and ephedrine hydrochloride without interference from coloring matter in an antitussive preparation using smart spectrophotometric methods. J. AOAC Int. 2018, 101, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Andresen, A.T.; Krogh, M.; Rasmussen, K.E. On-line dialysis and weak cation-exchange enrichment of dialysate: Automated high-performance liquid chromatography of pholcodine in human plasma and whole blood. J. Chromatogr. B 1992, 582, 123–130. [Google Scholar] [CrossRef]
- Johansen, M.; Rasmussen, K.E.; Christophersen, A.S. Determination of pholcodine and its metabolites in urine by capillary gas chromatography. J. Chromatogr. B 1990, 532, 277–284. [Google Scholar] [CrossRef]
- Meadway, C.; George, S.; Braithwaite, R. Interpretation of GC–MS opiate results in the presence of pholcodine. Forensic Sci. Int. 2002, 127, 131–135. [Google Scholar] [CrossRef]
- Maurer, H.H.; Fritz, C.F. Toxicological detection of pholcodine and its metabolites in urine and hair using radio immunoassay, fluorescence polarisation immunoassay, enzyme immunoassay and gas chromatography-mass spectrometry. Int. J. Leg. Med. 1990, 104, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Petkovska, A.; Babunovska, H.; Stefova, M. Fast and selective HPLC-DAD method for determination of pholcodine and related substances. Maced. J. Chem. Chem. Eng. 2011, 30, 139–150. [Google Scholar] [CrossRef]
- Johansen, M.; Tønnesen, F.; Rasmussen, K.E. Column-switching high-performance liquid chromatographic detection of pholcodine and its metabolites in urine with fluorescence and electrochemical detection. J. Chromatogr. B 1992, 573, 283–288. [Google Scholar] [CrossRef]
- Mansour, F.R.; Khairy, M.A. Stability indicating RP-HPLC method for simultaneous determination of pholcodine and guaiacol in pharmaceutical syrup. Pharm. Chem. J. 2019, 53, 775–779. [Google Scholar] [CrossRef]
- Acevska, J.; Dimitrovska, A.; Stefkov, G.; Brezovska, K.; Marija Karapandzova, M.; Kulevanova, S. Development and validation of a reversed-Phase HPLC method for determination of alkaloids from Papaver somniferum L. (Papaveraceae). J. AOAC Int. 2012, 95, 399–405. [Google Scholar] [CrossRef]
- Hongpei, C.; Derong, W.; Xiaojing, S. Assay of compound pholcodine syrup by HPLC. Chin. J. Pharm. Anal. 1999, 19, 397–398. [Google Scholar]
- Berg, T.; Lundanes, E.; Christophersen, A.S.; Strand, D.H. Determination of opiates and cocaine in urine by high pH mobile phase reversed phase UPLC–MS/MS. J. Chromatogr. B 2009, 877, 421. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.A.; Salem, H.; Hegazy, M.; Mahmoud, O.A. Simultaneous determination of carbinoxamine, pholcodine, and ephedrine in antitussive preparation by high-performance liquid chromatography and thin-layer chromatography–densitometry. J. Planar Chromatogr. Mod. TLC 2015, 28, 307–315. [Google Scholar] [CrossRef]
- Taylor, R.; Low, A.; Reid, R. Determination of opiates in urine by capillary electrophoresis. J. Chromatogr. B 1996, 675, 213–223. [Google Scholar] [CrossRef]
- Chatten, L.G. Recent applications of electrochemical techniques to the analysis of pharmaceuticals. J. Pharm. Biomed. Anal. 1983, 1, 491–495. [Google Scholar] [CrossRef]
- Kovács, Z.; Hosztafi, S.; Noszál, B. Site-specific acid–base properties of pholcodine and related compounds. Anal. Bioanal. Chem. 2006, 386, 1709–1716. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Badr, I.H.A.; Kamel, A.H.; Mohammad, M.S. A novel poly(vinyl chloride) matrix membrane sensor for batch and flow-injection determinations of thiocyanate, cyanide and some metal ions. Anal. Sci. 2009, 25, 911–917. [Google Scholar] [CrossRef]
- Kamel, A.H.; Hassan, A.M.E. Solid contact potentiometric sensors based on host-tailored molecularly imprinted polymers for creatine assessment. Int. J. Electrochem. Sci. 2016, 11, 8938–8949. [Google Scholar] [CrossRef]
- El-Naby, E.H.; Kamel, A.H. Potential transducers based man-tailored biomimetic sensors for selective recognition of dextromethorphan as an antitussive drug. Mater. Sci. Eng. C 2015, 54, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.H.; Galal, H.R. MIP-based biomimetic sensors for static and hydrodynamic potentiometric transduction of sitagliptin in biological fluids. Int. J. Electrochem. Sci. 2014, 9, 4361–4373. [Google Scholar]
- Kamel, A.H.; Soror, T.Y.; Al-Romian, F.M. Graphite solid-contact mepiquat potentiometric sensors based on molecularly imprinted polymers and their application to flow through analysis. Anal. Methods 2012, 4, 3007–3012. [Google Scholar] [CrossRef]
- Abd-Rabboh, H.S.M.; Kamel, A.H. Mimicking receptor for cyanide based on ion imprinting and their applications in potential transduction. Electroanalysis 2012, 24, 1409–1415. [Google Scholar] [CrossRef]
- Kamel, A.H.; Mahmoud, W.H.; Mostafa, M.S. Biomimetic ciprofloxacin sensors made of molecularly imprinted network receptors for potential measurements. Anal. Methods 2011, 3, 957–964. [Google Scholar] [CrossRef]
- Kamel, A.H.; Sayour, H.E.M. Miniaturized potentiometric sensors based on molecularly imprinted polymers for flow-through assay of quinine in soft drinks and urine. Electroanalysis 2009, 21, 2701–2708. [Google Scholar] [CrossRef]
- El-Kosasy, A.M.; Kamel, A.H.; Hussin, L.H.; Ayad, M.F.; Fares, N.V. Mimicking new receptors based on molecular imprinting and their application to potentiometric assessment of 2, 4-dichlorophenol as a food taint. Food Chem. 2018, 250, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Jasmin, J.P.; Miserque, F.; Dumas, E.; Vickridge, I.; Ganem, J.J.; Cannizzo, C.; Chausse, A. XPS and NRA investigations during the fabrication of gold nanostructured functionalized screen-printed sensors for the detection of metallic pollutants. Appl. Surf. Sci. 2017, 397, 159–166. [Google Scholar] [CrossRef]
- Liu, X.; Yao, Y.; Ying, Y.; Ping, J. Recent advances in nanomaterial-enabled screen-printed electrochemical sensors for heavy metal detection. TrAC Trends Anal. Chem. 2019, 115, 187–202. [Google Scholar] [CrossRef]
- Yin, T.; Yu, H.; Ding, J.; Qin, W. An integrated screen-printed potentiometric strip for determination of Ca2+ in seawater. J. Electrochem. Soc. 2019, 166, B589–B593. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Gao, Y.; Wang, P. A general approach to one-step fabrication of single piece nanocomposite membrane based Pb2+-selective electrodes. Sens. Actuators B Chem. 2019, 281, 705–712. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Fan, K.; Tang, W.; Wu, J.; Ying, Y. High-performance flexible potentiometric sensing devices using free-standing graphene paper. J. Mater. Chem. B 2013, 1, 4781–4791. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ying, Y.; Ping, J. Recent advances in solid-contact ion-selective electrodes: Functional materials, transduction mechanisms, and development trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef]
- Zdrachek, E.; Bakker, E. Potentiometric sensing. Anal. Chem. 2019, 91, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liang, R.; Wang, F.; Ding, J.; Qin, W. An all-solid-state potentiometric microelectrode for detection of copper in coastal sediment pore water. Sens. Actuators B Chem. 2019, 279, 369–373. [Google Scholar] [CrossRef]
- Ghosh, T.; Chung, H.J.; Rieger, J. All-solid-state sodium-selective electrode with a solid contact of chitosan/prussian blue nanocomposite. Sensors 2017, 17, 2536. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, H.; Shi, L.; Li, D.; Long, Y. Ion-selective gold–thiol film on integrated screen-printed electrodes for analysis of Cu(Ⅱ) ions. Analyst 2014, 139, 643–648. [Google Scholar] [CrossRef]
- Rius-Ruiz, F.X.; Crespo, G.A.; Bejarano-Nosas, D.; Blondeau, P.; Riu, J.; Rius, F.X. Potentiometric strip cell based on carbon nanotubes as transducer layer: Toward low-cost decentralized measurements. Anal. Chem. 2011, 83, 8810–8815. [Google Scholar] [CrossRef]
- Wei, Y.B.; Tang, Q.; Gong, C.B.; Lam, M.H.W. Review of the recent progress in photoresponsive molecularly imprinted polymers containing azobenzene chromophores. Anal. Chim. Acta 2015, 900, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Wulff, G. Enzyme-like catalysis by molecularly imprinted polymers. Chem. Rev. 2002, 102, 1–28. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, M.Y.; Shen, X.J.; Li, S.J. A cascade-reaction nanoreactor composed of a bifunctional molecularly imprinted polymer that contains Pt nanoparticles. Chem. Eur. J. 2015, 21, 7532–7539. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.D.; Zheng, A.X.; Gong, C.B.; Ma, X.B.; Lam, M.H.W.; Chow, C.F.; Tang, Q. A photoswitchable organocatalyst based on a catalyst-imprinted polymer containing azobenzene. RSC Adv. 2015, 5, 62539–62542. [Google Scholar] [CrossRef]
- Nematollahzadeh, A.; Lindemann, P.; Sun, W.; Stute, J.; Lutkemeyer, D.; Sellergren, B. Robust and selective nano cavities for protein separation: An interpenetrating polymer network modified hierarchically protein imprinted hydrogel. J. Chromatogr. A 2014, 1345, 154–163. [Google Scholar] [CrossRef]
- Li, Z.Y.; Quan, H.J.; Gong, C.B.; Yang, Y.Z.; Tang, Q.; Wei, Y.B.; Ma, X.B.; Lam, M.H.W. Photocontrolled solid-phase extraction of guanine from complex samples using a novel photoresponsive molecularly imprinted polymer. Food Chem. 2015, 172, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Karimian, N.M.; Vagin, M.; Zavar, M.H.A.; Chamsaz, M.; Turner, A.P.F.; Tiwari, A. An ultrasensitive molecularly-imprinted human cardiac troponin sensor. Biosens. Bioelectron. 2013, 50, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Liu, Y.; Yang, Y.; Yu, F.; Liu, J.; Song, H.; Liu, J.; Tang, H.; Ye, B.C.; Sun, Z.P. Novel electrochemical sensing platform based on a molecularly imprinted polymer decorated 3D nanoporous nickel skeleton for ultrasensitive and selective determination of metronidazole. ACS Appl. Mater. Interfaces 2015, 7, 15474–15480. [Google Scholar] [CrossRef]
- Tang, Q.; Nie, Y.T.; Gong, C.B.; Chow, C.F.; Peng, J.D.; Lam, M.H.W. Photo-responsive molecularly imprinted hydrogels for the detection of melamine in aqueous media. J. Mater. Chem. 2012, 22, 19812–19820. [Google Scholar] [CrossRef]
- Kulsing, C.; Knob, R.; Mackac, M.; Junor, P.; Boysen, R.I.; Hearn, M.T.W. Molecular imprinted polymeric porous layers in open tubular capillaries for chiral separations. J. Chromatogr. A 2014, 1354, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Guinovart, T.; Bandodkar, A.J.; Windmiller, J.R.; Andrade, J.R.; Wang, J. A potentiometric tattoo sensor for monitoring ammonium in sweat. Analyst 2013, 138, 7031–7038. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Reimhult, K.; Krozer, A.; Mosbach, K.; Sode, K.; Ye, L. Uniform molecularly imprinted microspheres and nanoparticles prepared by precipitation polymerization: The control of particle size suitable for different analytical applications. Anal. Chim. Acta 2007, 584, 112–121. [Google Scholar] [CrossRef]
- Wang, T.; Liang, R.; Yin, T.; Yao, R.; Qin, W. An all-solid-state imprinted polymer-based potentiometric sensor for determination of bisphenol S. RSC Adv. 2016, 6, 73308–73312. [Google Scholar] [CrossRef]
- Liang, R.N.; Song, D.A.; Zhang, R.M.; Qin, W. Potentiometric sensing of neutral species based on a uniform-sized molecularly imprinted polymer as a receptor. Angew. Chem. Int. Ed. 2010, 49, 2556–2559. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/pholcodine (accessed on 27 March 2021).
- Bakker, E. Determination of improved selectivity coefficients of polymer membrane ion-selective electrodes by conditioning with a discriminated ion. J. Electrochem. Soc. 1996, 143, L83–L85. [Google Scholar] [CrossRef]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Lindner, E.; Cosofret, V.V.; Ufer, S.; Buck, R.P.; Kusy, R.P.; Ash, R.B.; Nagle, H.T. Flexible (Kapton-based) microsensor arrays of high stability for cardiovascular applications. Faraday Trans. 1993, 89, 361–367. [Google Scholar] [CrossRef]
- British Pharmacopoeia, (Ph. Eur. Monograph 0522), Medicines and Healthcare products Regulatory Agency(MHRA). 2013. Available online: https://www.pharmacopoeia.com (accessed on 2 April 2021).

| Interfering Ion, B | log KPotPHO,b ± SD * |
|---|---|
| Morphine | −1.5 ± 0.2 |
| Ethylmorphine | −1.7 ± 0.4 |
| Ephedrine | −3.5 ± 0.3 |
| Codeine | −3.7 ± 0.2 |
| Dextromethorphan | −4.1 ± 0.5 |
| Carbinoxamine | −4.5 ± 0.3 |
| Caffeine | −4.7 ± 0.4 |
| Ketamine | −4.5 ± 0.6 |
| K+ | −5.1 ± 0.4 |
| Ca2+ | −5.5 ± 0.2 |
| Na+ | −5.2 ± 0.1 |
| Sample | Amount Spiked, µM | Amount Found, µM ± SD * | F-Test | |||
|---|---|---|---|---|---|---|
| Potentiometry | Recovery, % | Reference Method, [60] | Recovery, % | |||
| 1 | 1.0 | 0.94 ± 0.04 | 94.0 | 0.97 ± 0.01 | 97 | 2.21 |
| 2 | 2.0 | 1.91 ± 0.3 | 95.5 | 1.98 ± 0.2 | 99.0 | 3.55 |
| 3 | 3.0 | 2.73 ± 0.4 | 91.0 | 3.02 ± 0.3 | 100.6 | 4.25 |
| 4 | 5.0 | 4.65 ± 0.5 | 93.0 | 4.92 ± 0.1 | 98.4 | 2.35 |
| 5 | 10.0 | 9.54 ± 0.4 | 95.4 | 10.13 ± 0.3 | 101.3 | 4.11 |
| Sample | Labeled Amount, mg/mL | Amount Found, mg/mL ± SD * | F-Test | |||
|---|---|---|---|---|---|---|
| Potentiometry | Recovery, % | Reference Method, [60] | Recovery, % | |||
| Cyrinol, Apic Pharm. Co., Egypt (Syrup) | 4.0 | 3.62 ± 0.2 | 90.5 | 3.98 ± 0.2 | 99.5 | 1.61 |
| Marynol, Glaxo Wellcome, Egypt (Suspension) | 4.0 | 3.74 ± 0.3 | 93.5 | 3.93 ± 0.3 | 98.2 | 3.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Rabboh, H.S.M.; E. Amr, A.E.-G.; Almehizia, A.A.; Kamel, A.H. All-Solid-State Potentiometric Ion-Sensors Based on Tailored Imprinted Polymers for Pholcodine Determination. Polymers 2021, 13, 1192. https://doi.org/10.3390/polym13081192
Abd-Rabboh HSM, E. Amr AE-G, Almehizia AA, Kamel AH. All-Solid-State Potentiometric Ion-Sensors Based on Tailored Imprinted Polymers for Pholcodine Determination. Polymers. 2021; 13(8):1192. https://doi.org/10.3390/polym13081192
Chicago/Turabian StyleAbd-Rabboh, Hisham S. M., Abd El-Galil E. Amr, Abdulrahman A. Almehizia, and Ayman H. Kamel. 2021. "All-Solid-State Potentiometric Ion-Sensors Based on Tailored Imprinted Polymers for Pholcodine Determination" Polymers 13, no. 8: 1192. https://doi.org/10.3390/polym13081192
APA StyleAbd-Rabboh, H. S. M., E. Amr, A. E.-G., Almehizia, A. A., & Kamel, A. H. (2021). All-Solid-State Potentiometric Ion-Sensors Based on Tailored Imprinted Polymers for Pholcodine Determination. Polymers, 13(8), 1192. https://doi.org/10.3390/polym13081192








