Synthesis, Optical, Chemical and Thermal Characterizations of PMMA-PS/CeO2 Nanoparticles Thin Film
Abstract
1. Introduction
2. Experimental Details and Techniques
3. Result and Discussion
3.1. Optical Properties of PMMA-PS/Ce NPs Thin Film
3.1.1. Transmittance and Reflectance
3.1.2. Extinction Coefficient and Refractive Index
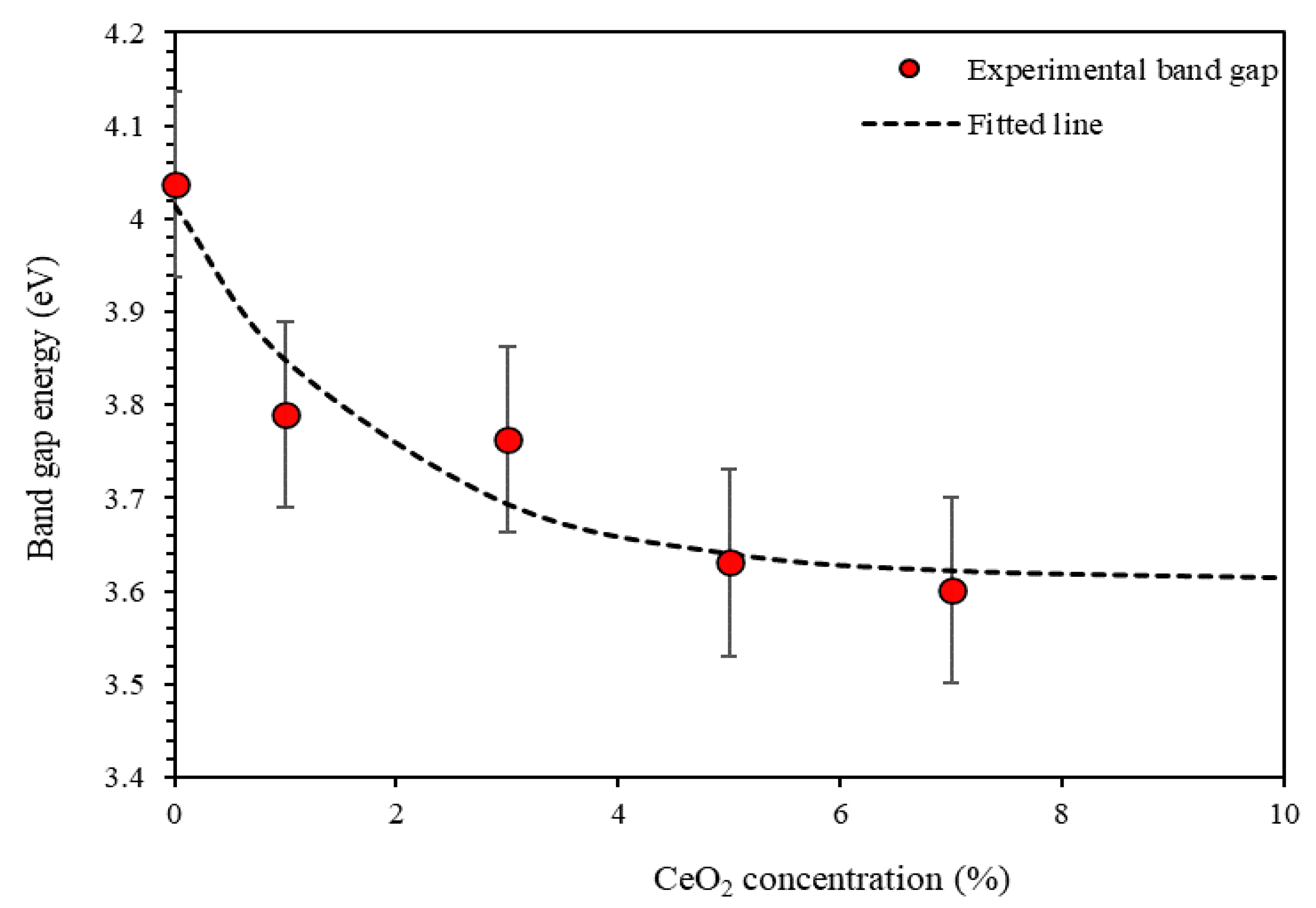
3.1.3. Band Gap Energy Eg
Wemple DiDomenico Model
Sellmeier Oscillator Parameters
Urbach Energy
3.2. FTIR Analysis
3.3. Thermogravimetric Analysis (TGA)
3.4. Surface Morphology of PMMA-PS/CeO2 Thin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Asbahi, B.A. Influence of SiO2/TiO2 nanocomposite on the optoelectronic properties of PFO/MEH-PPV-based OLED devices. Polymers 2018, 10, 800. [Google Scholar] [CrossRef]
- Wu, S.; Peng, S.; Wang, C.H. Multifunctional Polymer Nanocomposites Reinforced by Aligned Carbon Nanomaterials. Polymers 2018, 10, 542. [Google Scholar] [CrossRef]
- Kumar, S.; Sarita; Nehra, M.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Recent advances and remaining challenges for polymeric nanocomposites in healthcare applications. Prog. Polym. Sci. 2018, 80, 1–38. [Google Scholar] [CrossRef]
- Reyes-Acosta, M.A.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Flores-Vela, A.I.; Dorantes-Rosales, H.J.; Andraca-Adame, J.A. Thermal, Mechanical and UV-Shielding Properties of Poly(Methyl Methacrylate)/Cerium Dioxide Hybrid Systems Obtained by Melt Compounding. Polymers 2015, 7, 1638–1659. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, C.; Wang, J.-X.; Wang, D.; Zeng, X.-F.; Chen, J.-F. Synthesis of Transparent Aqueous ZrO2 Nanodispersion with a Controllable Crystalline Phase without Modification for a High-Refractive-Index Nanocomposite Film. Langmuir 2018, 34, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, B.; Jiang, S.; Bai, H.; Zhang, S. Use of CeO2 Nanoparticles to Enhance UV-Shielding of Transparent Regenerated Cellulose Films. Polymers 2019, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Kim, Y.G.; Kim, D.; Hyeon, T. Inorganic nanoparticles with enzyme-mimetic activities for biomedical applications. Co-ord. Chem. Rev. 2020, 403, 213092. [Google Scholar] [CrossRef]
- James, J.; Unni, A.B.; Taleb, K.; Chapel, J.-P.; Kalarikkal, N.; Varghese, S.; Vignaud, G.; Grohens, Y. Surface engineering of polystyrene–cerium oxide nanocomposite thin films for refractive index enhancement. Nano-Struct. Nano-Objects 2019, 17, 34–42. [Google Scholar] [CrossRef]
- Lee, L.-H.; Chen, W.-C. High-Refractive-Index Thin Films Prepared from Trialkoxysilane-Capped Poly(methyl methacrylate)—Titania Materials. Chem. Mater. 2001, 13, 1137–1142. [Google Scholar] [CrossRef]
- Shambat, G.; Ellis, B.; Majumdar, A.; Petykiewicz, J.; Mayer, M.; Sarmiento, T.; Harris, J.; Haller, E.; Vuckovic, J. Ultrafast directly modulated single-mode photonic crystal nanocavity light-emitting diode. Solid State Devices Mater. 2012, 2, 1–6. [Google Scholar] [CrossRef][Green Version]
- Schneiderman, D.K.; Hillmyer, M.A. 50th Anniversary Perspective: There Is a Great Future in Sustainable Polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Hong, M.; Chen, E.Y.-X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Carotenuto, G.; Giannini, C.; Siliqi, D.; Nicolais, L. Nanocomposites Based on Metal and Metal Sulfide Clusters Embedded in Polystyrene. Polymers 2011, 3, 1352–1362. [Google Scholar] [CrossRef]
- Girault, P.; Lorrain, N.; Poffo, L.; Guendouz, M.; Lemaitre, J.; Carré, C.; Gadonna, M.; Bosc, D.; Vignaud, G. Integrated polymer micro-ring resonators for optical sensing applications. J. Appl. Phys. 2015, 117, 104504. [Google Scholar] [CrossRef]
- Lü, C.; Yang, B. High refractive index organic–inorganic nanocomposites: Design, synthesis and application. J. Mater. Chem. 2009, 19, 2884–2901. [Google Scholar] [CrossRef]
- Zhang, Q.; Fang, Z.; Cao, Y.; Du, H.; Wu, H.; Beuerman, R.; Chan-Park, M.B.; Duan, H.; Xu, R. High Refractive Index Inorganic–Organic Interpenetrating Polymer Network (IPN) Hydrogel Nanocomposite toward Artificial Cornea Implants. ACS Macro Lett. 2012, 1, 876–881. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, K.; Chan-Park, M.B.; Wu, H.; Wang, D.; Xu, R. Development of high refractive ZnS/PVP/PDMAA hydrogel nanocomposites for artificial cornea implants. Acta Biomater. 2014, 10, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.G.; Gaudiana, R.A.; Hollinsed, W.C.; Kalyanaraman, P.S.; Manello, J.S.; McGowan, C.; Minns, R.A.; Sahatjian, R. Highly amorphous, birefringent, para-linked aromatic polyamides. Macromolecules 1985, 18, 1058–1068. [Google Scholar] [CrossRef]
- Yang, C.J.; Jenekhe, S.A. Effects of Structure on Refractive Index of Conjugated Polyimines. Chem. Mater. 1994, 6, 196–203. [Google Scholar] [CrossRef]
- Caglar, M.; Ilican, S.; Caglar, Y.; Yakuphanoglu, F. Electrical conductivity and optical properties of ZnO nanostructured thin film. Appl. Surf. Sci. 2009, 255, 4491–4496. [Google Scholar] [CrossRef]
- Soni, G.; Srivastava, S.; Soni, P.; Kalotra, P.; Vijay, Y.K. Optical, mechanical and structural properties of PMMA/SiO2 nanocomposite thin films. Mater. Res. Express 2017, 5, 015302. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Mohamed, M.B.; Mostafa, N.Y.; El-Naggar, A. Structural and Optical Properties of Cd 1 − x Mn x Fe2O4/PMMA Nanocomposites. J. Inorg. Organomet. Polym. Mater. 2019, 30, 1–9. [Google Scholar]
- Soni, G.; Gouttam, N.; Soni, P. Optical properties of PMMA/ZnO/SiO2 composite thin film. Mater. Today Proc. 2020, 30, 35–38. [Google Scholar] [CrossRef]
- Kumar, M.; Arun, S.; Upadhyaya, P.; Pugazhenthi, G. Properties of PMMA/clay nanocomposites prepared using various compatibilizers. Int. J. Mech. Mater. Eng. 2015, 10, 205. [Google Scholar] [CrossRef]
- Khaled, S.M.; Sui, R.; Charpentier, P.A.; Rizkalla, A.S. Synthesis of TiO2−PMMA Nanocomposite: Using Methacrylic Acid as a Coupling Agent. Langmuir 2007, 23, 3988–3995. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kader, M.H.; Mohamed, M.B. Exploring the direct effect of intermediate band semiconductor materials on the structural, thermal and optical properties of PMMA nanocomposite. Appl. Phys. A 2020, 126, 1–11. [Google Scholar] [CrossRef]
- Shih, Y.-F.; Kotharangannagari, V.K.; Tsou, T.-C. Development of eco-friendly modified cellulose nanofiber reinforced polystyrene nanocomposites: Thermal, mechanical, and optical properties. J. Polym. Res. 2020, 27, 1–10. [Google Scholar] [CrossRef]
- Okamoto, M. Biodegradable polymer/layered silicate nanocomposites: A review. J. Ind. Eng. Chem. 2004, 10, 1156–1181. [Google Scholar]
- Kargarzadeh, H.; Huang, J.; Lin, N.; Ahmad, I.; Mariano, M.; Dufresne, A.; Thomas, S.; Gałęski, A. Recent developments in nanocellulose-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites. Prog. Polym. Sci. 2018, 87, 197–227. [Google Scholar] [CrossRef]
- Vignaud, G.; Chebil, M.S.; Bal, J.K.; Delorme, N.; Beuvier, T.; Grohens, Y.; Gibaud, A. Densification and Depression in Glass Transition Temperature in Polystyrene Thin Films. Langmuir 2014, 30, 11599–11608. [Google Scholar] [CrossRef]
- Unni, A.B.; Vignaud, G.; Chapel, J.-P.; Giermanska, J.; Bal, J.K.; Delorme, N.; Beuvier, T.; Thomas, S.; Grohens, Y.; Gibaud, A. Probing the Density Variation of Confined Polymer Thin Films via Simple Model-Independent Nanoparticle Adsorption. Macromolecules 2017, 50, 1027–1036. [Google Scholar] [CrossRef]
- Lin, S.-S.; Huang, J.-L. Effect of thickness on the structural and optical properties of ZnO films by r.f. magnetron sputtering. Surf. Coatings Technol. 2004, 185, 222–227. [Google Scholar] [CrossRef]
- Hameed, S.A.; Saadmahdi, Z.; Jasim, A.N.; Taha, A.F.; Habeeb, A.A. Effect of Thickness on Structural and Optical Properties of CdO Thin Films Prepared by Chemical Spray Pyrolysis Method. NeuroQuantology 2020, 18, 20. [Google Scholar] [CrossRef]
- Wu, H.; Shen, S.; Li, J.; Chen, X.; Zhang, Z.; Ou-Yang, W. Boosted field emission properties and thickness effect of conductive polymers coated silicon carbide matrices for vacuum electronic devices. Vacuum 2020, 180, 109594. [Google Scholar] [CrossRef]
- Menazea, A.; Mostafa, A.M.; Al-Ashkar, E.A. Effect of nanostructured metal oxides (CdO, Al2O3, Cu2O) embedded in PVA via Nd:YAG pulsed laser ablation on their optical and structural properties. J. Mol. Struct. 2020, 1203, 127374. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, C.; Yang, B. A Review on High Refractive Index Nanocomposites for Optical Applications. Recent Pat. Mater. Sci. 2011, 4, 15–27. [Google Scholar] [CrossRef]
- Liu, J.-G.; Ueda, M. High refractive index polymers: Fundamental research and practical applications. J. Mater. Chem. 2009, 19, 8907–8919. [Google Scholar] [CrossRef]
- Tao, P.; Li, Y.; Rungta, A.; Viswanath, A.; Gao, J.; Benicewicz, B.C.; Siegel, R.W.; Schadler, L.S. TiO2 nanocomposites with high refractive index and transparency. J. Mater. Chem. 2011, 21, 18623–18629. [Google Scholar] [CrossRef]
- İncel, A.; Güner, T.; Parlak, O.; Demir, M.M. Null extinction of ceria@ silica hybrid particles: Transparent polystyrene composites. ACS Appl. Mater. Interfaces 2015, 7, 27539–27546. [Google Scholar] [CrossRef]
- Nadeem, M.; Khan, R.; Afridi, K.; Nadhman, A.; Ullah, S.; Faisal, S.; Mabood, Z.U.; Hano, C.; Abbasi, B.H. Green Synthesis of Cerium Oxide Nanoparticles (CeO2 NPs) and Their Antimicrobial Applications: A Review. Int. J. Nanomed. 2020, 15, 5951–5961. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dowding, J.M.; Klump, K.E.; McGinnis, J.F.; Self, W.; Seal, S. Cerium oxide nanoparticles: Applications and prospects in nanomedicine. Nanomedicine 2013, 8, 1483–1508. [Google Scholar] [CrossRef] [PubMed]
- Liying, H.; Yumin, S.; Lanhong, J.; Shikao, S. Recent advances of cerium oxide nanoparticles in synthesis, luminescence and biomedical studies: A review. J. Rare Earths 2015, 33, 791–799. [Google Scholar]
- Chiu, F.-C.; Lai, C.-M. Optical and electrical characterizations of cerium oxide thin films. J. Phys. D Appl. Phys. 2010, 43, 075104. [Google Scholar] [CrossRef]
- Vigneselvan, S.; Manikandan, V.; Petrila, I.; Vanitha, A.; Chandrasekaran, J. Effect of copper substitution on structural, optical and humidity-sensing characteristics of cerium oxide nanoparticles. J. Phys. Chem. Solids 2020, 136, 109173. [Google Scholar] [CrossRef]
- Albery, W.B. Acceleration in other axes affects +Gz tolerance: Dynamic centrifuge simulation of agile flight. Aviat. Space Environ. Med. 2004, 75, 1–6. [Google Scholar] [PubMed]
- Viezbicke, B.D.; Patel, S.; Davis, B.E.; Birnie, D.P. Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system (Phys. Status Solidi B 8/2015). Phys. Status Solidi B 2015, 252, 1700–1710. [Google Scholar] [CrossRef]
- Ahmad, A.; Alsaad, A.; Al-Bataineh, Q. Optical and structural characterization of dip synthesized Al-B Co-doped ZnO seeded platforms for ZnO nanostructures. Appl. Phys. A 2017. [Google Scholar] [CrossRef]
- Alsaad, A.; Ahmad, A.; Qattan, I.; Al-Bataineh, Q.M.; Albataineh, Z. Structural, Optoelectrical, Linear, and Nonlinear Optical Characterizations of Dip-Synthesized Undoped ZnO and Group III Elements (B, Al, Ga, and In)-Doped ZnO Thin Films. Crystals 2020, 10, 252. [Google Scholar] [CrossRef]
- Al-Bataineh, Q.M.; Alsaad, A.M.; Ahmad, A.A.; Al-Sawalmih, A. Structural, Electronic and Optical Characterization of ZnO Thin Film-Seeded Platforms for ZnO Nanostructures: Sol–Gel Method Versus Ab Initio Calculations. J. Electron. Mater. 2019, 48, 5028–5038. [Google Scholar] [CrossRef]
- Al-Bataineh, Q.M.; Alsaad, A.; Ahmad, A.; Telfah, A. A novel optical model of the experimental transmission spectra of nanocomposite PVC-PS hybrid thin films doped with silica nanoparticles. Heliyon 2020, 6, e04177. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Sharma, I. Optical properties of quaternary a-Ge15-x Sbx Se50 Te35 thermally evaporated thin-films: Refractive index dispersion and single oscillator parameters. Optik 2020, 200, 163415. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Hussein, A.M.; Ahmed, H.M. From Insulating PMMA Polymer to Conjugated Double Bond Behavior: Green Chemistry as a Novel Approach to Fabricate Small Band Gap Polymers. Polymers 2017, 9, 626. [Google Scholar] [CrossRef] [PubMed]
- Hussen, S.A. Structural and optical characterization of pure and SnZrO3 doped PS based polymer nanocomposite. Mater. Res. Express 2020, 7. [Google Scholar] [CrossRef]
- Oriaku, C.; Osuwa, J.; Njoku, C. Single oscillator parameters and optical energies of laser irradiated Cu doped cds thin films. Non-Oxide Glasses 2011, 3, 25–30. [Google Scholar]
- Yakuphanoglu, F.; Cukurovali, A.; Yilmaz, I. Single-oscillator model and determination of optical constants of some optical thin film materials. Phys. B Condens. Matter 2004, 353, 210–216. [Google Scholar] [CrossRef]
- Hassanien, A.; Akl, A.A. Influence of composition on optical and dispersion parameters of thermally evaporated non-crystalline Cd50S50−xSex thin films. J. Alloys Compd. 2015, 648, 280–290. [Google Scholar] [CrossRef]
- Güneri, E.; Kariper, A. Optical properties of amorphous CuS thin films deposited chemically at different pH values. J. Alloys Compd. 2012, 516, 20–26. [Google Scholar] [CrossRef]
- Wemple, S.H.; DiDomenico, M. Optical Dispersion and the Structure of Solids. Phys. Rev. Lett. 1969, 23, 1156–1160. [Google Scholar] [CrossRef]
- Badran, H.A.; Al-Ahmad, A.Y.; Hassan, Q.M.A.; Emshary, C.A. Determination of optical constants and nonlinear optical coefficients of Violet 1-doped polyvinyl alcohol thin film. Pramana 2015, 86, 135–145. [Google Scholar] [CrossRef]
- Bakr, N.A.; Jandow, N.N.; Habubi, N.F. Optical and Dispersion Parameters of ZnS Thin Films Prepared by Flash Evaporation Method. Int. Lett. Chem. Phys. Astron. 2014, 39, 52–63. [Google Scholar] [CrossRef]
- Badran, H.A. Study on Optical Constants and Refractive Index Dispersion of Neutral red Doped Polymer Film. Am. J. Appl. Sci. 2012, 9, 250–253. [Google Scholar] [CrossRef]
- Okutan, M.; San, S.E.; Köysal, O.; Yakuphanoglu, F. Investigation of refractive index dispersion and electrical properties in carbon nano-balls’ doped nematic liquid crystals. Phys. B Condens. Matter 2005, 362, 180–186. [Google Scholar] [CrossRef]











| Parameter | P-P | P-P/1% CeO2 NPs | P-P/3% CeO2 NPs | P-P/5% CeO2 NPs | P-P/7% CeO2 NPs |
|---|---|---|---|---|---|
| Dispersion energy Ed (eV) | 6.893 | 14.484 | 12.268 | 7.832 | 9.288 |
| Effective single oscillator E0 (eV) | 4.063 | 6.333 | 5.433 | 4.065 | 4.542 |
| Zero-frequency refractive index n0 | 1.642 | 1.812 | 1.804 | 1.710 | 1.744 |
| Zero-frequency dielectric constant ε0 | 2.696 | 3.286 | 3.257 | 2.926 | 3.044 |
| Optical oscillator strength f (eV)2 | 28.011 | 91.743 | 66.666 | 31.847 | 42.194 |
| Optical moments M−1 | 1.696 | 2.286 | 2.257 | 1.926 | 2.044 |
| Optical moments M−3 (eV−2) | 0.102 | 0.056 | 0.076 | 0.116 | 0.099 |
| Oscillator length strength S0 × 10−5 | 1.804 | 5.728 | 4.139 | 2.119 | 2.523 |
| Average oscillator wavelength λ0 | 306.200 | 199.389 | 232.683 | 302.325 | 282.096 |
| Urbach energy EU (meV) | 182.495 | 186.216 | 187.360 | 194.476 | 207.675 |
| Vibrational Band | PMMA-PS | PMMA-PS/CeO2 1% | PMMA-PS/CeO2 3% | PMMA-PS/CeO2 5% | PMMA-PS/CeO2 7% |
|---|---|---|---|---|---|
| Ce–O | -- | 541.42 | 541.42 | 541.42 | 541.42 |
| C–H bending | 704.03 | 706.03 | 693.74 | 697.86 | 697.86 |
| 753.44 | 7.45.20 | 753.44 | 751.38 | 753.44 | |
| 841.96 | 844.02 | 844.02 | 844.02 | 841.96 | |
| 965.47 | 963.41 | 963.41 | 963.41 | 963.41 | |
| C–O stretching | 1035.46 | 1037.52 | 1037.52 | 1037.52 | 1035.46 |
| 1068.40 | 1068.40 | 1066.34 | 1068.40 | 1070.46 | |
| 1148.68 | 1148.68 | 1148.68 | 1148.68 | 1150.47 | |
| 1187.79 | 1191.91 | 1191.91 | 1191.91 | 1191.91 | |
| 1251.61 | 1243.38 | 1243.38 | 1245.44 | 1245.44 | |
| 1280.43 | 1274.26 | 1274.26 | 1276.32 | 1276.32 | |
| –CH3 bending | 1371.01 | 1366.89 | 1366.89 | 1366.89 | 1368.95 |
| 1389.54 | 1385.42 | 1387.48 | 1385.42 | 1385.42 | |
| –CH2 bending | 1449.24 | 1451.29 | 1447.18 | 1449.24 | 1451.29 |
| C=C | 1484.23 | 1486.29 | 1486.29 | 1490.41 | 1492.47 |
| C=O | 1601.57 | 1599.51 | 1603.63 | 1603.63 | 1601.57 |
| 1725.03 | 1725.08 | 1725.08 | 1725.08 | 1725.08 | |
| C–H stretching | 2800–3200 | 2800–3200 | 2800–3200 | 2800–3200 | 2800–3200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bani-Salameh, A.A.; Ahmad, A.A.; Alsaad, A.M.; Qattan, I.A.; Aljarrah, I.A. Synthesis, Optical, Chemical and Thermal Characterizations of PMMA-PS/CeO2 Nanoparticles Thin Film. Polymers 2021, 13, 1158. https://doi.org/10.3390/polym13071158
Bani-Salameh AA, Ahmad AA, Alsaad AM, Qattan IA, Aljarrah IA. Synthesis, Optical, Chemical and Thermal Characterizations of PMMA-PS/CeO2 Nanoparticles Thin Film. Polymers. 2021; 13(7):1158. https://doi.org/10.3390/polym13071158
Chicago/Turabian StyleBani-Salameh, Areen A., A. A. Ahmad, A. M. Alsaad, I. A. Qattan, and Ihsan A. Aljarrah. 2021. "Synthesis, Optical, Chemical and Thermal Characterizations of PMMA-PS/CeO2 Nanoparticles Thin Film" Polymers 13, no. 7: 1158. https://doi.org/10.3390/polym13071158
APA StyleBani-Salameh, A. A., Ahmad, A. A., Alsaad, A. M., Qattan, I. A., & Aljarrah, I. A. (2021). Synthesis, Optical, Chemical and Thermal Characterizations of PMMA-PS/CeO2 Nanoparticles Thin Film. Polymers, 13(7), 1158. https://doi.org/10.3390/polym13071158