Designing Self-Sustainable Icephobic Layer by Introducing a Lubricating Un-Freezable Water Hydrogel from Sodium Polyacrylate on the Polyolefin Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimens Preparation
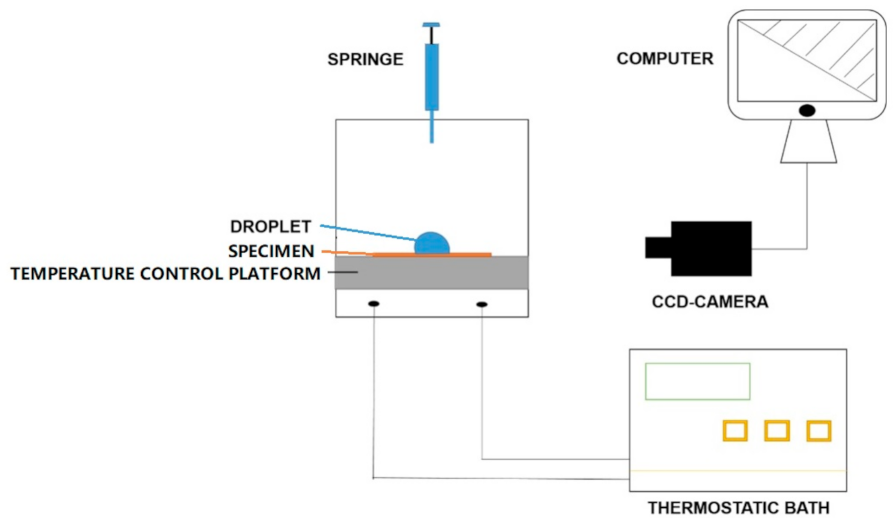
2.3. Static and Dynamic Contact Angles and Delayed Icing Time
2.4. Contact Angle Hysteresis (CAH)
2.5. Ice Adhesion Strength and Anti-Icing Durability
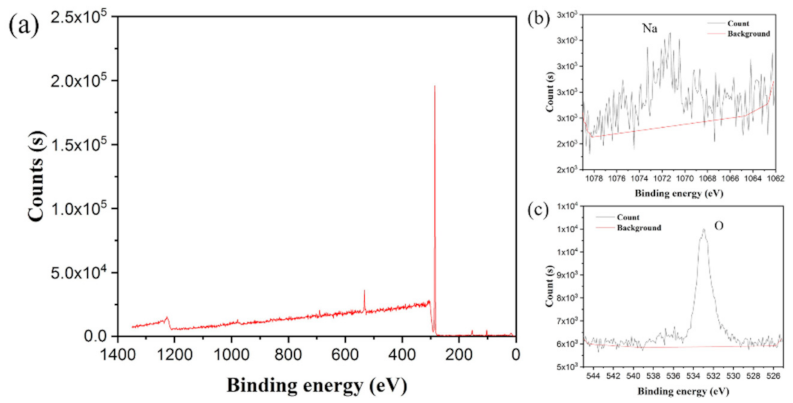
2.6. Surface Morphology and Elemental Analysis
2.7. Water Swelling Ratio
2.8. DMA
3. Results and Discussion
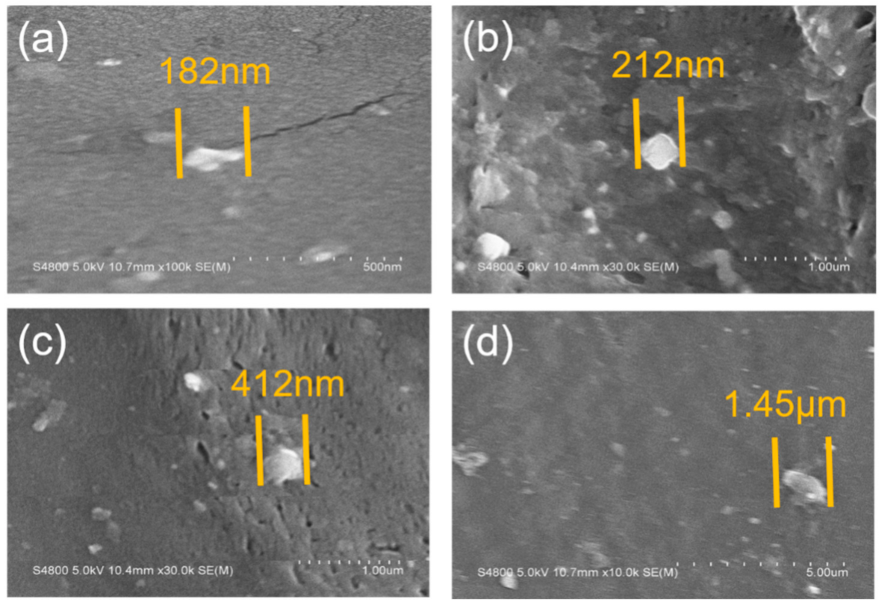
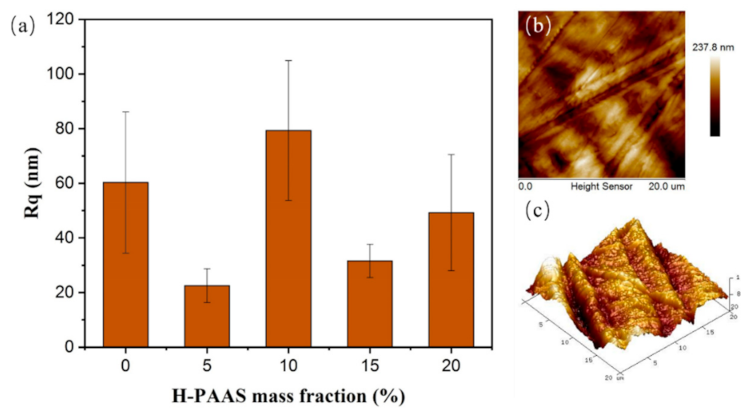
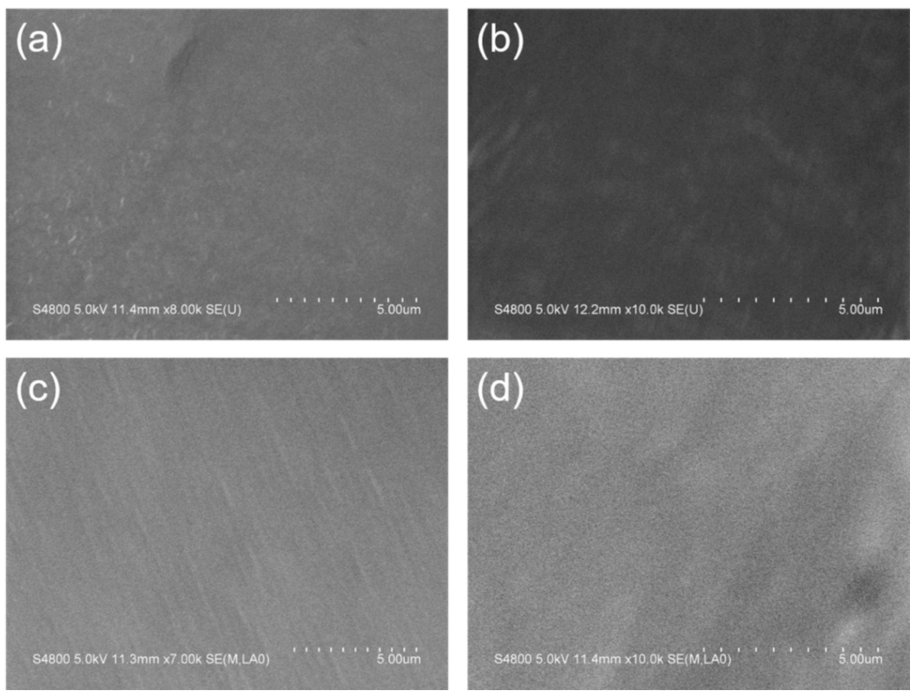
3.1. Surface Morphology
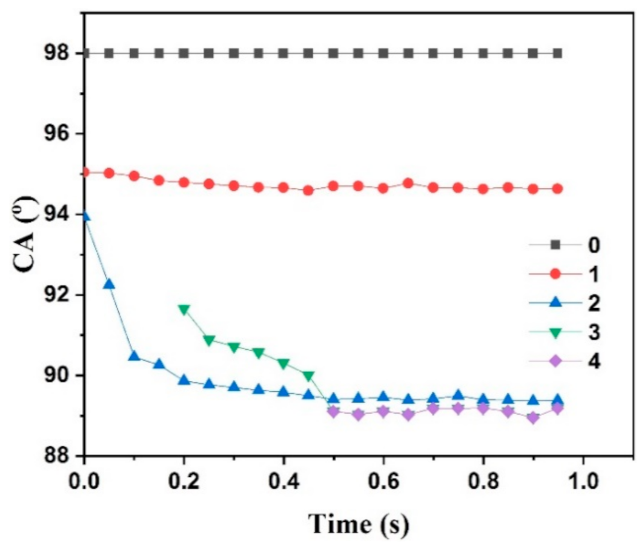
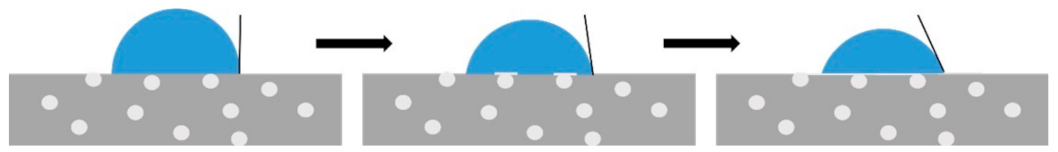
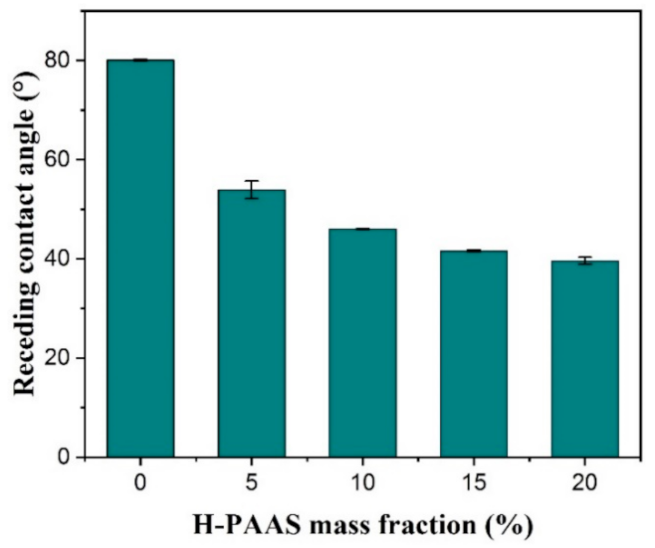
3.2. Static and Dynamic Contact Angles
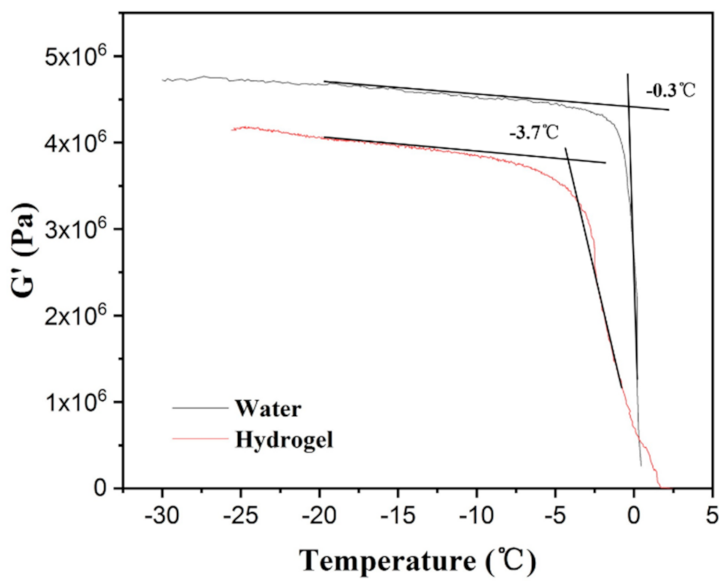
3.3. Dynamic Mechanic Analysis of Water and Hydrogel
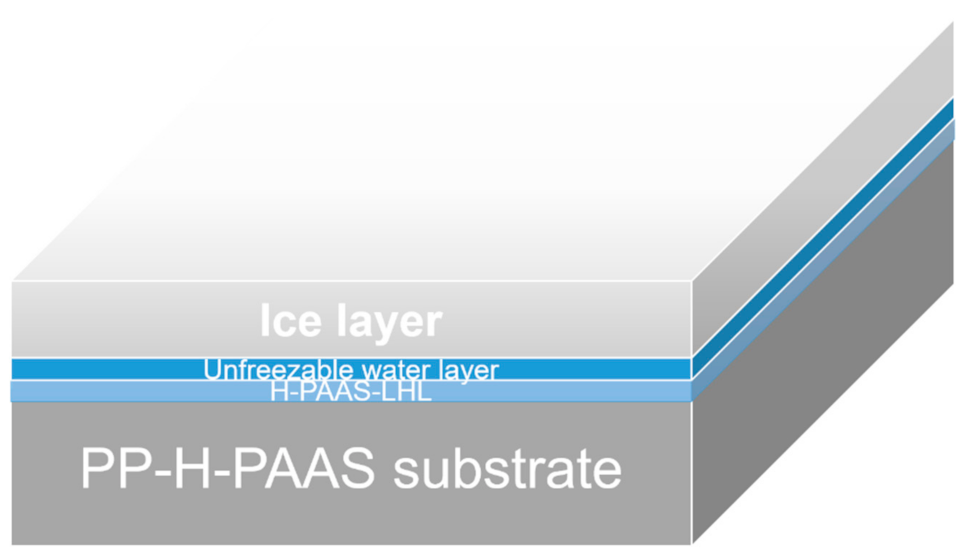
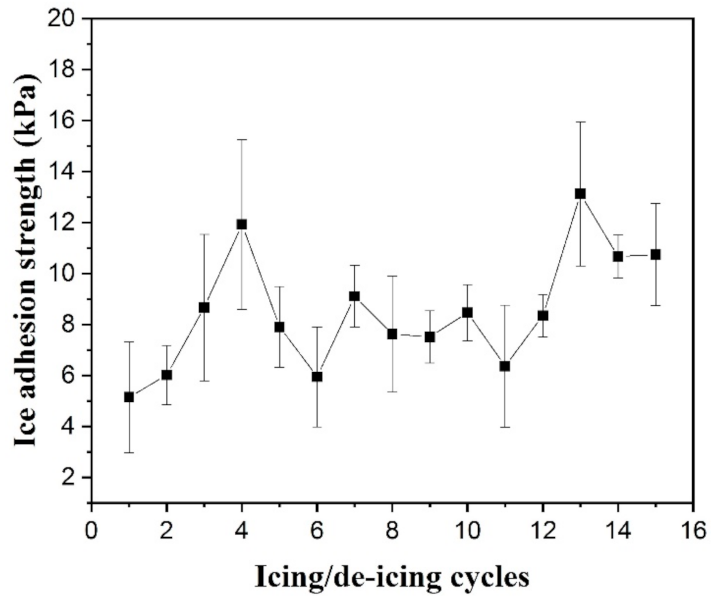
3.4. Anti-Icing Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emelyanenko, A.M.; Boinovich, L.B.; Bezdomnikov, A.A.; Chulkova, E.V.; Emelyanenko, K.A. Reinforced Superhydrophobic Coating on Silicone Rubber for Longstanding Anti-Icing Performance in Severe Conditions. ACS Appl. Mater. Inter. 2017, 9, 24210–24219. [Google Scholar] [CrossRef]
- Kim, M.-H.; Kim, D.R.; Lee, K.-S. Stochastic Approach to the Anti-freezing Behaviors of Superhydrophobic Surfaces. Int. J. Heat Mass Transf. 2017, 106, 841–846. [Google Scholar] [CrossRef]
- Jung, S.; Tiwari, M.K.; Doan, N.V.; Poulikakos, D. Mechanism of Supercooled Droplet Freezing on Surfaces. Nat. Commun. 2012, 3, 1–8. [Google Scholar] [CrossRef]
- Roberge, P.; Lemay, J.; Ruel, J.; Begin-Drolet, A. Field Analysis, Modeling and Characterization of Wind Turbine Hot Air Ice Protection Systems. Cold Reg. Sci. Technol. 2019, 163, 19–26. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y. Numerical Simulation on the Ice-induced Fatigue Damage of Ship Structural Members in Broken Ice Fields. Mar. Struct. 2019, 66, 83–105. [Google Scholar] [CrossRef]
- Shen, Y.Z.; Wu, X.H.; Tao, J.; Zhu, C.L.; Lai, Y.K.; Chen, Z. Icephobic Materials: Fundamentals, Performance Evaluation, and Applications. Prog. Mater. Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Zheng, H.K.; Chang, S.N.; Zhao, Y.Y. Anti-icing & Icephobic Mechanism and Applications of Superhydrophobic/Ultra Slippery Surface. Prog. Chem. 2017, 29, 102–118. [Google Scholar]
- He, Z.W.; Zhuo, Y.Z.; He, J.Y.; Zhang, Z.L. Design and Preparation of Sandwich-like Polydimethylsiloxane (PDMS) Sponges with Super-low Ice Adhesion. Soft Matter 2018, 14, 4846–4851. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Ma, Y.B.; Sun, Y.Y.; Xiong, Z.Y.; Qi, C.H.; Zhang, Y.H.; Liu, Y.Q. Robust Superhydrophobic Surface Based on Multiple Hybrid Coatings for Application in Corrosion Protection. ACS Appl. Mater. Inter. 2019, 11, 6512–6526. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.L.; Jin, J.F.; Liu, J.A.; Yan, Y.Y.; Han, Z.W.; Ren, L.Q. Anti-icing Property of Bio-inspired Micro-structure Superhydrophobic Surfaces and Heat Transfer Model. Appl. Surf. Sci. 2017, 400, 498–505. [Google Scholar] [CrossRef]
- Wu, S.; Du, Y.; Alsaid, Y.; Wu, D.; Hua, M.; Yan, Y.; Yao, B.; Ma, Y.; Zhu, X.; He, X. Superhydrophobic Photothermal Icephobic Surfaces Based on Candle Soot. Proc. Natl. Acad. Sci. USA 2020, 117, 11240–11246. [Google Scholar] [CrossRef]
- Lv, J.Y.; Song, Y.L.; Jiang, L.; Wang, J.J. Bio-Inspired Strategies for Anti-icing. ACS Nano 2014, 8, 3152–3169. [Google Scholar] [CrossRef]
- Fillion, R.M.; Riahi, A.R.; Edrisy, A. A Review of Icing Prevention in Photovoltaic Devices by Surface Engineering. Renew. Sust. Energy Rev. 2014, 32, 797–809. [Google Scholar] [CrossRef]
- Azimi Yancheshme, A.; Momen, G.; Jafari Aminabadi, R. Mechanisms of Ice Formation and Propagation on Superhydrophobic Surfaces: A Review. Adv. Colloid Interface Sci. 2020, 279, 102–155. [Google Scholar] [CrossRef]
- Wang, P.; Yao, T.; Li, Z.Q.; Wei, W.D.; Xie, Q.; Duan, W.; Han, H.L. A Superhydrophobic/Electrothermal Synergistically Anti-icing Strategy Based on Graphene Composite. Compos. Sci. Technol. 2020, 198, 108307. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, J.; Wang, Y.Y.; Chen, Q.M.; Ding, J.F.; Wang, Q.J. Icephobic Coatings Based on Silicon-Oil-Infused Polydimethylsiloxane. ACS Appl. Mater. Inter. 2013, 5, 4053–4062. [Google Scholar] [CrossRef]
- Liu, Y.F.; Wang, C.Y.; Jarrell, R.M.; Nair, S.; Wynne, K.J.; Di, D.L. Icephobic, Pt-Cured, Polydimethylsiloxane Nanocomposite Coatings. ACS Appl. Mater. Inter. 2020, 12, 11180–11189. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, M.J.; Lee, B.; Chun, J.M.; Patil, V.; Kim, Y.S. Durable Ice-lubricating Surfaces Based on Polydimethylsiloxane Embedded Silicone Oil Infused Silica Aerogel. Appl. Surf. Sci. 2020, 512, 1457–1466. [Google Scholar] [CrossRef]
- Ozbay, S.; Yuceel, C.; Erbil, H.Y. Improved Icephobic Properties on Surfaces with a Hydrophilic Lubricating Liquid. ACS Appl. Mater. Inter. 2015, 7, 22067–22077. [Google Scholar] [CrossRef]
- Wang, P.; Wei, W.D.; Li, Z.Q.; Duan, W.; Han, H.L.; Xie, Q. A Superhydrophobic Fluorinated PDMS Composite as a Wearable Strain Sensor with Excellent Mechanical Robustness and Liquid Impalement Resistance. J. Mater. Chem. A 2020, 8, 3509–3516. [Google Scholar] [CrossRef]
- Beemer, D.; Wang, W.; Kota, A. Durable Gels with Ultra-low Adhesion to Ice. J. Mater. Chem. A 2016, 4, 18253–18258. [Google Scholar] [CrossRef]
- Li, J.S.; Ueda, E.; Paulssen, D.; Levkin, P.A. Slippery Lubricant-infused Surfaces: Properties and Emerging Applications. Adv. Funct. Mater. 2019, 29, 2317–2340. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, K.Q.; Tao, C.; Zhao, Y.H.; Li, X.H.; Zhu, K.Y.; Yuan, X.Y. Strategies for Anti-icing: Low Surface Energy or Liquid-infused? RSC Adv. 2016, 6, 70251–70260. [Google Scholar] [CrossRef]
- Chen, D.Y.; Gelenter, M.D.; Hong, M.; Cohen, R.E.; McKinley, G.H. Icephobic Surfaces Induced by Interfacial Nonfrozen Water. ACS Appl. Mater. Inter. 2017, 9, 4202–4214. [Google Scholar] [CrossRef]
- Jamil, M.I.; Ali, A.; Haq, F.; Zhang, Q.H.; Zhan, X.L.; Chen, F.Q. Icephobic Strategies and Materials with Superwettability: Design Principles and Mechanism. Langmuir 2018, 34, 15425–15444. [Google Scholar] [CrossRef]
- Jamil, M.I.; Zhan, X.L.; Chen, F.Q.; Cheng, D.G.; Zhang, Q.H. Durable and Scalable Candle Soot Icephobic Coating with Nucleation and Fracture Mechanism. ACS Appl. Mater. Inter. 2019, 11, 31532–31542. [Google Scholar] [CrossRef]
- Ng, Y.H.; Tay, S.W.; Hong, L. Formation of Icephobic Surface with Micron-scaled Hydrophobic Heterogeneity on Polyurethane Aerospace Coating. ACS Appl. Mater. Inter. 2018, 10, 37517–37528. [Google Scholar] [CrossRef]
- Yin, L.; Xia, Q.; Xue, J.A.; Yang, S.Q.; Wang, Q.J.; Chen, Q.M. In Situ Investigation of Ice Formation on Surfaces with Representative Wettability. Appl. Surf. Sci. 2010, 256, 6764–6769. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Xue, J.; Wang, Q.J.; Chen, Q.M.; Ding, J.F. Verification of Icephobic/Anti-icing Properties of a Superhydrophobic Surface. ACS Appl. Mater. Inter. 2013, 5, 3370–3381. [Google Scholar] [CrossRef]
- Seyfi, A.; Afzalzadeh, R.; Hajnorouzi, A. Increase in Water Evaporation Rate with Increase in Static Magnetic Field Perpendicular to Water-air Interface. Chem. Eng. Process. 2017, 120, 195–200. [Google Scholar] [CrossRef]
- Wang, G.; Mu, J.; Zhou, H.; Miao, W.; Liu, Z.; Liu, Y. Research on the Test Technology for Vertical Ice Adhesion Strength. J. Eng. Thermophys. 2012, 33, 282–284. [Google Scholar]
- Zhang, J.; Liu, X.H.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Ma, J.; Wang, T.W. Preparation and Characterization of Water-absorbing Polyurethane Foam Composites with Microsized Sodium Polyacrylate Particles. J. Appl. Polym. Sci. 2018, 135, 6702–6709. [Google Scholar] [CrossRef]
- Zheng, P.; Cai, D.; Zhang, Z.; Yang, Y.; Yin, T.; Shen, W. Interactions between Sodium Polyacrylate and Mixed Micelles of Dodecyltrimethylammonium Bromide and Sodium Bis(2-ethylhexyl) Sulfosuccinate. Macromolecules 2013, 46, 247–256. [Google Scholar] [CrossRef]
- Golovin, K.; Dhyani, A.; Thouless, M.D.; Tuteja, A. Low-interfacial Toughness Materials for Effective Large-scale Deicing. Science 2019, 364, 371–375. [Google Scholar] [CrossRef]
- Jafari, R.; Chameau, M.; Farzaneh, M.; Momen, G. Superhydrophobic and Highly Oleophilic Polystyrene Fibers (PS) with Delayed Freezing Time and Effective Oil Adsorption. Adv. Mater. 2018, 941, 2232–2236. [Google Scholar]
- Li, T.; Ibanez-Ibanez, P.F.; Hakonsen, V.; Wu, J.; Xu, K.; Zhuo, Y.; Luo, S.; He, J.; Zhang, Z. Self-Deicing Electrolyte Hydrogel Surfaces with Pa-level Ice Adhesion and Durable Antifreezing/Antifrost Performance. ACS Appl. Mater. Inter. 2020, 12, 35572–35578. [Google Scholar] [CrossRef] [PubMed]
- Koop, T.; Luo, B.P.; Tsias, A.; Peter, T. Water Activity as the Determinant for Homogeneous Ice Nucleation in Aqueous Solutions. Nature 2000, 406, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.J.; Guo, H.S.; Chen, P.G.; Zhu, Y.N.; Wen, C.Y.; Gao, Y.H.; Yang, J.; Zhang, X.Y.; Zhang, L. Zwitterionic Osmolyte-Based Hydrogels with Antifreezing Property, High Conductivity, and Stable Flexibility at Subzero Temperature. Adv. Funct. Mater. 2020, 30, 1907–1916. [Google Scholar] [CrossRef]
- Logutenko, O.A.; Titkov, A.I.; Vorob’yov, A.M.; Balaev, D.A.; Shaikhutdinov, K.A.; Semenov, S.V.; Yukhin, Y.M.; Lyakhov, N.Z. Effect of Molecular Weight of Sodium Polyacrylates on the Size and Morphology of Nickel Nanoparticles Synthesized by the Modified Polyol Method and their Magnetic Properties. Eur. Polym. J. 2018, 99, 102–110. [Google Scholar] [CrossRef]
- Meuler, A.J.; Smith, J.D.; Varanasi, K.K.; Mabry, J.M.; McKinley, G.H.; Cohen, R.E. Relationships between Water Wettability and Ice Adhesion. ACS Appl. Mater. Inter. 2010, 2, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Q.; Xia, Q.A.; Zhu, L.; Xue, J.A.; Wang, Q.J.; Chen, Q.M. Research on the Icephobic Properties of Fluoropolymer-based Materials. Appl. Surf. Sci. 2011, 257, 4956–4962. [Google Scholar] [CrossRef]
- Zhuo, Y.Z.; Hakonsen, V.; He, Z.W.; Xiao, S.B.; He, J.Y.; Zhang, Z.L. Enhancing the Mechanical Durability of Icephobic Surfaces by Introducing Autonomous Self-Healing Function. ACS Appl. Mater. Inter. 2018, 10, 11972–11978. [Google Scholar] [CrossRef] [PubMed]








| Specimen | ||||||
|---|---|---|---|---|---|---|
| 0 | 98.0 | 0.0 | 1.00 | 0.00 | 98.0 | 98.0 |
| 1 | 98.0 | 0.0 | 0.95 | 0.05 | 95.8 | 95.0 |
| 2 | 98.0 | 0.0 | 0.90 | 0.10 | 93.5 | 93.9 |
| 3 | 98.0 | 0.0 | 0.85 | 0.15 | 91.3 | 91.7 |
| 4 | 98.0 | 0.0 | 0.80 | 0.20 | 89.0 | 89.1 |
| Specimen | Icing Time/s | Delayed Icing Time/s |
|---|---|---|
| 0 | 24 ± 3.2 | - |
| 1 | 29.8 ± 6.4 | 5.8 ± 6.4 |
| 2 | 69.8 ± 9.1 | 45.8 ± 9.1 |
| 3 | 106.8 ± 5.9 | 82.8 ± 5.9 |
| 4 | 3072 ±376.4 | 3048 ± 376.4 |
| Process Status | Na Atomic Fraction/% | O Atomic Fraction/% |
|---|---|---|
| Before water absorption | 0.21 | 3.53 |
| After water absorption | 6.11 | 26.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Cao, C.; Zhang, L.; Quan, Y.; Wang, Q.; Xie, H. Designing Self-Sustainable Icephobic Layer by Introducing a Lubricating Un-Freezable Water Hydrogel from Sodium Polyacrylate on the Polyolefin Surface. Polymers 2021, 13, 1126. https://doi.org/10.3390/polym13071126
Shi J, Cao C, Zhang L, Quan Y, Wang Q, Xie H. Designing Self-Sustainable Icephobic Layer by Introducing a Lubricating Un-Freezable Water Hydrogel from Sodium Polyacrylate on the Polyolefin Surface. Polymers. 2021; 13(7):1126. https://doi.org/10.3390/polym13071126
Chicago/Turabian StyleShi, Junqi, Chongjian Cao, Lu Zhang, Yiwu Quan, Qingjun Wang, and Hongfeng Xie. 2021. "Designing Self-Sustainable Icephobic Layer by Introducing a Lubricating Un-Freezable Water Hydrogel from Sodium Polyacrylate on the Polyolefin Surface" Polymers 13, no. 7: 1126. https://doi.org/10.3390/polym13071126
APA StyleShi, J., Cao, C., Zhang, L., Quan, Y., Wang, Q., & Xie, H. (2021). Designing Self-Sustainable Icephobic Layer by Introducing a Lubricating Un-Freezable Water Hydrogel from Sodium Polyacrylate on the Polyolefin Surface. Polymers, 13(7), 1126. https://doi.org/10.3390/polym13071126








