Electrospun Carbon Nanofibers from Biomass and Biomass Blends—Current Trends
Abstract
:1. Introduction
2. Biomass as Precursor for Carbon Nanofibers
3. Preparation of Carbon Nanofibers
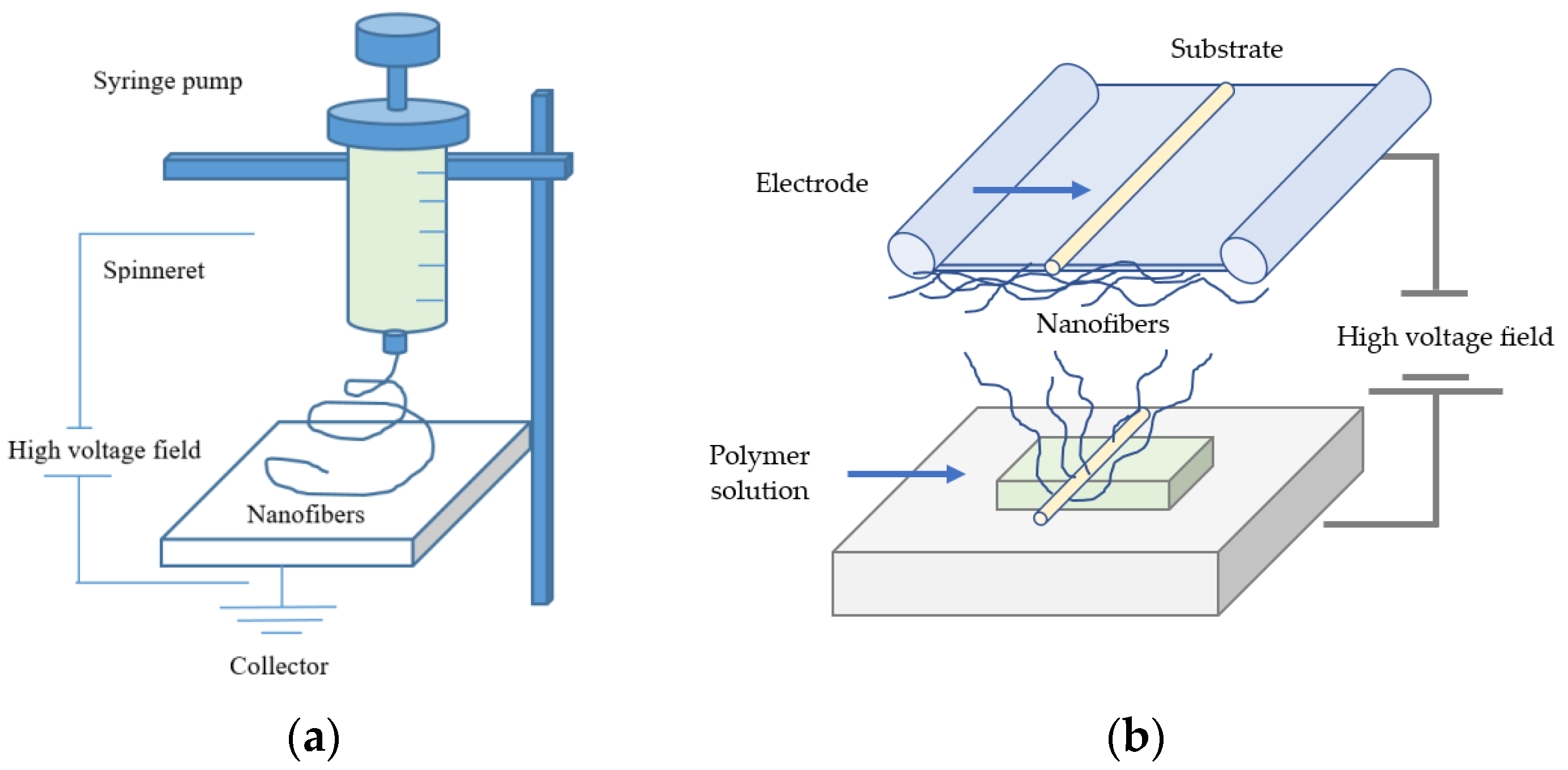
3.1. Electrospinning
3.2. Stabilization and Carbonization of Nanofibers
4. Application of Carbon Nanofibers
4.1. For Energy Storage
4.1.1. Fuel Cells
4.1.2. Electrochemical Batteries
4.1.3. Supercapacitors
4.2. Environmental Science
4.2.1. Wastewater Treatment
4.2.2. CO2 Capture
4.3. Biotechnological and Medical Fields
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azwar, E.; Wan Mahari, W.A.; Chuah, J.H.; Vo, D.-V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrogen Energy 2018, 43, 20811–20821. [Google Scholar] [CrossRef]
- Wang, T.; Rony, A.H.; Sun, K.; Gong, W.; He, X.; Lu, W.; Tang, M.; Ye, R.; Yu, J.; Kang, L.; et al. Carbon Nanofibers Prepared from Solar Pyrolysis of Pinewood as Binder-free Electrodes for Flexible Supercapacitors. Cell Rep. Phys. Sci. 2020, 1, 100079. [Google Scholar] [CrossRef]
- Du, B.; Chen, C.; Sun, Y.; Yu, M.; Liu, B.; Wang, X.; Zhou, J. Lignin bio-oil-based electrospun nanofibers with high substitution ratio property for potential carbon nanofibers applications. Polym. Test. 2020, 89, 106591. [Google Scholar] [CrossRef]
- Omoriyekomwan, J.E.; Tahmasebi, A.; Dou, J.; Wang, R.; Yu, J. A review on the recent advances in the production of carbon nanotubes and carbon nanofibers via microwave-assisted pyrolysis of biomass. Fuel Process. Technol. 2020, 214, 106686. [Google Scholar] [CrossRef]
- Du, B.; Chen, C.; Sun, Y.; Yang, M.; Yu, M.; Liu, B.; Wang, X.; Zhou, J. Unlocking the response of lignin structure by depolymerization process improved lignin-based carbon nanofibers preparation and mechanical strength. Int. J. Biol. Macromol. 2020, 156, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Dineshkumar, M.; Meera Sheriffa Begum, K.M.; Shrikar, B.; Ramanathan, A. Synthesis and characterization study of solid carbon biocatalyst produced from novel biomass char in a microwave pyrolysis. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Duran-Jimenez, G.; Monti, T.; Titman, J.J.; Hernandez-Montoya, V.; Kingman, S.W.; Binner, E.R. New insights into microwave pyrolysis of biomass: Preparation of carbon-based products from pecan nutshells and their application in wastewater treatment. J. Anal. Appl. Pyrolysis 2017, 124, 113–121. [Google Scholar] [CrossRef]
- Wan, W.; Zhao, W.; Wu, Y.; Dai, C.; Zhu, X.; Wang, Y.; Qin, J.; Chen, T.; Lü, Z. A highly efficient biomass based electrocatalyst for cathodic performance of lithium–oxygen batteries: Yeast derived hydrothermal carbon. Electrochim. Acta 2020, 349, 136411. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, H.; Wang, J.; Zhu, S.; Xiong, Y. Catalytic cracking of biomass tar using Ni nanoparticles embedded carbon nanofiber/porous carbon catalysts. Energy 2021, 216, 119285. [Google Scholar] [CrossRef]
- Unur, E.; Brutti, S.; Panero, S.; Scrosati, B. Nanoporous carbons from hydrothermally treated biomass as anode materials for lithium ion batteries. Microporous Mesoporous Mater. 2013, 174, 25–33. [Google Scholar] [CrossRef]
- Huang, W.; Tong, Z.; Wang, R.; Liao, Z.; Bi, Y.; Chen, Y.; Ma, M.; Lyu, P.; Ma, Y. A review on electrospinning nanofibers in the field of microwave absorption. Ceram. Int. 2020, 46, 26441–26453. [Google Scholar] [CrossRef]
- Shabafrooz, V.; Mozafari, M.; Vashaee, D.; Tayebi, L. Electrospun nanofibers: From filtration membranes to highly specialized tissue engineering scaffolds. J. Nanosci. Nanotechnol. 2014, 14, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, J.; Zhang, L.; Qiu, Y. Recent advances in energy materials by electrospinning. Renew. Sustain. Energy Rev. 2018, 81, 1825–1858. [Google Scholar] [CrossRef]
- Sabantina, L.; Wehlage, D.; Klöcker, M.; Mamun, A.; Grothe, T.; García-Mateos, F.J.; Rodríguez-Mirasol, J.; Cordero, T.; Finsterbusch, K.; Ehrmann, A. Stabilization of electrospun PAN/gelatin nanofiber mats for carbonization. J. Nanomater. 2018, 6131085. [Google Scholar] [CrossRef] [Green Version]
- Sabantina, L.; Rodríguez-Cano, M.Á.; Klöcker, M.; García-Mateos, F.J.; Ternero-Hidalgo, J.J.; Mamun, A.; Beermann, F.; Schwakenberg, M.; Voigt, A.L.; Rodríguez-Mirasol, J.; et al. Fixing PAN nanofiber mats during stabilization for carbonization and creating novel metal/carbon composites. Polymers 2018, 10, 735. [Google Scholar] [CrossRef] [Green Version]
- Bier, M.C.; Kohn, S.; Stierand, A.; Grimmelsmann, N.; Homburg, S.V.; Rattenholl, A.; Ehrmann, A. Investigation of eco-friendly casein fibre production methods. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 192004. [Google Scholar] [CrossRef]
- Grimmelsmann, N.; Grothe, T.; Homburg, S.V.; Ehrmann, A. Electrospinning and stabilization of chitosan nanofiber mats. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 102006. [Google Scholar] [CrossRef]
- Shang, Z.; An, X.; Liu, L.; Yang, J.; Zhang, W.; Dai, H.; Cao, H.; Xu, Q.; Liu, H.; Ni, Y. Chitin nanofibers as versatile bio-templates of zeolitic imidazolate frameworks for N-doped hierarchically porous carbon electrodes for supercapacitor. Carbohydr. Polym. 2021, 251, 117107. [Google Scholar] [CrossRef]
- Sabantina, L.; Kinzel, F.; Hauser, T.; Többer, A.; Klöcker, M.; Döpke, C.; Böttjer, R.; Wehlage, D.; Rattenholl, A.; Ehrmann, A. Comparative Study of Pleurotus ostreatus Mushroom Grown on Modified PAN Nanofiber Mats. Nanomaterirals 2019, 9, 475. [Google Scholar] [CrossRef] [Green Version]
- Cao, M.; Cheng, W.; Ni, X.; Hu, Y.; Han, G. Lignin-based multi-channels carbon nanofibers @ SnO2 nanocomposites for high-performance supercapacitors. Electrochim. Acta 2020, 345, 136172. [Google Scholar] [CrossRef]
- Sharma, A.; Mandal, T.; Goswami, S. Fabrication of cellulose acetate nanocomposite films with lignocelluosic nanofiber filler for superior effect on thermal, mechanical and optical properties. Nano Struct. Nano Objects 2021, 25, 100642. [Google Scholar] [CrossRef]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef]
- Mamun, A. Review of possible applications of nanofibrous mats for wound dressings. Tekstilec 2019, 62, 89–100. [Google Scholar] [CrossRef]
- Ranjan, V.D.; Zeng, P.; Li, B.; Zhang, Y. In vitro cell culture in hollow microfibers with porous structures. Biomater. Sci. 2020, 8, 2175–2188. [Google Scholar] [CrossRef]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, B.; Yalcinkaya, F.; Chaloupek, J. Thin Film Nanofibrous Composite Membrane for Dead-End Seawater Desalination. J. Nanomater. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Roche, R.; Yalcinkaya, F. Electrospun Polyacrylonitrile Nanofibrous Membranes for Point-of-Use Water and Air Cleaning. ChemistryOpen 2019, 8, 97–103. [Google Scholar] [CrossRef]
- Torres-Mendieta, R.; Yalcinkaya, F.; Boyraz, E.; Havelka, O.; Wacławek, S.; Maryška, J.; Černík, M.; Bryjak, M. PVDF nanofibrous membranes modified via laser-synthesized Ag nanoparticles for a cleaner oily water separation. Appl. Surf. Sci. 2020, 526, 146575. [Google Scholar] [CrossRef]
- Choi, J.; Moon, D.S.; Ryu, S.G.; Lee, B.; Ying, W.B.; Lee, K. J. N-chloro hydantoin functionalized polyurethane fibers toward protective cloth against chemical warfare agents. Polymer 2018, 138, 146–155. [Google Scholar] [CrossRef]
- Grothe, T.; Wehlage, D.; Böhm, T.; Remche, A.; Ehrmann, A. Needleless electrospinning of PAN nanofiber mats. Tekstilec 2017, 60, 290–295. [Google Scholar] [CrossRef]
- Maver, T.; Kurečič, M.; Smrke, D.M.; Kleinschek, K.S.; Maver, U. Electrospun nanofibrous CMC/PEO as a part of an effective pain-relieving wound dressing. J. Sol-Gel Sci. Technol. 2016, 79, 475–486. [Google Scholar] [CrossRef]
- Ebrahimi-Hosseinzadeh, B.; Pedram, M.; Hatamian-Zarmi, A.; Salahshour-Kordestani, S.; Rasti, M.; Mokhtari-Hosseini, Z.B.; Mir-Derikvand, M. In vivo evaluation of gelatin/hyaluronic acid nanofiber as Burn-wound healing and its comparison with ChitoHeal gel. Fibers Polym. 2016, 17, 820–826. [Google Scholar] [CrossRef]
- Na, K.H.; Kim, W.T.; Park, D.C.; Shin, H.G.; Lee, S.H.; Park, J.; Song, T.H.; Choi, W.Y. Fabrication and characterization of the magnetic ferrite nanofibers by electrospinning process. Thin Solid Films 2018, 660, 358–364. [Google Scholar] [CrossRef]
- Matos, R.J.R.; Chaparro, C.I.P.; Silva, J.C.; Valente, M.A.; Borges, J.P.; Soares, P.I.P. Electrospun composite cellulose acetate/iron oxide nanoparticles non-woven membranes for magnetic hyperthermia applications. Carbohydr. Polym. 2018, 198, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, Y.; Yuan, M.; Sun, G.; Liao, Q.; Zhang, Y. Solid and macroporous Fe3C/N-C nanofibers with enhanced electromagnetic wave absorbability. Sci. Rep. 2018, 8, 16832. [Google Scholar] [CrossRef]
- Lekota, M.W.; Mpupa, A.; Dimpe, K.M.; Nomngongo, P.N. Preparation of ferric oxide-aluminium oxide carbon nanofiber nanocomposites for ultrasound-assisted dispersive magnetic solid phase extraction of 17-beta estradiol in wastewater. Emerg. Contam. 2020, 6, 162–171. [Google Scholar] [CrossRef]
- Ponnamma, D.; Aljarod, O.; Parangusan, H.; Ali Al-Maadeed, M. Al Electrospun nanofibers of PVDF-HFP composites containing magnetic nickel ferrite for energy harvesting application. Mater. Chem. Phys. 2020, 239, 122257. [Google Scholar] [CrossRef]
- Amini, F.; Blachowicz, T.; Ehrmann, A. Systematic study of magnetization reversal in beaded fibers from different magnetic materials. J. Magn. Magn. Mater. 2021, 529, 167855. [Google Scholar] [CrossRef]
- Kerker, E.; Steinhäußer, D.; Mamun, A.; Trabelsi, M.; Fiedler, J.; Sabantina, L.; Juhász Junger, I.; Schiek, M.; Ehrmann, A.; Kaschuba, R. Spectroscopic investigation of highly-scattering nanofiber mats during drying and film formation. Optik 2020, 208, 164081. [Google Scholar] [CrossRef]
- Garg, C.; Yang, S.H.; Phung, T.; Pushp, A.; Parkin, S.S.P. Dramatic influence of curvature of nanowire on chiral domain wall velocity. Sci. Adv. 2017, 3, e1602804. [Google Scholar] [CrossRef] [Green Version]
- Blachowicz, T.; Ehrmann, A. Magnetization reversal in bent nanofibers of different cross sections. J. Appl. Phys. 2018, 124, 152112. [Google Scholar] [CrossRef] [Green Version]
- Döpke, C.; Grothe, T.; Steblinski, P.; Klöcker, M.; Sabantina, L.; Kosmalska, D.; Blachowicz, T.; Ehrmann, A. Magnetic nanofiber mats for data storage and transfer. Nanomaterials 2019, 9, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asmatulu, R.; Khan, W.S. Chapter 7—Electrospun nanofibers for filtration applications. In Synthesis and Applications of Electrospun Nanofibers; Asmatulu, R., Khan, W.S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–152. ISBN 978-0-12-813914-1. [Google Scholar]
- Xiong, J.; Zhou, M.; Zhang, H.; Quan, Z.; Wang, R.; Qin, X. Sandwich-structured fibrous membranes with low filtration resistance for effective PM 2.5 capture via one-step needleless electrospinning. Mater. Res. Express 2018, 6, 035027. [Google Scholar] [CrossRef]
- Yalcinkaya, F. A review on advanced nanofiber technology for membrane distillation. J. Eng. Fiber. Fabr. 2019, 14, 1558925018824901. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Yaacob, M.H. Chapter 20—Polyaniline-graphite nanocomposite based modified cladding optical fiber gas sensors. In Handbook of Polymer Nanocomposites for Industrial Applications; Hussain, C.M., Ed.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 545–570. ISBN 978-0-12-821497-8. [Google Scholar]
- Chi, Q.; Zhou, Y.; Feng, Y.; Cui, Y.; Zhang, Y.; Zhang, T.; Chen, Q. Excellent energy storage performance of polyetherimide filled by oriented nanofibers with optimized diameters. Mater. Today Energy 2020, 18, 100516. [Google Scholar] [CrossRef]
- Behera, A.; Mallick, P. Chapter 20—Application of nanofibers in aerospace industry. In Fiber-Reinforced Nanocomposites: Fundamentals and Applications; Han, B., Sharma, S., Nguyen, T.A., Longbiao, L., Bhat, K.S., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 449–457. ISBN 978-0-12-819904-6. [Google Scholar]
- Wang, Y.; Wei, Y.; Wang, B.; Jing, P.; Zhang, Y.; Zhang, Y.; Wang, Q.; Wu, H. Bio-assisted engineering of hierarchical porous carbon nanofiber host in-situ embedded with iron carbide nanocatalysts toward high-performance Li–S batteries. Carbon N. Y. 2021, 177, 60–70. [Google Scholar] [CrossRef]
- Serbezeanu, D.; Popa, A.M.; Stelzig, T.; Sava, I.; Rossi, R.M.; Fortunato, G. Preparation and characterization of thermally stable polyimide membranes by electrospinning for protective clothing applications. Text. Res. J. 2015, 85, 1763–1775. [Google Scholar] [CrossRef]
- Mathew, J.; Joy, J.; George, S.C. Potential applications of nanotechnology in transportation: A review. J. King Saud Univ. Sci. 2019, 31, 586–594. [Google Scholar] [CrossRef]
- Kalalinia, F.; Taherzadeh, Z.; Jirofti, N.; Amiri, N.; Foroghinia, N.; Beheshti, M.; Bazzaz, B.S.F.; Hashemi, M.; Shahroodi, A.; Pishavar, E.; et al. Evaluation of wound healing efficiency of vancomycin-loaded electrospun chitosan/poly ethylene oxide nanofibers in full thickness wound model of rat. Int. J. Biol. Macromol. 2021, 177, 100–110. [Google Scholar] [CrossRef]
- Luu, Y.K.; Kim, K.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA–PEG block copolymers. J. Control. Release 2003, 89, 341–353. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef] [Green Version]
- Barnes, C.P.; Sell, S.A.; Knapp, D.C.; Walpoth, B.H.; Brand, D.D.; Bowlin, G.L. Preliminary Investigation of Electrospun Collagen and Polydioxanone for Vascular Tissue Engineering Applications. Int. J. Electrospun Nanofibers Appl. 2007, 1, 73–87. [Google Scholar]
- Welle, A.; Kröger, M.; Döring, M.; Niederer, K.; Pindel, E.; Chronakis, I.S. Electrospun aliphatic polycarbonates as tailored tissue scaffold materials. Biomaterials 2007, 28, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ali, J.; Zhang, C.; Mailhot, G.; Pan, G. Simultaneously enhanced photocatalytic and antibacterial activities of TiO2/Ag composite nanofibers for wastewater purification. J. Environ. Chem. Eng. 2020, 8, 102104. [Google Scholar] [CrossRef]
- Wu, X.; Li, P.; Cong, L.; Yu, H.; Zhang, D.; Yue, Y.; Xu, H.; Xu, K.; Zheng, X.; Wang, X. Electrospun poly(vinyl alcohol) nanofiber films containing menthol/β-cyclodextrin inclusion complexes for smoke filtration and flavor retention. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 605, 125378. [Google Scholar] [CrossRef]
- Kurian, M.; Paul, A. Recent Trends in the Use of Green Sources for Carbon Dot Synthesis—A Short Review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Scott, E.; Peter, F.; Sanders, J. Biomass in the manufacture of industrial products-the use of proteins and amino acids. Appl. Microbiol. Biotechnol. 2007, 75, 751–762. [Google Scholar] [CrossRef] [Green Version]
- García-Mateos, F.J.; Cordero-Lanzac, T.; Berenguer, R.; Morallón, E.; Cazorla-Amorós, D.; Rodríguez-Mirasol, J.; Cordero, T. Lignin-derived Pt supported carbon (submicron)fiber electrocatalysts for alcohol electro-oxidation. Appl. Catal. B Environ. 2017, 211, 18–30. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Berenguer, R.; Valero-Romero, M.J.; Rodríguez-Mirasol, J.; Cordero, T. Phosphorus functionalization for the rapid preparation of highly nanoporous submicron-diameter carbon fibers by electrospinning of lignin solutions. J. Mater. Chem. A 2018, 6, 1219–1233. [Google Scholar] [CrossRef]
- Berenguer, R.; García-Mateos, F.J.; Ruiz-Rosas, R.; Cazorla-Amorós, D.; Morallón, E.; Rodríguez-Mirasol, J.; Cordero, T. Biomass-derived binderless fibrous carbon electrodes for ultrafast energy storage. Green Chem. 2016, 18, 1506–1515. [Google Scholar] [CrossRef] [Green Version]
- García-Mateos, F.J.; Ruiz-Rosas, R.; María Rosas, J.; Morallón, E.; Cazorla-Amorós, D.; Rodríguez-Mirasol, J.; Cordero, T. Activation of electrospun lignin-based carbon fibers and their performance as self-standing supercapacitor electrodes. Sep. Purif. Technol. 2020, 241, 116724. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Jiang, K.; Thundat, T. Carbonized nanocellulose sustainably boosts the performance of activated carbon in ionic liquid supercapacitors. Nano Energy 2016, 25, 161–169. [Google Scholar] [CrossRef]
- Deng, L.; Young, R.J.; Kinloch, I.A.; Abdelkader, A.M.; Holmes, S.M.; De Haro-Del Rio, D.A.; Eichhorn, S.J. Supercapacitance from cellulose and carbon nanotube nanocomposite fibers. ACS Appl. Mater. Interfaces 2013, 5, 9983–9990. [Google Scholar] [CrossRef]
- Lai, C.; Zhou, Z.; Zhang, L.; Wang, X.; Zhou, Q.; Zhao, Y.; Wang, Y.; Wu, X.F.; Zhu, Z.; Fong, H. Free-standing and mechanically flexible mats consisting of electrospun carbon nanofibers made from a natural product of alkali lignin as binder-free electrodes for high-performance supercapacitors. J. Power Sources 2014, 247, 134–141. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Woo, M.W.; Zhang, L.; Xu, K.Z. Preparation of steam activated carbon from black liquor by flue gas precipitation and its performance in hydrogen sulfide removal: Experimental and simulation works. Rev. Mex. Urol. 2016, 76, 395–404. [Google Scholar] [CrossRef]
- Kumar, M.; Hietala, M.; Oksman, K. Lignin-based electrospun carbon nanofibers. Front. Mater. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, Y.; Chen, J.; Zhu, M.; Yang, C.; Guo, H.; Song, Y.; Li, Y.; Zhou, J. Electrospun biomass based carbon nanofibers as high-performance supercapacitors. Ind. Crops Prod. 2020, 148, 112181. [Google Scholar] [CrossRef]
- Yang, C.; Chen, C.; Pan, Y.; Li, S.; Wang, F.; Li, J.; Li, N.; Li, X.; Zhang, Y.; Li, D. Flexible highly specific capacitance aerogel electrodes based on cellulose nanofibers, carbon nanotubes and polyaniline. Electrochim. Acta 2015, 182, 264–271. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of chitin nanofibers with a uniform width as α-chitin from crab shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Luo, M.; Ming, Y.; Wang, L.; Li, Y.; Li, B.; Chen, J.; Shi, S. Local delivery of deep marine fungus-derived equisetin from polyvinylpyrrolidone (PVP) nanofibers for anti-MRSA activity. Chem. Eng. J. 2018, 350, 157–163. [Google Scholar] [CrossRef]
- Deng, L.; Zhong, W.; Wang, J.; Zhang, P.; Fang, H.; Yao, L.; Liu, X.; Ren, X.; Li, Y. The enhancement of electrochemical capacitance of biomass-carbon by pyrolysis of extracted nanofibers. Electrochim. Acta 2017, 228, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Duan, B.; Gao, X.; Yao, X.; Fang, Y.; Huang, L.; Zhou, J.; Zhang, L. Unique elastic N-doped carbon nanofibrous microspheres with hierarchical porosity derived from renewable chitin for high rate supercapacitors. Nano Energy 2016, 27, 482–491. [Google Scholar] [CrossRef]
- Widiyastuti, W.; Fahrudin Rois, M.; Suari, N.M.I.P.; Setyawan, H. Activated carbon nanofibers derived from coconut shell charcoal for dye removal application. Adv. Powder Technol. 2020, 31, 3267–3273. [Google Scholar] [CrossRef]
- Chung, S.; Shin, D.; Choun, M.; Kim, J.; Yang, S.; Choi, M.; Kim, J.W.; Lee, J. Improved water management of Pt/C cathode modified by graphitized carbon nanofiber in proton exchange membrane fuel cell. J. Power Sources 2018, 399, 350–356. [Google Scholar] [CrossRef]
- He, Z.; Li, M.; Li, Y.; Zhu, J.; Jiang, Y.; Meng, W.; Zhou, H.; Wang, L.; Dai, L. Flexible electrospun carbon nanofiber embedded with TiO2 as excellent negative electrode for vanadium redox flow battery. Electrochim. Acta 2018, 281, 601–610. [Google Scholar] [CrossRef]
- Abdelkareem, M.A.; Al Haj, Y.; Alajami, M.; Alawadhi, H.; Barakat, N.A.M. Ni-Cd carbon nanofibers as an effective catalyst for urea fuel cell. J. Environ. Chem. Eng. 2018, 6, 332–337. [Google Scholar] [CrossRef]
- Zhao, R.; Yong, X.; Pan, M.; Deng, J.; Pan, K. Aldehyde-containing nanofibers electrospun from biomass vanillin-derived polymer and their application as adsorbent. Sep. Purif. Technol. 2020, 246, 116916. [Google Scholar] [CrossRef]
- Zainab, G.; Babar, A.A.; Ali, N.; Aboalhassan, A.A.; Wang, X.; Yu, J.; Ding, B. Electrospun carbon nanofibers with multi-aperture/opening porous hierarchical structure for efficient CO2 adsorption. J. Colloid Interface Sci. 2020, 561, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Hellert, C.; Wortmann, M.; Frese, N.; Grötsch, G.; Cornelißen, C.; Ehrmann, A. Adhesion of Electrospun Poly(acrylonitrile) Nanofibers on Conductive and Isolating Foil Substrates. Coatings 2021, 11, 249. [Google Scholar] [CrossRef]
- Banner, J.; Dautzenberg, M.; Feldhans, T.; Hofmann, J.; Plümer, P.; Ehrmann, A. Water resistance and morphology of electrospun gelatine blended with citric acid and coconut oil. Tekstilec 2018, 61, 129–135. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Mohideen, M.; Ramakrishna, S. (Eds.) Chapter 1—Development of melt electrospinning: The past, present, and future. In Melt Electrospinning; Academic Press: Cambridge, MA, USA, 2019; pp. 1–5. ISBN 978-0-12-816220-0. [Google Scholar]
- Kijeńska, E.; Swieszkowski, W. 2—General requirements of electrospun materials for tissue engineering: Setups and strategy for successful electrospinning in laboratory and industry. In Electrospun Materials for Tissue Engineering and Biomedical Applications; Uyar, T., Kny, E., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 43–56. ISBN 978-0-08-101022-8. [Google Scholar]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Khalili Amand, F.; Esmaeili, A. Investigating the properties of electrospun nanofibers made of hybride polymer containing anticoagulant drugs. Carbohydr. Polym. 2020, 228, 115397. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Jadhav, A.; Wang, L.; Wang, X. Electrospinning from a convex needle with multiple jet toward better controlling and enhanced production rate. J. Appl. Polym. Sci. 2019, 136, 1–7. [Google Scholar] [CrossRef]
- Hwang, M.; Karenson, M.O.; Elabd, Y.A. High Production Rate of High Purity, High Fidelity Nafion Nanofibers via Needleless Electrospinning. ACS Appl. Polym. Mater. 2019, 1, 2731–2740. [Google Scholar] [CrossRef]
- Rosenthal, T.; Weller, J.M.; Chan, C.K. Needleless Electrospinning for High Throughput Production of Li7La3Zr2O12 Solid Electrolyte Nanofibers. Ind. Eng. Chem. Res. 2019, 58, 17399–17405. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Komarek, M. Polyvinyl Butyral (PVB) Nanofiber/Nanoparticle-Covered Yarns for Antibacterial Textile Surfaces. Int. J. Mol. Sci. 2019, 20, 4317. [Google Scholar] [CrossRef] [Green Version]
- Bavatharani, C.; Muthusankar, E.; Wabaidur, S.M.; Alothman, Z.A.; Alsheetan, K.M.; mana AL-Anazy, M.; Ragupathy, D. Electrospinning technique for production of polyaniline nanocomposites/nanofibres for multi-functional applications: A review. Synth. Met. 2021, 271, 116609. [Google Scholar] [CrossRef]
- Sundera Murthe, S.; Mohamed Saheed, M.S.; Perumal, V.; Mohamed Saheed, M.S.; Mohamed, N.M. 11—Electrospun Nanofibers for Biosensing Applications. In Nanobiosensors for Biomolecular Targeting; Gopinath, S.C.B., Lakshmipriya, T., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–267. ISBN 978-0-12-813900-4. [Google Scholar]
- Trabelsi, M.; Mamun, A.; Klöcker, M.; Sabantina, L.; Großerhode, C.; Blachowicz, T.; Ehrmann, A. Increased mechanical properties of carbon nanofiber mats for possible medical applications. Fibers 2019, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Sabantina, L.; Hes, L.; Mirasol, J.R.; Cordero, T.; Ehrmann, A. Water vapor permeability through PAN nanofiber mat with varying membrane-like areas. Fibres Text. East. Eur. 2019, 27, 12–15. [Google Scholar] [CrossRef]
- Molnár, K.; Szolnoki, B.; Toldy, A.; Vas, L.M. Thermochemical stabilization and analysis of continuously electrospun nanofibers: Carbon nanotube-loaded polyacrylonitrile nanofibers for high performance carbon nanofiber mass production. J. Therm. Anal. Calorim. 2014, 117, 1123–1135. [Google Scholar] [CrossRef]
- Liu, C.; Lafdi, K. Fabrication and characterization of carbon nanofibers from polyacrylonitrile/pitch blends. J. Appl. Polym. Sci. 2017, 134, 45388. [Google Scholar] [CrossRef]
- Gergin, I.; Ismar, E.; Sarac, A.S. Oxidative stabilization of polyacrylonitrile nanofibers and carbon nanofibers containing graphene oxide (GO): A spectroscopic and electrochemical study. Beilstein J. Nanotechnol. 2017, 8, 1616–1628. [Google Scholar] [CrossRef] [Green Version]
- Sabantina, L.; Klöcker, M.; Wortmann, M.; Mirasol, J.R.; Cordero, T.; Moritzer, E.; Finsterbusch, K.; Ehrmann, A. Stabilization of polyacrylonitrile nanofiber mats obtained by needleless electrospinning using dimethyl sulfoxide as solvent. J. Ind. Text. 2020, 50, 224–239. [Google Scholar] [CrossRef]
- Sabantina, L.; Mirasol, J.R.; Cordero, T.; Finsterbusch, K.; Ehrmann, A. Investigation of needleless electrospun PAN nanofiber mats. AIP Conf. Proc. 2018, 1952, 020085. [Google Scholar] [CrossRef]
- Bashir, Z. A critical review of the stabilisation of polyacrylonitrile. Carbon N. Y. 1991, 29, 1081–1090. [Google Scholar] [CrossRef]
- Dalton, S.; Heatley, F.; Budd, P.M. Thermal stabilization of polyacrylonitrile fibres. Polymer 1999, 40, 5531–5543. [Google Scholar] [CrossRef]
- Ibupoto, A.S.; Qureshi, U.A.; Ahmed, F.; Khatri, Z.; Khatri, M.; Maqsood, M.; Brohi, R.Z.; Kim, I.S. Reusable carbon nanofibers for efficient removal of methylene blue from aqueous solution. Chem. Eng. Res. Des. 2018, 136, 744–752. [Google Scholar] [CrossRef]
- Jo, E.; Yeo, J.G.; Kim, D.K.; Oh, J.S.; Hong, C.K. Preparation of well-controlled porous carbon nanofiber materials by varying the compatibility of polymer blends. Polym. Int. 2014, 63, 1471–1477. [Google Scholar] [CrossRef]
- Ji, L.; Yao, Y.; Toprakci, O.; Lin, Z.; Liang, Y.; Shi, Q.; Medford, A.J.; Millns, C.R.; Zhang, X. Fabrication of carbon nanofiber-driven electrodes from electrospun polyacrylonitrile/polypyrrole bicomponents for high-performance rechargeable lithium-ion batteries. J. Power Sources 2010, 195, 2050–2056. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Controlling the Composition, Morphology, Porosity, and Surface Chemistry of Lignin-Based Electrospun Carbon Materials. Front. Mater. 2019, 6, 114. [Google Scholar] [CrossRef] [Green Version]
- Miao, F.; Shao, C.; Li, X.; Wang, K.; Liu, Y. Flexible solid-state supercapacitors based on freestanding nitrogen-doped porous carbon nanofibers derived from electrospun polyacrylonitrile@polyaniline nanofibers. J. Mater. Chem. A 2016, 4, 4180–4187. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Chen, L.; Dong, Y.; Xie, P.; Li, Q. Hydrogen production through hydrolysis of sodium borohydride: Highly dispersed CoB particles immobilized in carbon nanofibers as a novel catalyst. Int. J. Hydrogen Energy 2020, 45, 32145–32156. [Google Scholar] [CrossRef]
- Mamun, A.; Trabelsi, M.; Klöcker, M.; Lukas Storck, J.; Böttjer, R.; Sabantina, L. Needleless electrospun polyacrylonitrile/konjac glucomannan nanofiber mats. J. Eng. Fiber. Fabr. 2020, 15, 1558925020964806. [Google Scholar] [CrossRef]
- Ma, C.; Li, Z.; Li, J.; Fan, Q.; Wu, L.; Shi, J.; Song, Y. Lignin-based hierarchical porous carbon nanofiber films with superior performance in supercapacitors. Appl. Surf. Sci. 2018, 456, 568–576. [Google Scholar] [CrossRef]
- Calvo-Muñoz, E.M.; García-Mateos, F.J.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Biomass waste carbon materials as adsorbents for CO 2 capture under post-combustion conditions. Front. Mater. 2016, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Kang, K.; Wu, J.; Hu, Q.; Harper, D.P.; Du, G.; Wang, S.; Xu, K. Comparative effects of electrospinning ways for fabricating green, sustainable, flexible, porous, nanofibrous cellulose/chitosan carbon mats as anode materials for lithium-ion batteries. J. Mater. Res. Technol. 2021, 11, 50–61. [Google Scholar] [CrossRef]
- Tao, L.; Huang, Y.; Zheng, Y.; Yang, X.; Liu, C.; Di, M.; Larpkiattaworn, S.; Nimlos, M.R.; Zheng, Z. Porous carbon nanofiber derived from a waste biomass as anode material in lithium-ion batteries. J. Taiwan Inst. Chem. Eng. 2019, 95, 217–226. [Google Scholar] [CrossRef]
- Song, K.; Wu, Q.; Zhang, Z.; Ren, S.; Lei, T.; Negulescu, I.I.; Zhang, Q. Porous Carbon Nanofibers from Electrospun Biomass Tar/Polyacrylonitrile/Silver Hybrids as Antimicrobial Materials. ACS Appl. Mater. Interfaces 2015, 7, 15108–15116. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadi, F.; Amiri, M. Fabrication of MEA from Biomass-Based Carbon Nanofibers Composited with Nickel-Cobalt Oxides as a New Electrocatalyst for Oxygen Reduction Reaction in Passive Direct Methanol Fuel Cells. Electrocatalysis 2021, 11, 485–496. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Shi, G.-F.; Chen, B.; Wang, G.-Y.; Guo, T.-C. Biomass-Based Carbon Nanofibers Prepared by Electrospinning for Supercapacitor. J. Nanosci. Nanotechnol. 2018, 18, 5731–5737. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, H.; Cao, Q.; Zheng, H.; Xu, D.; Guo, H.; Wang, S.; Li, Y.; Zhou, J. Electrospun Lignin-Based Carbon Nanofibers as Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 12831–12841. [Google Scholar] [CrossRef]
- Jung, K.H.; Deng, W.; Smith, D.W.; Ferraris, J.P. Carbon nanofiber electrodes for supercapacitors derived from new precursor polymer: Poly(acrylonitrile-co-vinylimidazole). Electrochem. Commun. 2012, 23, 149–152. [Google Scholar] [CrossRef]
- Guo, J.; Sun, G.; Wang, Q.; Wang, G.; Zhou, Z.; Tang, S.; Jiang, L.; Zhou, B.; Xin, Q. Carbon nanofibers supported Pt-Ru electrocatalysts for direct methanol fuel cells. Carbon N. Y. 2006, 44, 152–157. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, D.; Dong, C.; Li, H.; Xiong, K.; Fei, L.; Qian, Y. Carbon nanofibers: Synthesis, characterization, and electrochemical properties. Carbon N. Y. 2006, 44, 828–832. [Google Scholar] [CrossRef]
- García-Díaz, I.; López, F.A.; Alguacil, F.J. Carbon nanofibers: A new adsorbent for copper removal from wastewater. Metals 2018, 8, 914. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, A.; Deva, D.; Sharma, A.; Verma, N. Adsorbents based on carbon microfibers and carbon nanofibers for the removal of phenol and lead from water. J. Colloid Interface Sci. 2011, 359, 228–239. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef]
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon nanotubes as optical biomedical sensors. Adv. Drug Deliv. Rev. 2013, 65, 1933–1950. [Google Scholar] [CrossRef] [PubMed]
- Sheikhpour, M.; Golbabaie, A.; Kasaeian, A. Carbon nanotubes: A review of novel strategies for cancer diagnosis and treatment. Mater. Sci. Eng. C 2017, 76, 1289–1304. [Google Scholar] [CrossRef]
- Liang, M.; Liu, Y.; Xiao, B.; Yang, S.; Wang, Z.; Han, H. An analytical model for the transverse permeability of gas diffusion layer with electrical double layer effects in proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2018, 43, 17880–17888. [Google Scholar] [CrossRef]
- Liang, M.; Fu, C.; Xiao, B.; Luo, L.; Wang, Z. A fractal study for the effective electrolyte diffusion through charged porous media. Int. J. Heat Mass Transf. 2019, 137, 365–371. [Google Scholar] [CrossRef]
- Chan, S.; Jankovic, J.; Susac, D.; Saha, M.S.; Tam, M.; Yang, H.; Ko, F. Electrospun carbon nanofiber catalyst layers for polymer electrolyte membrane fuel cells: Structure and performance. J. Power Sources 2018, 392, 239–250. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Skupov, K.M.; Naumkin, A.V.; Basu, V.G.; Zhigalina, O.M.; Razorenov, D.Y.; Ponomarev, I.I.; Volkova, Y.A. Probing of complex carbon nanofiber paper as gas-diffusion electrode for high temperature polymer electrolyte membrane fuel cell. RSC Adv. 2019, 9, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Bui, H.T.; Kim, D.Y.; Kim, D.W.; Suk, J.; Kang, Y. Carbon nanofiber@platinum by a coaxial electrospinning and their improved electrochemical performance as a Li−O2 battery cathode. Carbon N. Y. 2018, 130, 94–104. [Google Scholar] [CrossRef]
- Yoon, K.R.; Shin, K.; Park, J.; Cho, S.H.; Kim, C.; Jung, J.W.; Cheong, J.Y.; Byon, H.R.; Lee, H.M.; Kim, I.D. Brush-Like Cobalt Nitride Anchored Carbon Nanofiber Membrane: Current Collector-Catalyst Integrated Cathode for Long Cycle Li-O2 Batteries. ACS Nano 2018, 12, 128–139. [Google Scholar] [CrossRef]
- Xia, J.; Yuan, Y.; Yan, H.; Liu, J.; Zhang, Y.; Liu, L.; Zhang, S.; Li, W.; Yang, X.; Shu, H.; et al. Electrospun SnSe/C nanofibers as binder-free anode for lithium–ion and sodium-ion batteries. J. Power Sources 2020, 449, 227559. [Google Scholar] [CrossRef]
- Zhao, W.; Ci, S.; Hu, X.; Chen, J.; Wen, Z. Highly dispersed ultrasmall NiS2 nanoparticles in porous carbon nanofiber anodes for sodium ion batteries. Nanoscale 2019, 11, 4688–4695. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, M.; Wang, L.; Huang, Q.; Su, Z.; Li, L.; Wang, X.; Li, Y.; Zeng, C.; Guo, Z. Synthesis and Electrochemical Performance of ZnSe Electrospinning Nanofibers as an Anode Material for Lithium Ion and Sodium Ion Batteries. Front. Chem. 2019, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Qu, H.Q.; Liu, Z.; Jiang, R.Z.; Li, C.; Zhu, M.Q. Long cycle life and high rate capability of three dimensional CoSe 2 grain-attached carbon nanofibers for flexible sodium-ion batteries. Nano Energy 2019, 58, 715–723. [Google Scholar] [CrossRef]
- Dai, Z.; Ren, P.-G.; Jin, Y.-L.; Zhang, H.; Ren, F.; Zhang, Q. Nitrogen-sulphur Co-doped graphenes modified electrospun lignin/polyacrylonitrile-based carbon nanofiber as high performance supercapacitor. J. Power Sources 2019, 437, 226937. [Google Scholar] [CrossRef]
- Wang, P.; Gong, Z.; Ye, K.; Gao, Y.; Zhu, K.; Yan, J.; Wang, G.; Cao, D. The stable lithium metal cell with two-electrode biomass carbon. Electrochim. Acta 2020, 356, 136824. [Google Scholar] [CrossRef]
- Worch, E. Adsorption Technology in Water Treatment; Walter de Gruyter GmbH: Berlin, Germany; Co. KG: Boston, MA, USA, 2012; ISBN 9783110240221. [Google Scholar]
- Dey, S.; Bano, F.; Malik, A. Pharmaceuticals and personal care product (PPCP) contamination—A global discharge inventory. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Butterworth-Heinemann, Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 1–26. ISBN 9780128161890. [Google Scholar]
- Zhou, X.; Liu, B.; Chen, Y.; Guo, L.; Wei, G. Carbon nanofiber-based three-dimensional nanomaterials for energy and environmental applications. Mater. Adv. 2020, 1, 2163–2181. [Google Scholar] [CrossRef]
- Gan, L.; Geng, A.; Song, C.; Xu, L.; Wang, L.; Fang, X.; Han, S.; Cui, J.; Mei, C. Simultaneous removal of rhodamine B and Cr(VI) from water using cellulose carbon nanofiber incorporated with bismuth oxybromide: The effect of cellulose pyrolysis temperature on photocatalytic performance. Environ. Res. 2020, 185, 109414. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Babar, A.A.; Zhang, Y.; Iqbal, N.; Wang, X.; Yu, J.; Ding, B. Porous, flexible, and core-shell structured carbon nanofibers hybridized by tin oxide nanoparticles for efficient carbon dioxide capture. J. Colloid Interface Sci. 2020, 560, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.C.; Wu, C.Y.; Chen, Y.J. Effects of activation on the properties of electrospun carbon nanofibers and their adsorption performance for carbon dioxide. Sep. Purif. Technol. 2020, 233, 116040. [Google Scholar] [CrossRef]
- Chaniotakis, N.; Vamvakaki, V.; Tsagaraki, K.; Chaniotakis, N. Carbon Nanofiber-Based Glucose Biosensor Carbon Nanofiber-Based Glucose Biosensor. Anal. Chem. 2006, 78, 5538–5542. [Google Scholar]
- Tran, P.A.; Zhang, L.; Webster, T.J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1097–1114. [Google Scholar] [CrossRef] [PubMed]
- Swisher, L.Z.; Prior, A.M.; Gunaratna, M.J.; Shishido, S.; Madiyar, F.; Nguyen, T.A.; Hua, D.H.; Li, J. Quantitative electrochemical detection of cathepsin B activity in breast cancer cell lysates using carbon nanofiber nanoelectrode arrays toward identification of cancer formation. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1695–1704. [Google Scholar] [CrossRef] [Green Version]
- Olenic, L.; Mihailescu, G.; Pruneanu, S.; Lupu, D.; Biris, A.R.; Margineanu, P.; Garabagiu, S.; Biris, A.S. Investigation of carbon nanofibers as support for bioactive substances. J. Mater. Sci. Mater. Med. 2009, 20, 177–183. [Google Scholar] [CrossRef]
- Nguyen-Vu, T.D.B.; Chen, H.; Cassell, A.M.; Andrews, R.J.; Meyyappan, M.; Li, J. Vertically aligned carbon nanofiber architecture as a multifunctional 3-D neural electrical interface. IEEE Trans. Biomed. Eng. 2007, 54, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Waid, M.C.; McKenzie, J.L.; Price, R.L.; Ejiofor, J.U. Nano-biotechnology: Carbon nanofibres as improved neural and orthopaedic implants. Nanotechnology 2004, 15, 48–54. [Google Scholar] [CrossRef]
- Khang, D.; Sato, M.; Price, R.L.; Ribbe, A.E.; Webster, T.J. Selective adhesion and mineral deposition by osteoblasts on carbon nanofiber patterns. Int. J. Nanomed. 2006, 1, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, K.; Haniu, H.; Kim, Y.A.; Saito, N. The use of electrospun organic and carbon nanofibers in bone regeneration. Nanomaterials 2020, 10, 562. [Google Scholar] [CrossRef] [Green Version]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An Overview on Application of Natural Substances Incorporated with Electrospun Nanofibrous Scaffolds to Development of Innovative Wound Dressings. Mini Rev. Med. Chem. 2017, 18, 414–427. [Google Scholar] [CrossRef]
- Sylvester, M.A.; Amini, F.; Keat, T.C. Electrospun nanofibers in wound healing. Mater. Today Proc. 2019, 29, 1–6. [Google Scholar] [CrossRef]
- Saito, N.; Aoki, K.; Usui, Y.; Shimizu, M.; Hara, K.; Narita, N.; Ogihara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; et al. Application of carbon fibers to biomaterials: A new era of nano-level control of carbon fibers after 30-years of development. Chem. Soc. Rev. 2011, 40, 3824–3834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Aboagye, A.; Kelkar, A.; Lai, C.; Fong, H. A review: Carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480. [Google Scholar] [CrossRef]
- Smolka, W.; Panek, A.; Gubernat, M.; Szczypta-Fraczek, A.; Jelen, P.; Paluszkiewicz, C.; Markowski, J.; Blazewicz, M. Structure and Biological Properties of Surface-Engineered Carbon Nanofibers. J. Nanomater. 2019, 2019, 4146190. [Google Scholar] [CrossRef]
- Li, Z.; Milionis, A.; Zheng, Y.; Yee, M.; Codispoti, L.; Tan, F.; Poulikakos, D.; Yap, C.H. Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. In Proceedings of the Nature Communications; Springer: New York, NY, USA, 2019; Volume 10. [Google Scholar]
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Zanjanizadeh Ezazi, N.; Liu, D.; Santos, H.A. Electrospun Fibrous Architectures for Drug Delivery, Tissue Engineering and Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1802852. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lopresti, F.; Botta, L. Nanocarbons in electrospun polymeric nanomats for tissue engineering: A review. Polymers 2017, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Ngadiman, N.H.A.; Noordin, M.Y.; Idris, A.; Kurniawan, D. A review of evolution of electrospun tissue engineering scaffold: From two dimensions to three dimensions. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 597–616. [Google Scholar] [CrossRef]
- Ma, G.; Fang, D.; Liu, Y.; Zhu, X.; Nie, J. Electrospun sodium alginate/poly(ethylene oxide) core-shell nanofibers scaffolds potential for tissue engineering applications. Carbohydr. Polym. 2012, 87, 737–743. [Google Scholar] [CrossRef]
- Xu, C.; Inai, R.; Kotaki, M.; Ramakrishna, S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004, 10, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Ndreu, A.; Nikkola, L.; Ylikauppila, H.; Ashammakhi, N.; Hasirci, V. Electrospun biodegradable nanofibrous mats for tissue engineering. Nanomedicine 2008, 3, 45–60. [Google Scholar] [CrossRef]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C 2019, 103, 109768. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent advances in carbon nanofibers and their applications—A review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Nekounam, H.; Allahyari, Z.; Gholizadeh, S.; Mirzaei, E.; Shokrgozar, M.A.; Faridi-Majidi, R. Simple and robust fabrication and characterization of conductive carbonized nanofibers loaded with gold nanoparticles for bone tissue engineering applications. Mater. Sci. Eng. C 2020, 117, 111226. [Google Scholar] [CrossRef]
- Samadian, H.; Mobasheri, H.; Hasanpour, S.; Ai, J.; Azamie, M.; Faridi-Majidi, R. Electro-conductive carbon nanofibers as the promising interfacial biomaterials for bone tissue engineering. J. Mol. Liq. 2020, 298, 112021. [Google Scholar] [CrossRef]
- Stocco, T.D.; Antonioli, E.; Romagnolli, M.L.; Sousa, G.F.; Ferretti, M.; Lobo, A.O. Aligned biomimetic scaffolds based on carbon nanotubes-reinforced polymeric nanofibers for knee meniscus tissue engineering. Mater. Lett. 2020, 264, 127351. [Google Scholar] [CrossRef]
- Patel, K.D.; Kim, T.H.; Mandakhbayar, N.; Singh, R.K.; Jang, J.H.; Lee, J.H.; Kim, H.W. Coating biopolymer nanofibers with carbon nanotubes accelerates tissue healing and bone regeneration through orchestrated cell- and tissue-regulatory responses. Acta Biomater. 2020, 108, 97–110. [Google Scholar] [CrossRef]
- Serafin, A.; Murphy, C.; Rubio, M.C.; Collins, M.N. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Mater. Sci. Eng. C 2021, 122, 111927. [Google Scholar] [CrossRef] [PubMed]
- Zhijiang, C.; Cong, Z.; Jie, G.; Qing, Z.; Kongyin, Z. Electrospun carboxyl multi-walled carbon nanotubes grafted polyhydroxybutyrate composite nanofibers membrane scaffolds: Preparation, characterization and cytocompatibility. Mater. Sci. Eng. C 2018, 82, 29–40. [Google Scholar] [CrossRef]
- Dai, J.; Luo, Y.; Nie, D.; Jin, J.; Yang, S.; Li, G.; Yang, Y.; Zhang, W. pH/photothermal dual-responsive drug delivery and synergistic chemo-photothermal therapy by novel porous carbon nanofibers. Chem. Eng. J. 2020, 397, 125402. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Taha, M.; Mousa, H.M.; Bartnikowski, M.; Hassan, M.L.; Dewidar, M.; Ivanovski, S. Engineering of electrically-conductive poly(ε-caprolactone)/ multi-walled carbon nanotubes composite nanofibers for tissue engineering applications. Ceram. Int. 2019, 45, 15736–15740. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Shrestha, S.; Tiwari, A.P.; Kim, J.I.; Ko, S.W.; Kim, H.J.; Park, C.H.; Kim, C.S. Bio-inspired hybrid scaffold of zinc oxide-functionalized multi-wall carbon nanotubes reinforced polyurethane nanofibers for bone tissue engineering. Mater. Des. 2017, 133, 69–81. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; Ortiz, A.G.; Barrera, E.V. Carbon nano-onions reinforced multilayered thin film system for stimuli-responsive drug release. Pharmaceutics 2020, 12, 1208. [Google Scholar] [CrossRef] [PubMed]
- Mykhailiv, O.; Zubyk, H.; Plonska-Brzezinska, M.E. Carbon nano-onions: Unique carbon nanostructures with fascinating properties and their potential applications. Inorg. Chim. Acta 2017, 468, 49–66. [Google Scholar] [CrossRef]
- Ahlawat, J.; Masoudi Asil, S.; Guillama Barroso, G.; Nurunnabi, M.; Narayan, M. Application of carbon nano onions in the biomedical field: Recent advances and challenges. Biomater. Sci. 2021, 9, 626–644. [Google Scholar] [CrossRef]
- Jin, H.; Wu, S.; Li, T.; Bai, Y.; Wang, X.; Zhang, H.; Xu, H.; Kong, C.; Wang, H. Synthesis of porous carbon nano-onions derived from rice husk for high-performance supercapacitors. Appl. Surf. Sci. 2019, 488, 593–599. [Google Scholar] [CrossRef]
- Breczko, J.; Winkler, K.; Plonska-Brzezinska, M.E.; Villalta-Cerdas, A.; Echegoyen, L. Electrochemical properties of composites containing small carbon nano-onions and solid polyelectrolytes. J. Mater. Chem. 2010, 20, 7761–7768. [Google Scholar] [CrossRef]
- Breczko, J.; Plonska-Brzezinska, M.E.; Echegoyen, L. Electrochemical oxidation and determination of dopamine in the presence of uric and ascorbic acids using a carbon nano-onion and poly(diallyldimethylammonium chloride) composite. Electrochim. Acta 2012, 72, 61–67. [Google Scholar] [CrossRef]
- Giordani, S.; Bartelmess, J.; Frasconi, M.; Biondi, I.; Cheung, S.; Grossi, M.; Wu, D.; Echegoyen, L.; O’Shea, D.F. NIR fluorescence labelled carbon nano-onions: Synthesis, analysis and cellular imaging. J. Mater. Chem. B 2014, 2, 7459–7463. [Google Scholar] [CrossRef] [PubMed]



| Precursors | Heat Treatment | Reference |
|---|---|---|
| Polyacrylonitrile (PAN)/gelatine | Stabilization: between 240 °C and 300 °C, heating rates between 0.5 K min−1 and 4 K min−1, 1 h at the final temperature. Carbonization: at 800 °C, heating rate of 10 K min−1 in N2, 1 h at the final temperature. | [14] |
| PAN/mycelium | Stabilization: at 280 °C for 1 h, heating rate of 1 K min−1, 1 h at the final temperature. Carbonization: at 500 °C for 1 h, heating rate of 10 K min−1 in N2, 1 h at the final temperature. | [19] |
| PAN/konjac glucomannan (KGM) Amorphophallus konjac | Stabilization: at 280 °C for 1 h, heating rate of 1 K min−1, 1 h at the final temperature. Carbonization: at 500 °C for 1 h, heating rate of 10 K min−1 in N2, 1 h at the final temperature. | [111] |
| Mg(NO3)2. 6H2O/lignin | Stabilization: (1) Temperature was increased from 25 to 150 °C, heating rate of 1 K min−1, 24 h. (2) Temperature was increased from 150 to 350°, heating rate of 1 K min−1, 4 h. Carbonization: at 800 °C for 1 h, heating rate of 3 K min−1 in N2. | [112] |
| Lignin | Stabilization: at 200 °C, heating rate of 0.08 K min−1, 48 h at the final temperature. Carbonization: at 900 °C, in N2, heating rate of 10 K min−1, 2 h at the final temperature. | [113] |
| Cellulose acetate/lignin | Stabilization: at 220 °C, heating rate of 0.4 K min−1, 12 h at the final temperature. Carbonization: 600 °C, heating rate of 4.0 K min−1 in N2, 2 h at the final temperature. | [72] |
| H3PO4/lignin | Stabilization: (1) without H3PO4: at 200 °C, heating rate of 0.08 K min−1, 60 h at the final temperature. (2) with H3PO4: at 200 °C, heating rate of 1 K min−1, 1 h at the final temperature. Carbonization: at 900 °C, under low concentration of O2. | [64] |
| Lignin/polyvinyl acetate (PVA) | Stabilization: (1) Temperature was increased from 25 to 100 °C, heating rate of 10 K min−1, 2 h. (2) Temperature was increased from 100 to 180 °C, heating rate of 1 K min−1, 16 h. (3) Temperature was increased from 180 to 220 °C, heating rate of 0.5 K min−1, 8 h. Carbonization: Temperature was increased from 25 to 1200 °C, heating rate of 5 K min−1 in argon, 1 h. | [69] |
| Cellulose/ chitosan | Stabilization: at 270 °C, heating rate of 2 K min−1, 2.5 h at the final temperature. Carbonization: at 900 °C, heating rate 2 K min−1, 2 h at the final temperature. | [114] |
| PVA/ walnut shell powder | Carbonization in one-step: between 800 and 1200 °C, heating rate of 5 K min−1, 1 h at the final temperature. | [115] |
| Tar/PAN/ silver (Ag) | Stabilization: at 300 °C, heating rate of 1 K min−1, 1 h at the final temperature. Carbonization: 900 °C, heating rate of 5 K min−1 in N2, 1 h at the final temperature. | [116] |
| NFKP/Ni–Co Typha domingensis | Stabilization: 12 h at 200 °C. (unspecified heating rate) Carbonization: at 700 °C, argon, 3 h (unspecified heating rate) | [117] |
| Aconitum sinomontanum Nakai/PAN | Stabilization: at 280 °C, heating rate 1 K min−1, 3 h at the final temperature. Carbonization: at 800 °C, heating rate of 2 K min−1 in N2, 1 h at the final temperature. | [118] |
| Lignin/PAN; Lignin/PAN/KOH | Stabilization: at 220 °C, heating rate 0.5 K min−1, 4 h at the final temperature. Carbonization: (1) At 1000 °C in N2, heating rate of 4 K min−1, 4 h (2) Lignin/PAN + KOH; 800 °C, heating rate of 4 K min−1 in N2, 1 h | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moulefera, I.; Trabelsi, M.; Mamun, A.; Sabantina, L. Electrospun Carbon Nanofibers from Biomass and Biomass Blends—Current Trends. Polymers 2021, 13, 1071. https://doi.org/10.3390/polym13071071
Moulefera I, Trabelsi M, Mamun A, Sabantina L. Electrospun Carbon Nanofibers from Biomass and Biomass Blends—Current Trends. Polymers. 2021; 13(7):1071. https://doi.org/10.3390/polym13071071
Chicago/Turabian StyleMoulefera, Imane, Marah Trabelsi, Al Mamun, and Lilia Sabantina. 2021. "Electrospun Carbon Nanofibers from Biomass and Biomass Blends—Current Trends" Polymers 13, no. 7: 1071. https://doi.org/10.3390/polym13071071
APA StyleMoulefera, I., Trabelsi, M., Mamun, A., & Sabantina, L. (2021). Electrospun Carbon Nanofibers from Biomass and Biomass Blends—Current Trends. Polymers, 13(7), 1071. https://doi.org/10.3390/polym13071071









