Remarkable Thermal Conductivity of Epoxy Composites Filled with Boron Nitride and Cured under Pressure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- PCTP2: 2 μm, 10 μm, 0.2 g/cm3, 10 m2/g;
- PCTP30: 30 μm, 100 μm, 0.6 g/cm3, 1 m2/g;
- PCTP30D: 180 μm, 1600 μm, 0.6 g/cm3, 1 m2/g;
- CTS7M: 120 μm, 180 μm, 0.5 g/cm3, 3.5 m2/g.
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Thermal Conductivity
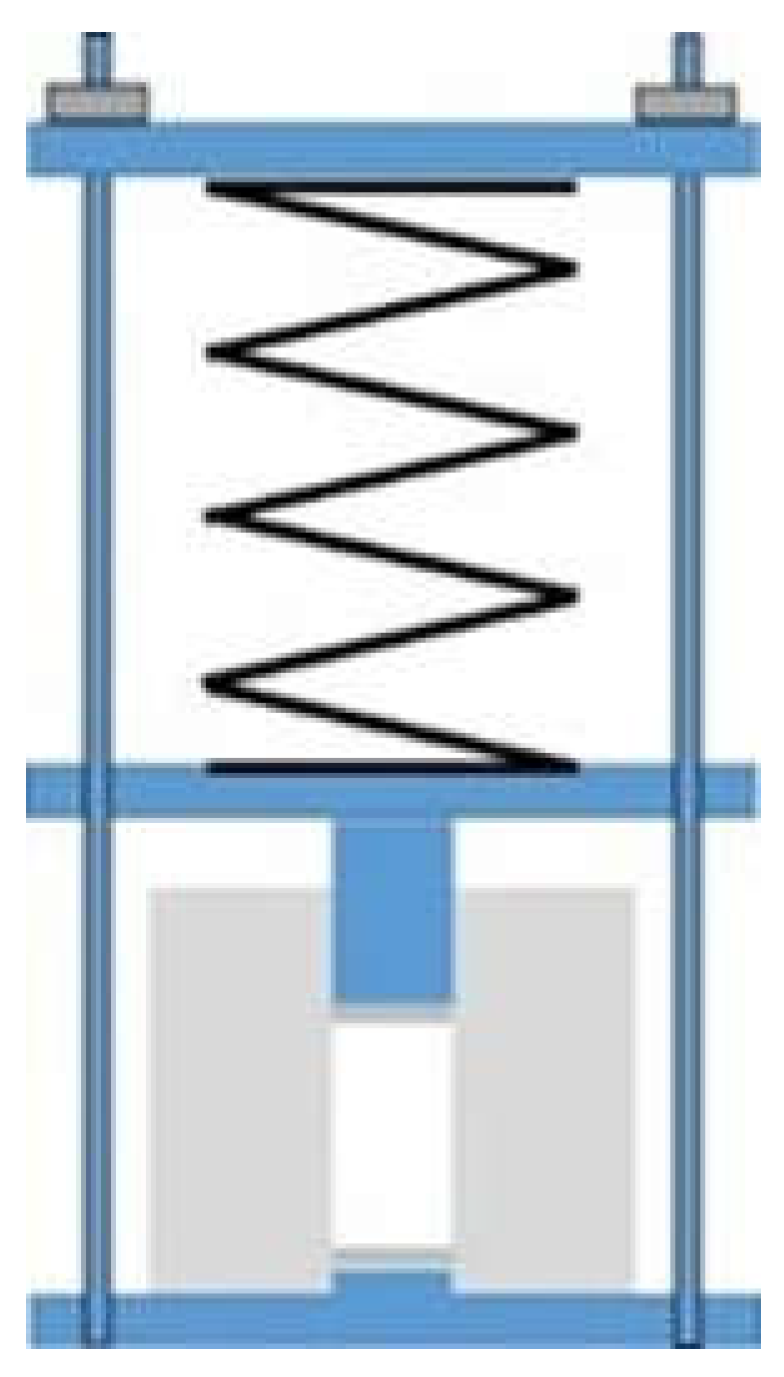
2.2.3. Compressed Samples
2.2.4. Density
2.2.5. Scanning Electron Microscopy (SEM)
3. Results and Discussion
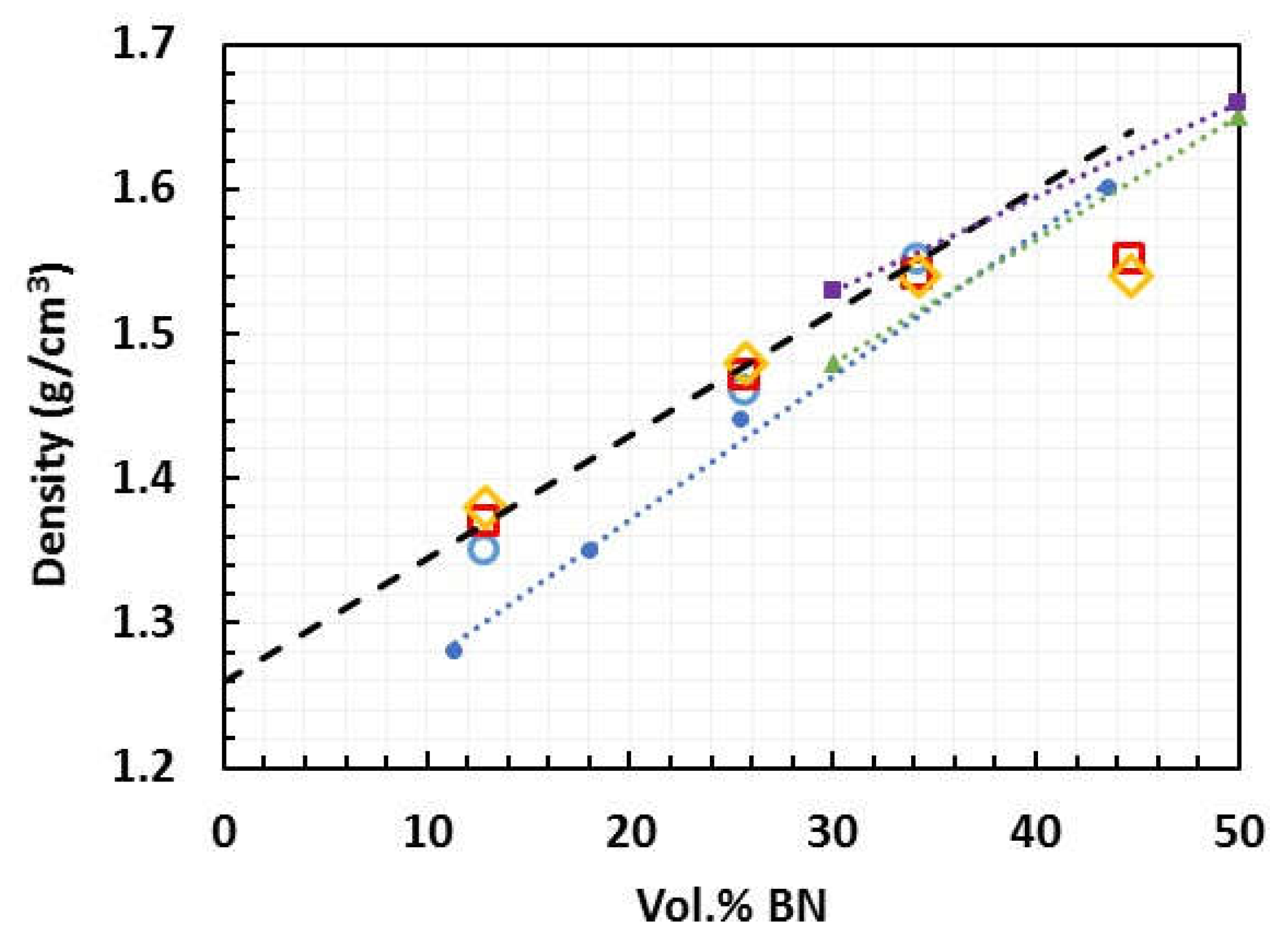
3.1. Density Measurements
3.2. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of thermal conductivity in composites: Mechanisms, parameters and theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; Moradi, S. Thermal conductivity and cure kinetics of epoxy-boron nitride composites: A review. Materials 2020, 13, 3634. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.M.; Román, F.; Folch, A. Epoxy-thiol systems filled with boron nitride for high thermal conductivity applications. Polymers 2018, 10, 340. [Google Scholar] [CrossRef]
- Moradi, S.; Calventus, Y.; Román, F.; Hutchinson, J.M. Achieving high thermal conductivity in epoxy composites: Effect of boron nitride particle size and matrix-filler interface. Polymers 2019, 11, 1156. [Google Scholar] [CrossRef]
- Moradi, S.; Calventus, Y.; Román, F.; Ruiz, P.; Hutchinson, J.M. Epoxy composites filled with boron nitride: Cure kinetics and the effect of particle shape on the thermal conductivity. J. Therm. Anal. Calorim. 2020, 142, 595–605. [Google Scholar] [CrossRef]
- Xu, Y.S.; Chung, D.D.L. Increasing the thermal conductivity of boron nitride and aluminum nitride particle epoxy-matrix composites by particle surface treatments. Compos. Interfaces 2000, 7, 243–256. [Google Scholar] [CrossRef]
- Hong, J.P.; Yoon, S.W.; Hwang, T.S.; Lee, Y.K.; Won, S.H.; Nam, J.D. Interphase control of boron nitride/epoxy composites for high thermal conductivity. Korea-Aust. Rheol. J. 2010, 22, 259–264. [Google Scholar]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. Effective surface treatments for enhancing the thermal conductivity of BN-filled epoxy composite. J. Appl. Polym. Sci. 2011, 119, 3234–3243. [Google Scholar] [CrossRef]
- Hou, J.; Li, G.; Yang, N.; Qin, L.; Grami, M.E.; Zhang, Q.; Wang, N.; Qu, X. Preparation and characterization of surface modified boron nitride epoxy composites with enhanced thermal conductivity. RSC Adv. 2014, 4, 44282–44290. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.; Hwang, Y.; Kim, J. Chemically modified boron nitride-epoxy terminated dimethylsiloxane composite for improving the thermal conductivity. Ceram. Int. 2014, 40, 2047–2056. [Google Scholar] [CrossRef]
- Chung, S.L.; Lin, J.S. Thermal conductivity of epoxy resin composites filled with combustion synthesized h-BN particles. Molecules 2016, 21, 670. [Google Scholar] [CrossRef]
- Jang, I.; Shin, K.H.; Yang, I.; Kim, H.; Kim, J.; Kim, W.H.; Jeon, S.W.; Kim, J.P. Enhancement of thermal conductivity of BN/epoxy composite through surface modification with silane coupling agents. Coll. Surf. A Physicochem. Eng. Asp. 2017, 518, 64–72. [Google Scholar] [CrossRef]
- Lee, J.; Shin, H.; Rhee, K.Y. Surface functionalization of boron nitride platelets via a catalytic oxidation/silanization process and thermomechanical properties of boron nitride epoxy composites. Compos. Part B 2019, 157, 276–282. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Q.; Dang, J.; Xie, C. Thermal conductivity epoxy resin composites filled with boron nitride. Polym. Adv. Technol. 2012, 23, 1025–1028. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Wu, C.; Wu, X.; Wang, G.; Jiang, P. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Donnay, M.; Tzavalas, S.; Logakis, E. Boron nitride filled epoxy with improved thermal conductivity and dielectric breakdown strength. Comp. Sci. Technol. 2015, 110, 152–158. [Google Scholar] [CrossRef]
- Caputo, A.; Turbini, L.J.; Perovic, D.D. Conductive Anodic Filament (CAF) formation. Part I: The influence of water-soluble flux on its formation. J. Electron. Mater. 2010, 39, 85–91. [Google Scholar] [CrossRef]
- Caputo, A.; Turbini, L.J.; Perovic, D.D. Conductive Anodic Filament Formation. Part II: Electrochemical reactions leading to CAF. J. Electron. Mater. 2010, 39, 92–96. [Google Scholar] [CrossRef]
- Xiao, C.; Guo, Y.; Tang, Y.; Ding, J.; Zhang, X.; Zheng, K.; Tian, X. Epoxy composite with significantly improved thermal conductivity by constructing a vertically aligned three-dimensional network of silicon carbide nanowires/boron nitride nanosheets. Compos. Part B 2020, 187, 107855. [Google Scholar] [CrossRef]
- Brown, I.G.; Wetton, R.E.; Richardson, M.J.; Savill, N.G. Glass transition and thermodynamic state of densified polymeric glasses. Polymer 1978, 19, 659–663. [Google Scholar] [CrossRef]
- Moradi, S.; Román, F.; Calventus, Y.; Hutchinson, J.M. Densification: A route towards enhanced thermal conductivity of epoxy composites. Polymers 2021, 13, 286. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, P.; Lv, P.; Xu, T.; Zheng, J.; Ma, C.; Yu, K.; Feng, W.; Wei, W.; Chen, L. Densely packed polymer/boron nitride composite for superior anisotropic thermal conductivity. Polym. Comp. 2018, 39, E1653–E1658. [Google Scholar] [CrossRef]
- Isarn, I.; Bonnaud, L.; Massagués, L.; Serra, A.; Ferrando, F. Enhancement of thermal conductivity in epoxy coatings through the combined addition of expanded graphite and boron nitride fillers. Prog. Org. Coat. 2019, 133, 299–308. [Google Scholar] [CrossRef]
- Lewis, J.S.; Barani, Z.; Sanchez Magana, A.; Kargar, F.; Balandin, A.A. Thermal and electrical conductivity control in hybrid composites with graphene and boron nitride fillers. Mater. Res. Express 2019, 6, 085325. [Google Scholar] [CrossRef]
- He, Y.; Wang, Q.; Liu, W.; Liu, Y. Functionalization of boron nitride nanoparticles and their utilization in epoxy composites with enhanced thermal conductivity. Phys. Status Solidi A 2014, 211, 677–684. [Google Scholar] [CrossRef]
- Sun, J.; Wang, D.; Yao, Y.; Zeng, X.; Pan, G.; Huang, Y.; Hu, J.; Sun, R.; Xu, J.; Wong, C. Boron nitride microsphere/epoxy composites with enhanced thermal conductivity. High Volt. 2017, 2, 147–153. [Google Scholar] [CrossRef]
- Mun, S.Y.; Lim, H.M.; Lee, S. Thermal and electrical properties of epoxy composite with expanded graphite-ceramic core-shell hybrids. Mater. Res. Bull. 2018, 97, 19–23. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Negi, A.; He, J.; Hu, G.; Tian, S.; Liu, J. Synergistic effects of boron nitride (BN) nanosheets and silver (Ag) nanoparticles on thermal conductivity and electrical properties of epoxy nanocomposites. Polymers 2020, 12, 426. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.P.; Yoon, S.W.; Hwang, T.; Oh, J.S.; Hong, S.C.; Lee, Y.; Nam, J.D. High thermal conductivity epoxy composites with bimodal distribution of aluminum nitride and boron nitride fillers. Thermochim. Acta 2012, 537, 70–75. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, J.; Ren, L.; Yao, Y.; Wang, M.; Zeng, X.; Sun, R.; Xu, J.; Wong, C. Nacre-inspired polymer composites with high thermal conductivity and enhanced mechanical strength. Comp. Part A 2019, 121, 92–99. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Liu, X. Novel functionalized BN nanosheets/epoxy composites with advanced thermal conductivity and mechanical properties. ACS Appl. Mater. Interfaces 2020, 12, 6503–6515. [Google Scholar] [CrossRef]
- Hu, J.; Huang, Y.; Zeng, X.; Li, Q.; Ren, L.; Sun, R.; Xu, J.B.; Wong, C.P. Polymer composite with enhanced thermal conductivity and mechanical strength through orientation manipulating of BN. Comp. Sci. Technol. 2018, 160, 127–137. [Google Scholar] [CrossRef]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. The adsorption of cationic surfactants on BN surface: Its effects on the thermal conductivity and mechanical properties of BN-epoxy composite. Coll. Surf. A Physicochem. Eng. Asp. 2010, 369, 203–210. [Google Scholar] [CrossRef]
- Wattanakul, K.; Manuspiya, H.; Yanumet, N. Thermal conductivity and mechanical properties of BN-filled epoxy composite: Effects of filler content, mixing conditions, and BN agglomerate size. J. Comp. Mater. 2011, 45, 1967–1980. [Google Scholar] [CrossRef]
- Xia, C.; Garcia, A.C.; Shi, S.Q.; Qiu, Y.; Warner, N.; Wu, Y.; Cai, L.; Rizvi, H.R.; D’Souza, N.A.; Nie, X. Hybrid boron nitride-natural fiber composites for enhanced thermal conductivity. Sci. Rep. 2016, 6, 34726. [Google Scholar] [CrossRef] [PubMed]
- CarboTherm Thermal Management Fillers, Product Data Sheet DS 0617. Available online: https://www.bn.saint-gobain.com/sites/imdf.bn.com/files/carbotherm-bn-thermal-fillers-ds_0.pdf (accessed on 18 January 2021).
- Hammerschmidt, U.; Meier, V. New transient hot-bridge sensor to measure thermal conductivity, thermal diffusivity, and volumetric specific heat. Int. J. Thermophys. 2006, 27, 840–865. [Google Scholar] [CrossRef]
- US Research Nanomaterials, Inc. Boron Nitride (BN) MicroPowder. Available online: https://www.us-nano.com/inc/sdetail/742 (accessed on 18 March 2021).
- Thomason, J.L. The interface region in glass fibre-reinforced epoxy resin composites: 1. Sample preparation, void content and interfacial strength. Composites 1995, 26, 467–475. [Google Scholar] [CrossRef]
- Gu, Y.; Li, M.; Zhang, Z.; Sun, Z. Void formation model and measuring method of void formation condition during hot pressing process. Polym. Comp. 2010, 31, 1562–1571. [Google Scholar] [CrossRef]
- Nakamae, K.; Nishino, T.; Xu, A.R.; Takatsuka, K. Pressure dependence of the curing behavior of epoxy resin. Polym. J. 1991, 23, 1157–1162. [Google Scholar] [CrossRef]
- Beloshenko, V.A.; Pakter, M.K.; Beresnev, B.I.; Zaika, T.P.; Slobodina, V.G.; Shepel, V.M. Properties of epoxy polymers modified by hydrostatic treatment. Mech. Comp. Mater. 1990, 26, 149–153. [Google Scholar] [CrossRef]
- Beloshenko, V.A.; Pacter, M.K.; Varyukhin, V.N. Modification of properties of epoxy polymers by high hydrostatic pressure. Acta Polym. 1995, 46, 328–333. [Google Scholar] [CrossRef]
- Hwang, S.J.; Chang, Y.S. P-V-T-C equation for epoxy molding compound. IEEE Transacions Compon. Packag. Technol. 2006, 29, 112–117. [Google Scholar] [CrossRef]
- Hopmann, C.; Wagner, P.N.; Böttcher, A. Development of a dilatometer for shrinkage analysis of thermosetting resin systems at accurate processing conditions of liquid composite moulding processes and exemplary results of the effects of varying processing conditions on chemical shrinkage of an epoxy resin. J. Comp. Mater. 2018, 52, 2451–2461. [Google Scholar] [CrossRef]
- Islam, A.M.; Lim, H.; You, N.; Ahn, S.; Goh, M.; Hahn, J.R.; Yeo, H.; Jang, S.G. Enhanced thermal conductivity of liquid crystalline epoxy resin using controlled linear polymerization. ACS Macro Lett. 2018, 7, 1180–1185. [Google Scholar] [CrossRef]
- Kargar, F.; Barani, Z.; Salgado, R.; Debnath, B.; Lewis, J.S.; Aytan, E.; Lake, R.K.; Balandid, A.A. Thermal percolation threshold and thermal properties of composites with high loading of graphene and boron nitride fillers. ACS Appl. Mater. Interfaces 2018, 10, 37555–37565. [Google Scholar] [CrossRef]
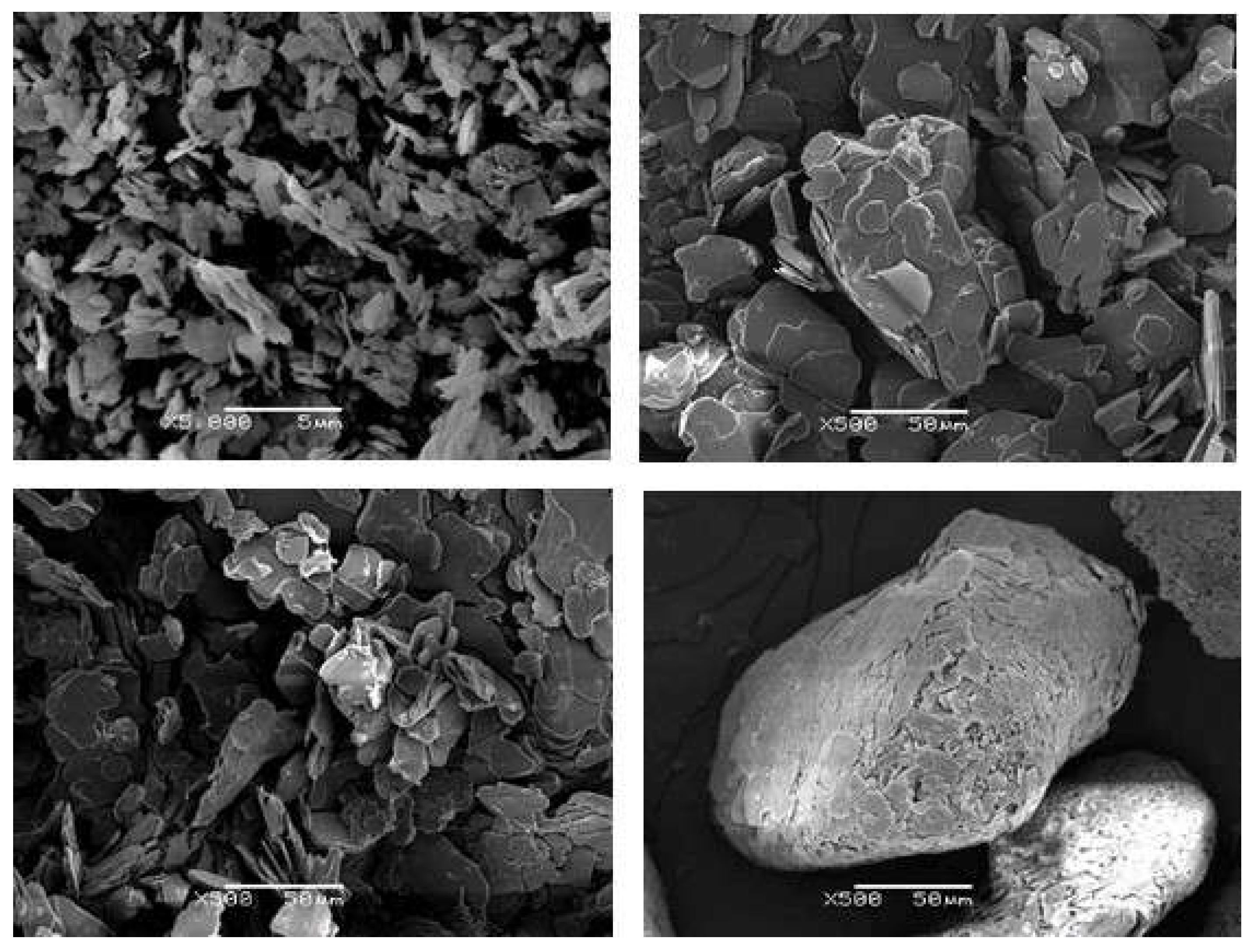

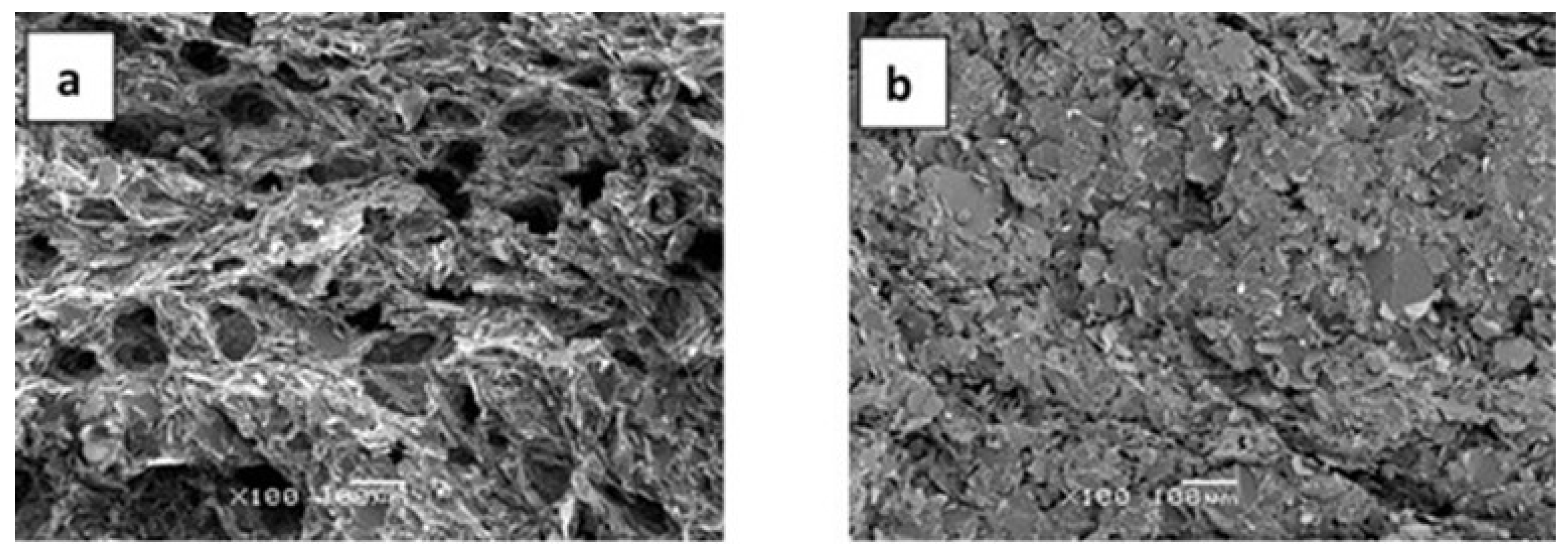
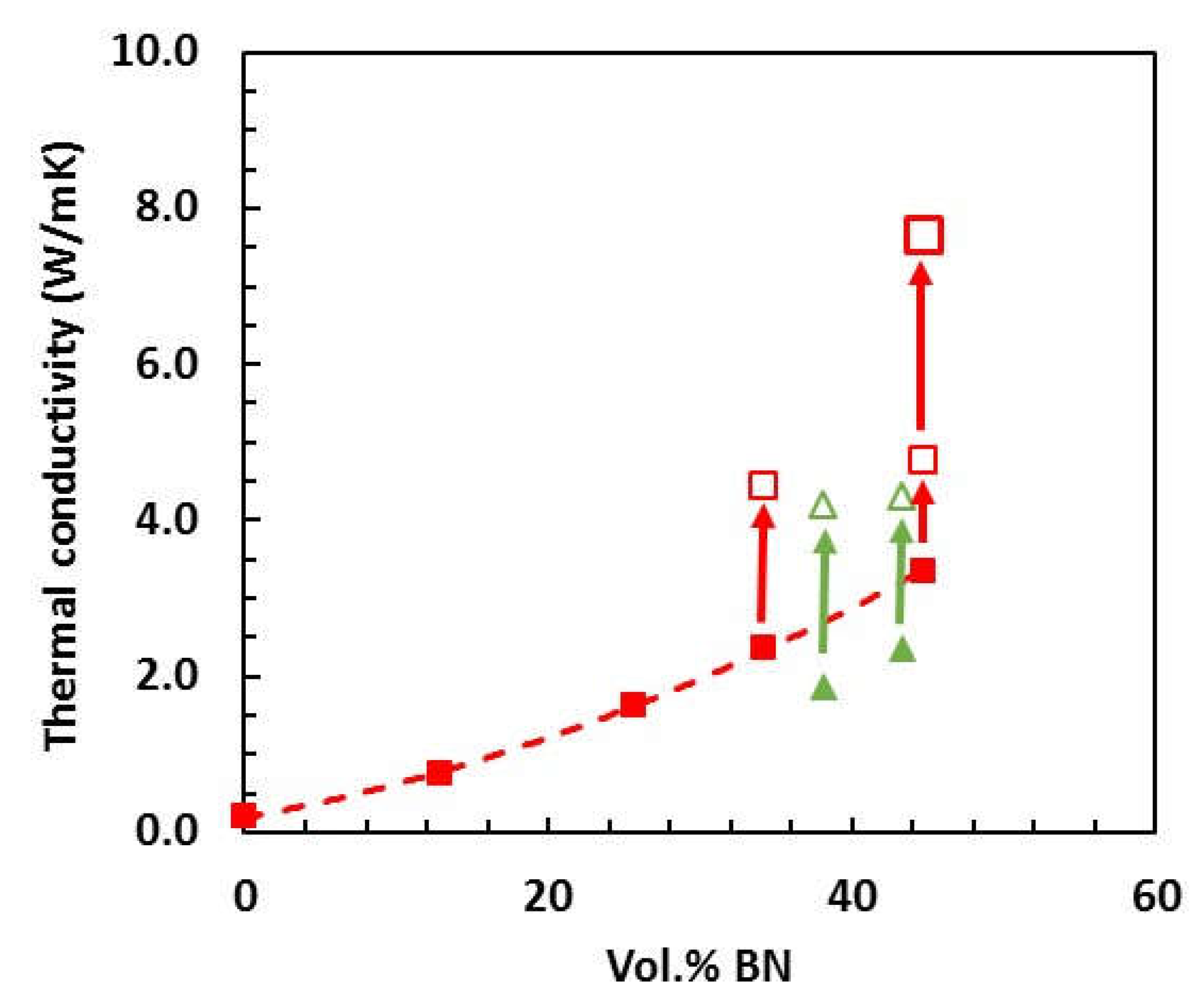


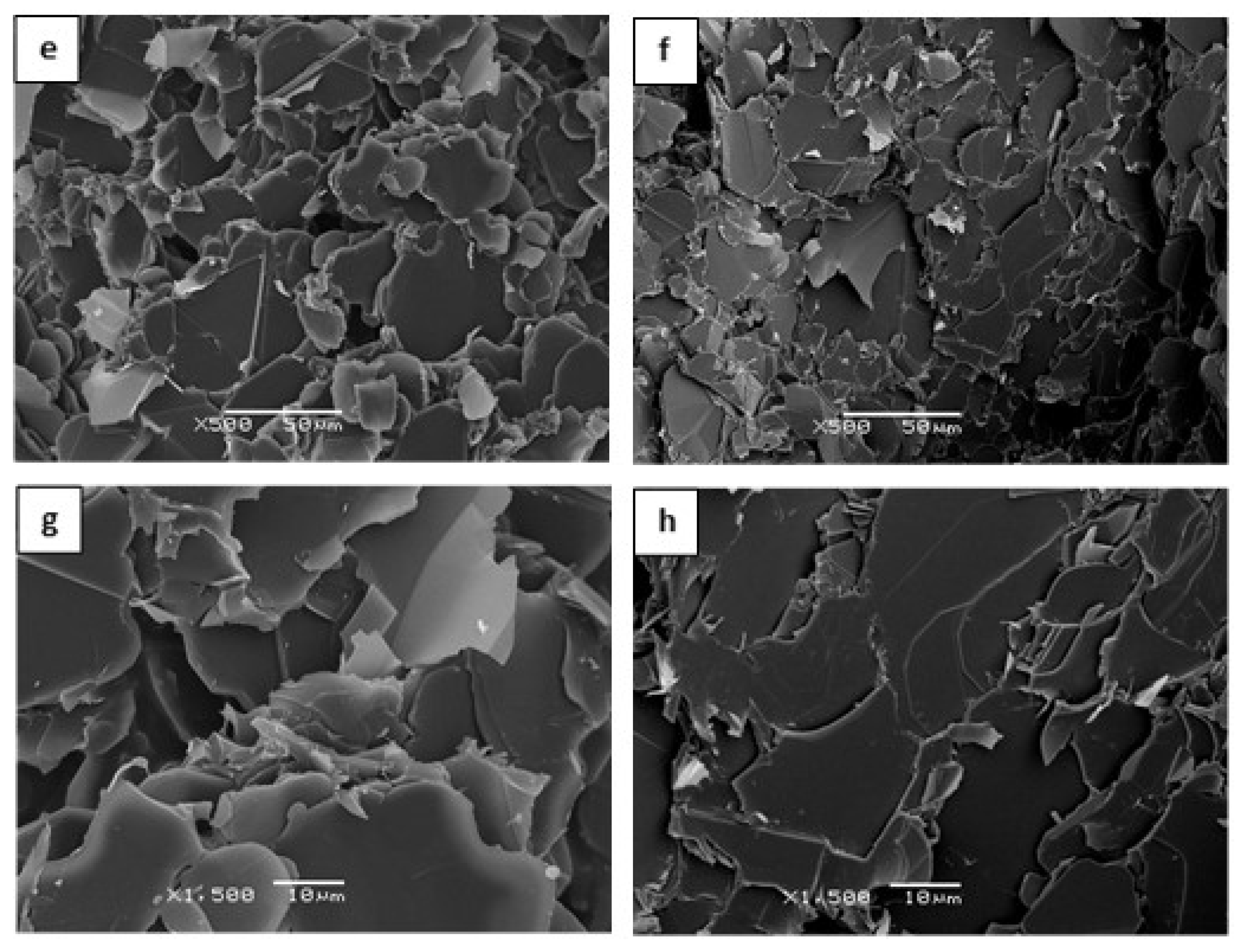
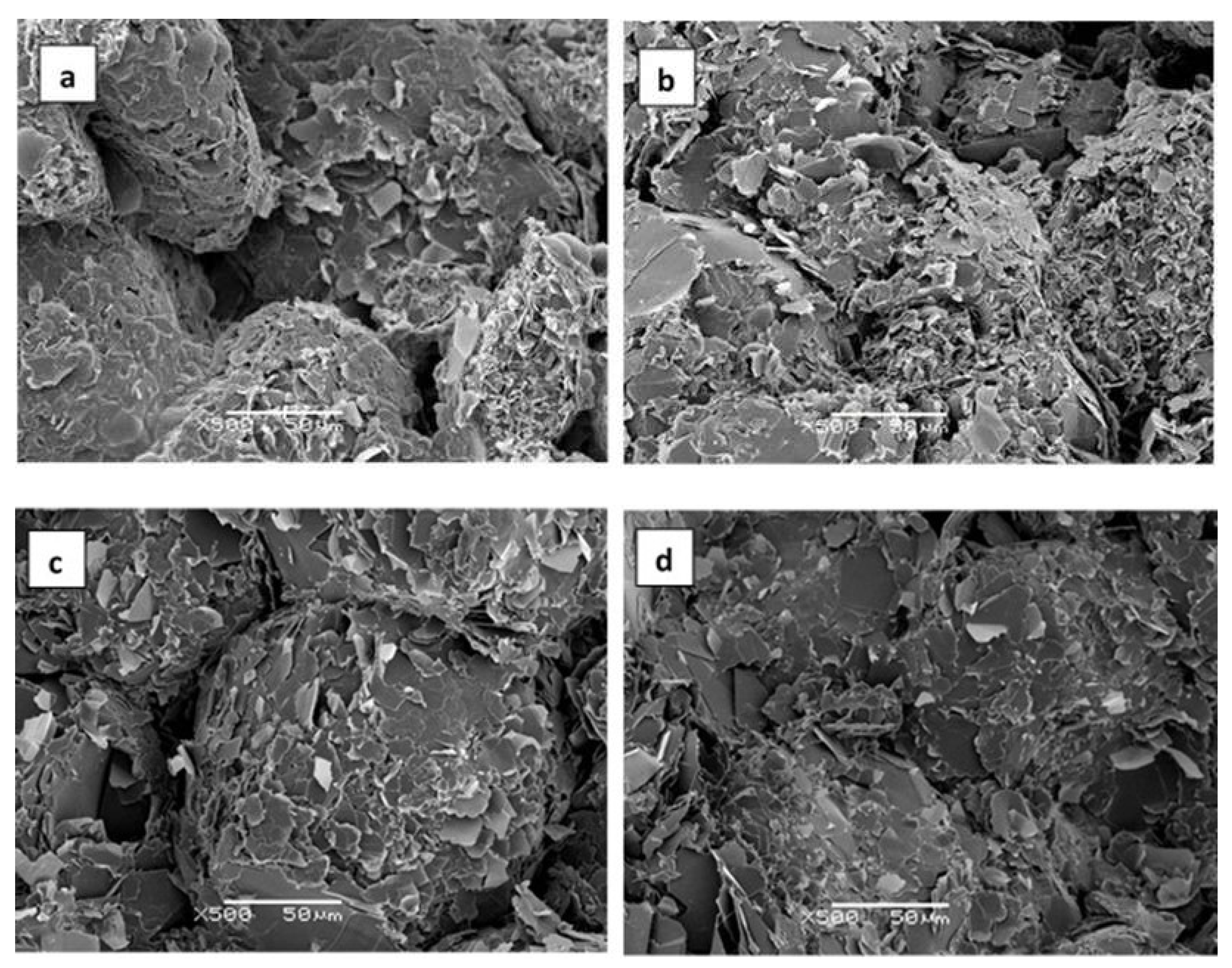
| Sample | Epoxy | BN | Thiol | LC-80 | Diamine | BN vol.% |
|---|---|---|---|---|---|---|
| ETL | 100 | 0 | 66.7 | 2.0 | - | 0 |
| ETLBNx-30 | 70 | 30 | 46.7 | 1.4 | - | 12.9 |
| ETLBNx-50 | 50 | 50 | 33.4 | 1.0 | - | 25.7 |
| ETLBNx-60 | 40 | 60 | 26.7 | 0.8 | - | 34.2 |
| ETLBNx-70 | 30 | 70 | 20.0 | 0.6 | - | 44.7 |
| EJ | 100 | 0 | - | - | 33.3 | 0 |
| EJBNx-60 | 40 | 60 | - | - | 13.3 | 38.1 |
| EJBNx-65 | 35 | 65 | - | - | 11.7 | 43.3 |
| Sample | Pamb | Density (g/cm3) 175 kPa | 2 MPa | Pamb | TC (W/mK) 175 kPa | 2 MPa |
|---|---|---|---|---|---|---|
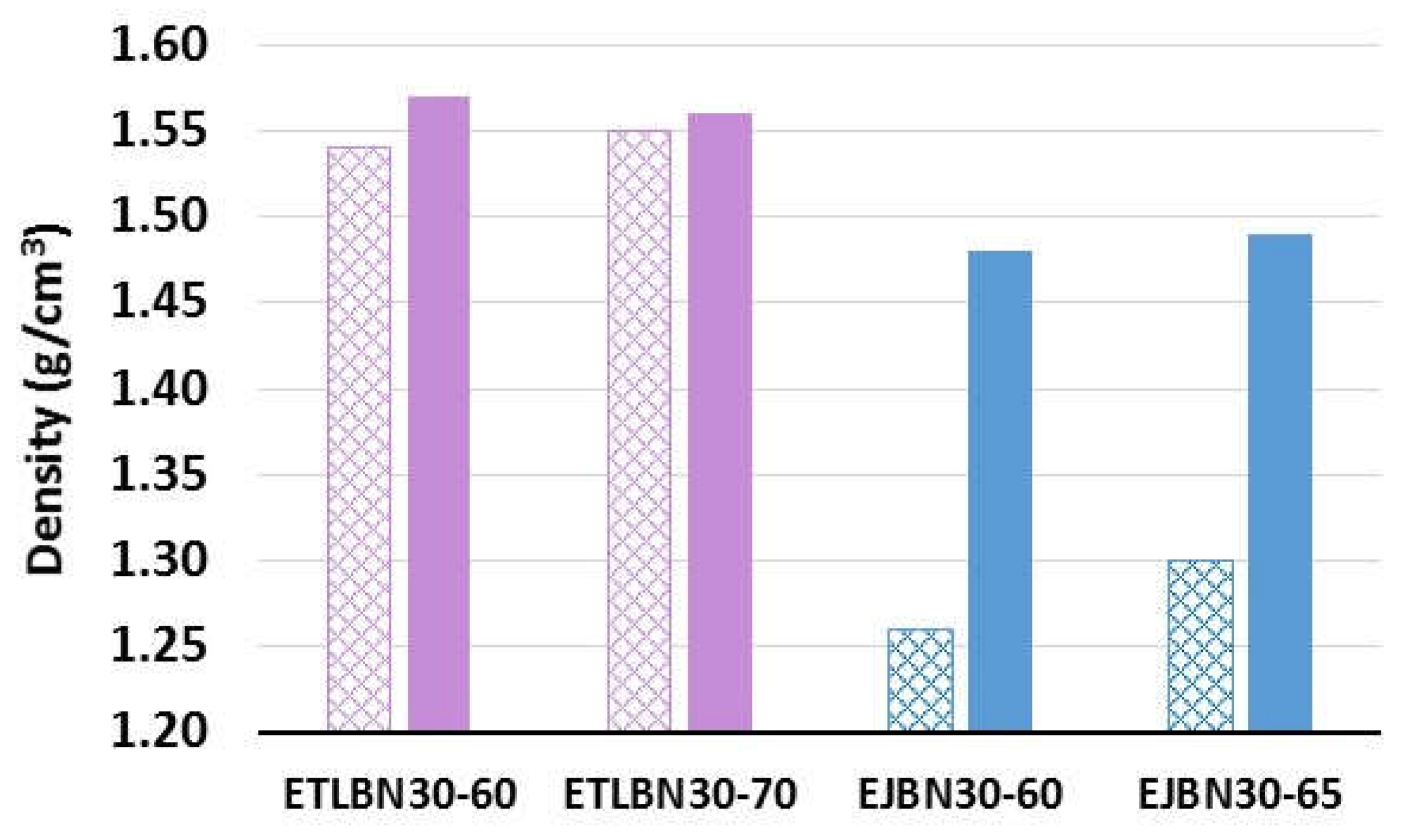
| ETLBN2-60 | 1.55 | 1.52 | - | 1.28 | 1.49 | - |
| ETLBN30-60 | 1.54 | 1.57 | - | 2.36 | 4.44 | - |
| ETLBN30-70 | 1.55 | 1.56 | 1.70 | 3.34 | 4.77 | 7.67 |
| ETLBN180-60 | 1.54 | 1.54 | - | 3.02 | 3.80 | - |
| ETLBN180-70 | 1.54 | 1.61 | 1.69 | 4.22 | 5.38 | 8.52 |
| ETLBN120s-60 | 1.42 | 1.43 | - | 3.42 | 5.34 | - |
| ETLBN120s-70 | - | 1.42 | 1.43 | - | 5.55 | 10.49 |
| EJBN30-60 | 1.26 | 1.48 | - | 1.87 | 4.19 | - |
| EJBN30-65 | 1.30 | 1.49 | - | 2.37 | 4.32 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradi, S.; Román, F.; Calventus, Y.; Hutchinson, J.M. Remarkable Thermal Conductivity of Epoxy Composites Filled with Boron Nitride and Cured under Pressure. Polymers 2021, 13, 955. https://doi.org/10.3390/polym13060955
Moradi S, Román F, Calventus Y, Hutchinson JM. Remarkable Thermal Conductivity of Epoxy Composites Filled with Boron Nitride and Cured under Pressure. Polymers. 2021; 13(6):955. https://doi.org/10.3390/polym13060955
Chicago/Turabian StyleMoradi, Sasan, Frida Román, Yolanda Calventus, and John M. Hutchinson. 2021. "Remarkable Thermal Conductivity of Epoxy Composites Filled with Boron Nitride and Cured under Pressure" Polymers 13, no. 6: 955. https://doi.org/10.3390/polym13060955
APA StyleMoradi, S., Román, F., Calventus, Y., & Hutchinson, J. M. (2021). Remarkable Thermal Conductivity of Epoxy Composites Filled with Boron Nitride and Cured under Pressure. Polymers, 13(6), 955. https://doi.org/10.3390/polym13060955







