Influence of the Catalyst Layer Structure Formed by Inkjet Coating Printer on PEFC Performance
Abstract
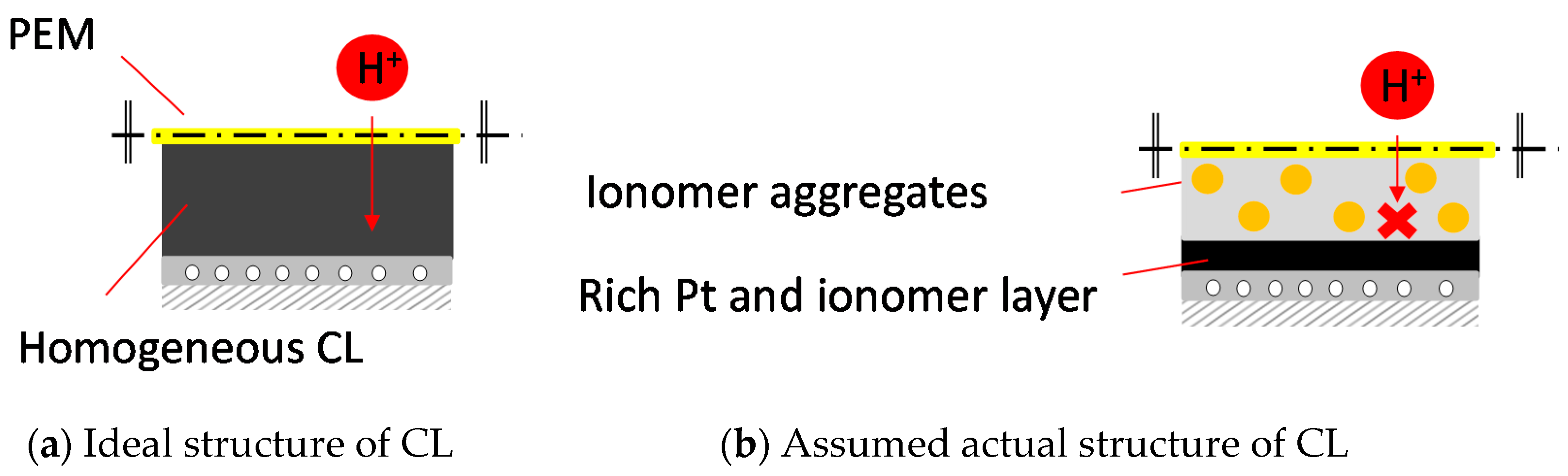
1. Introduction
2. Materials and Methods
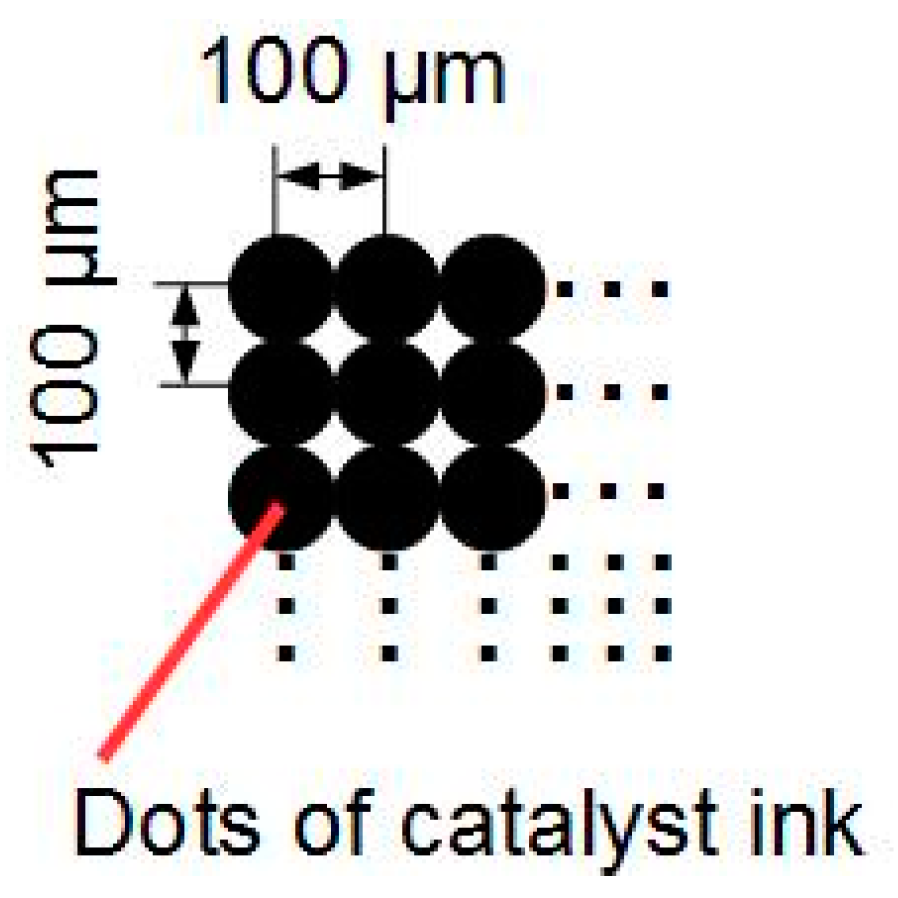
2.1. Settings on Inkjet Printer and Preparation of Catalyst Ink for Inkjet Printer
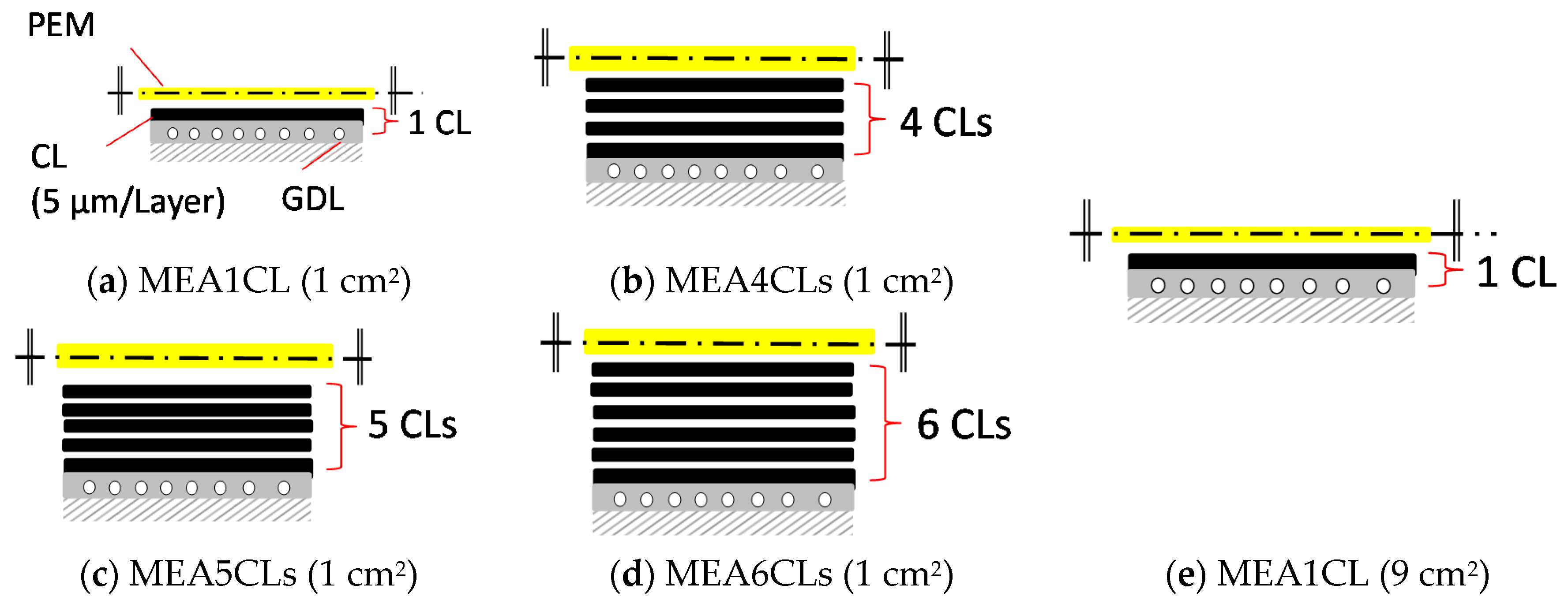
2.2. Application Method by Inkjet Printer and Preparation of MEA
2.3. Experimental Apparatus and Method
2.4. A Method of Overpotential Analysis
- (1)
- We assumed the relationship as Equation (1):where Vth is the theoretical open circuit voltage in the PEFC (1.23 V), Vact is an activation over potential, Vir is a resistance overpotential, Vdiff is a diffusion over potential and Vcell is a cell voltage, respectively.
- (2)
- The resistance over potential Vir is derived using Equation (2) from the resistance R measured by the measurement of I-V performance:where i A/cm2 is the current density, r Ωcm2 is an area resistance, I A is a current, Ae cm2 is an electrode area, R Ω is a measured resistance and Ac cm2 is a contact area, respectively.
- (3)
- I-V performance was converted to an IR-free curve, thereafter the I-V curve was rearranged to Tafel plot. Here, an activation overpotential (Vact) was derived by the difference between the regression curve of the voltage of the Tafel plot and the theoretical voltage in the low current density region.
- (4)
- A diffusion overpotential (Vdiff) was defined by the value in which the above-mentioned regression curve of voltage and IR-free are subtracted from the theoretical open circuit voltage.
3. Results and Discussion
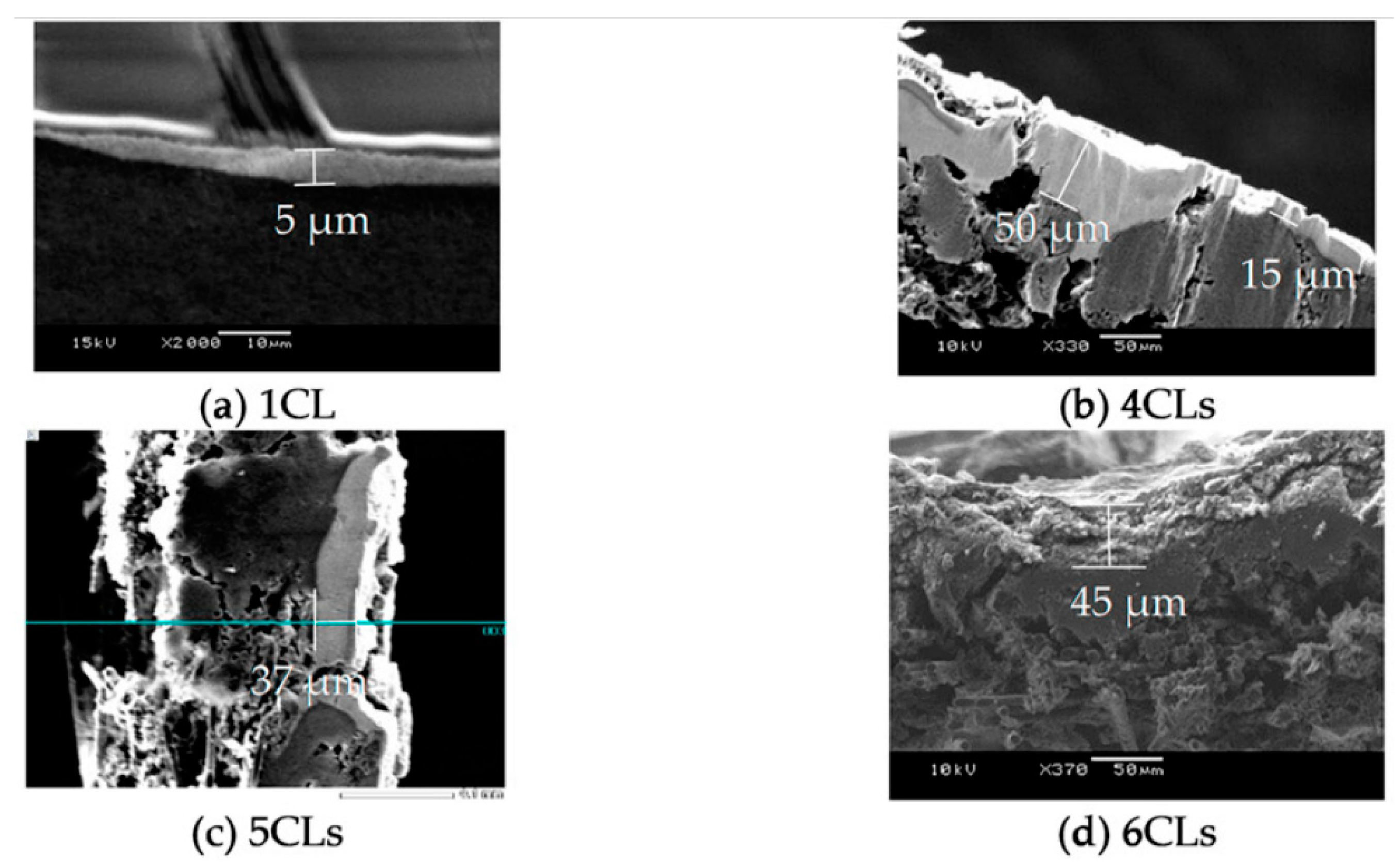
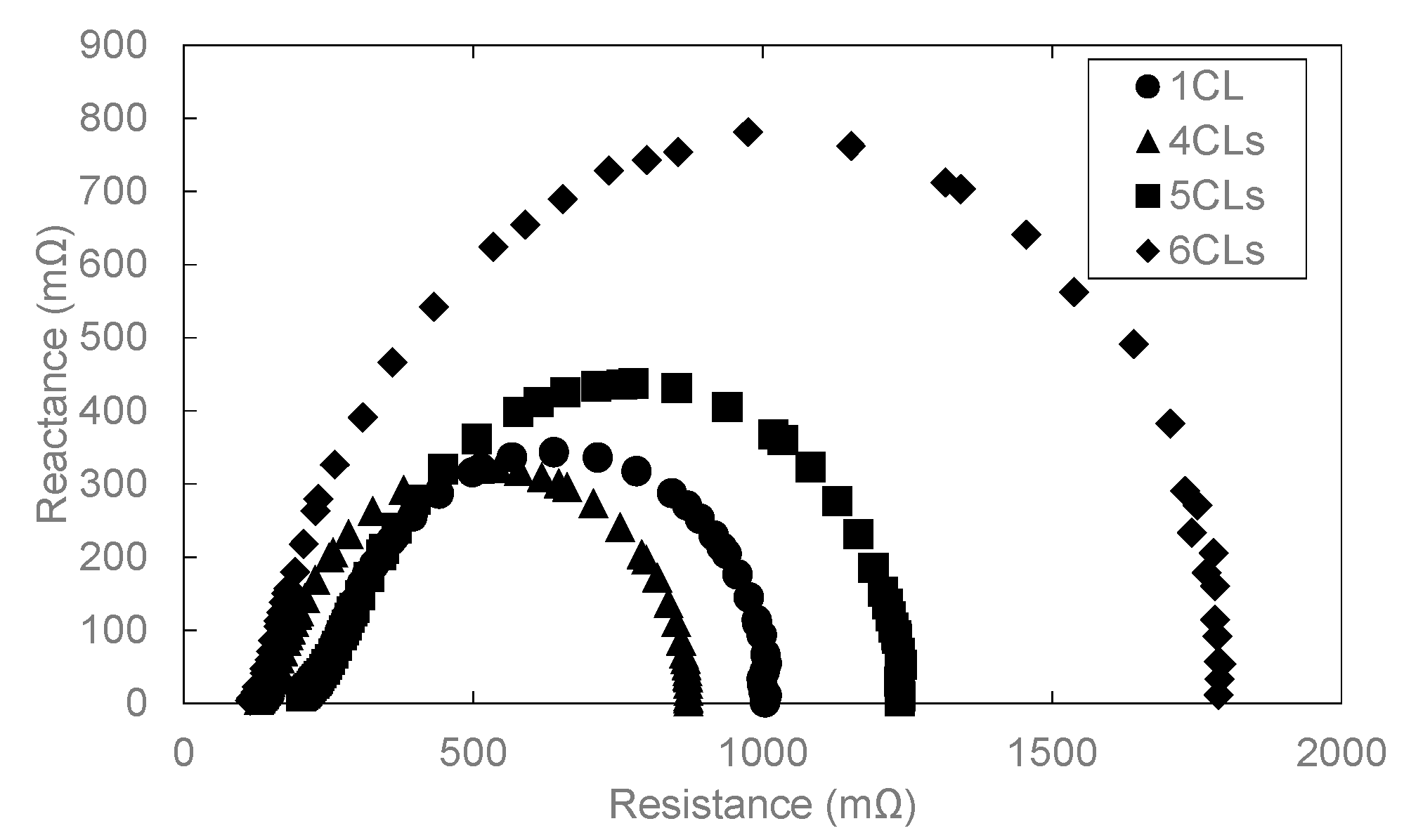
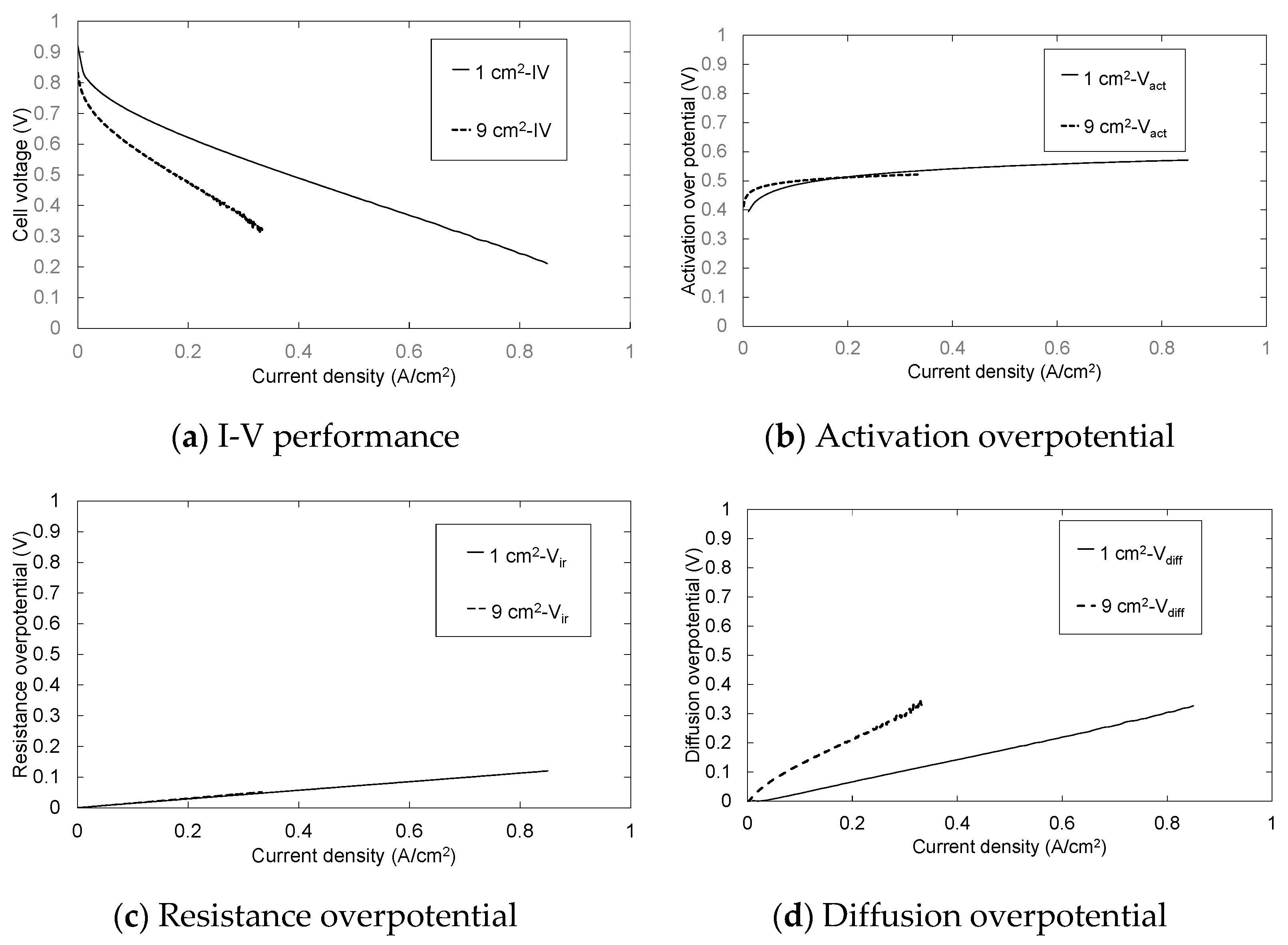
3.1. Influence of the CL Thickness on Cell Performance
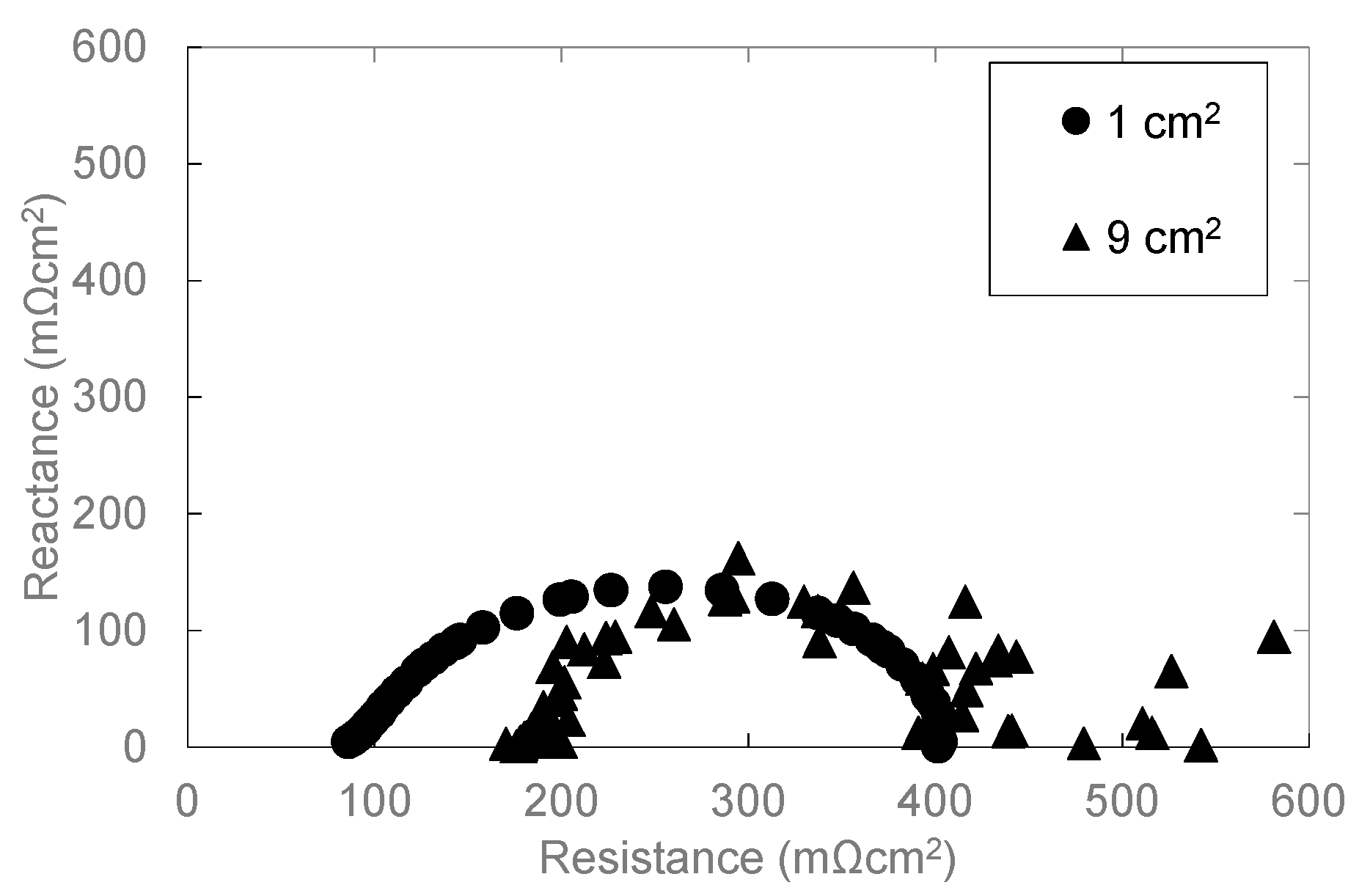
3.2. Influence of the Electrode Area on Cell Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Economy, Trade and Industry Hydrogen/Fuel Cell Strategy Council, Hydrogen/Fuel Cell Strategy Roadmap. Available online: https://www.meti.go.jp/press/2018/03/20190312001/20190312001-1.pdf (accessed on 21 February 2021).
- Kim, A.R.; Vinothkannan, M.; Song, M.H.; Lee, J.-Y.; Lee, H.-K.; Yoo, D.J. Amine functionalized carbon nanotube (ACNT) filled in sulfonated poly(ether ether ketone) membrane: Effects of ACNT in improving polymer electrolyte fuel cell performance under reduced relative humidity. Compos. Part B Eng. 2020, 188, 107890. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Lee, K.H.; Chu, J.Y.; Ryu, S.K.; Kim, H.G.; Lee, J.-Y.; Lee, H.-K.; Yoo, D.J. Ameliorated Performance of Sulfonated Poly(Arylene Ether Sulfone) Block Copolymers with Increased Hydrophilic Oligomer Ratio in Proton-Exchange Membrane Fuel Cells Operating at 80% Relative Humidity. Polymers 2020, 12, 1871. [Google Scholar] [CrossRef]
- Shi, G.; Tryk, D.A.; Iwataki, T.; Yano, H.; Uchida, M.; Iiyama, A.; Uchida, H. Unparalleled mitigation of membrane degradation in fuel cells via a counter-intuitive approach: Suppression of H2O2 production at the hydrogen anode using a Ptskin-PtCo catalyst. J. Mater. Chem. A 2020, 8, 1091–1094. [Google Scholar] [CrossRef]
- Takenaka, S.; Hirata, A.; Tanabe, E.; Matsune, H.; Kishida, M. Preparation of supported Pt–Co alloy nanoparticle catalysts for the oxygen reduction reaction by coverage with silica. J. Catal. 2010, 274, 228–238. [Google Scholar] [CrossRef]
- Henning, S.; Kühn, L.; Herranz, J.; Nachtegaal, M.; Hübner, R.; Werheid, M.; Eychmüller, A.; Schmidt, T.J. Effect of Acid Washing on the Oxygen Reduction Reaction Activity of Pt-Cu Aerogel Cata-lysts. Electrochim. Acta 2017, 233, 210–217. [Google Scholar] [CrossRef]
- Tamura, Y.; Taneda, K.; Ueda, M.; Ohtsuka, T. Effect of oxide film on oxygen reduction current for the platinum–cobalt alloy electrodes in PEFC. Corros. Sci. 2009, 51, 1560–1564. [Google Scholar] [CrossRef]
- Lu, Y.; Du, S.; Steinberger-Wilckens, R. Three-dimensional catalyst electrodes based on PtPd nanodendrites for oxygen reduction reaction in PEFC applications. Appl. Catal. B Environ. 2016, 187, 108–114. [Google Scholar] [CrossRef]
- Aoki, N.; Inoue, H.; Shirai, A.; Higuchi, S.; Matsui, Y.; Daimon, H.; Doi, T.; Inaba, M. Electrochemical and Chemical Treatment Methods for Enhancement of Oxygen Reduction Reaction Activity of Pt Shell-Pd Core Structured Catalyst. Electrochim. Acta 2017, 244, 146–153. [Google Scholar] [CrossRef]
- Higuchi, E.; Okada, K.; Chiku, M.; Inoue, H. Electrocatalytic Activity for Oxygen Reduction Reaction of Au Core/Pt Shell Nanoparticle-Loaded Carbon Black Catalyst with Different Core Sizes. Electrochim. Acta 2015, 179, 100–107. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hiyoshi, M.; Iijima, T.; Noguchi, H.; Uosaki, K. Investigation of the effects of Pt/Pd composition and PVP content on the activity of Pt/Pd core-shell catalysts. Electrochem. Commun. 2020, 115, 106736. [Google Scholar] [CrossRef]
- Takahashi, S.; Todoroki, N.; Myochi, R.; Nagao, T.; Taguchi, N.; Ioroi, T.; Feiten, F.E.; Wakisaka, Y.; Asakura, K.; Sekizawa, O.; et al. Effective surface termination with Au on PtCo@Pt core-shell nanoparticle: Microstructual investigations and oxygen reduction reaction properties. J. Electroanal. Chem. 2019, 842, 1–7. [Google Scholar] [CrossRef]
- Fukunaga, H.; Kachi, K.; Takimoto, D.; Mochizuki, D.; Sugimoto, W. Direct preparation of core-shell platinum cathode in membrane electrode assembly catalyst layer for polymer electrolyte fuel cell. Int. J. Hydrog. Energy 2020, 45, 14547–14551. [Google Scholar] [CrossRef]
- Ishihara, A.; Arao, M.; Matsumoto, M.; Tokai, T.; Nagai, T.; Kuroda, Y.; Matsuzawa, K.; Imai, H.; Mitsushima, S.; Ota, K.-I. Niobium-added titanium oxides powders as non-noble metal cathodes for polymer electrolyte fuel cells—Electrochemical evaluation and effect of added amount of niobium. Int. J. Hydrog. Energy 2020, 45, 5438–5448. [Google Scholar] [CrossRef]
- Arashi, T.; Seo, J.; Takanabe, K.; Kubota, J.; Domen, K. Nb-doped TiO2 cathode catalysts for oxygen reduction reaction of polymer electrolyte fuel cells. Catal. Today 2014, 233, 181–186. [Google Scholar] [CrossRef]
- Osmieri, L.; Ahluwalia, R.K.; Wang, X.; Chung, H.T.; Yin, X.; Kropf, A.J.; Park, J.; Cullen, D.A.; More, K.L.; Zelenay, P.; et al. Elucidation of Fe-N-C electrocatalyst active site functionality via in-situ X-ray absorption and operando determination of oxygen reduction reaction kinetics in a PEFC. Appl. Catal. B Environ. 2019, 257, 117929. [Google Scholar] [CrossRef]
- Jung, C.-Y.; Kim, W.-J.; Yi, S.-C. Optimization of catalyst ink composition for the preparation of a membrane electrode assembly in a proton exchange membrane fuel cell using the decal transfer. Int. J. Hydrog. Energy 2012, 37, 18446–18454. [Google Scholar] [CrossRef]
- Bayer, T.; Pham, H.C.; Sasaki, K.; Lyth, S.M. Spray deposition of Nafion membranes: Electrode-supported fuel cells. J. Power Sources 2016, 327, 319–326. [Google Scholar] [CrossRef]
- Millington, B.; Whipple, V.; Pollet, B.G. A novel method for preparing proton exchange membrane fuel cell electrodes by the ultra-sonic-splay technique. J. Power Sources 2011, 196, 8500–8508. [Google Scholar] [CrossRef]
- Shukla, S.; Domican, K.; Karan, K.; Bhattacharjee, S.; Secanell, M. Analysis of Low Platinum Loading Thin Polymer Electrolyte Fuel Cell Electrodes Prepared by Inkjet Printing. Electrochim. Acta 2015, 156, 289–300. [Google Scholar] [CrossRef]
- Shukla, S.; Stanier, D.; Saha, M.S.; Stumper, J.; Secanell, M. Analysis of Inkjet Printed PEFC Electrodes with Varying Platinum Loading. J. Electrochem. Soc. 2016, 163, F677–F687. [Google Scholar] [CrossRef]
- Bae, I.; Kim, B.; Kim, D.Y.; Kim, H.; Oh, K.H. In-plane 2-D patterning of microporous layer by inkjet printing for water management of polymer elec-trolyte fuel cell. Renew. Energy 2020, 146, 960–967. [Google Scholar] [CrossRef]
- Morioka, K.; Sugiura, K. Evaluation of cell performance of MEA for PEFC using inkjet coating printer. J. Mech. Eng. Res. Dev. 2020, 43, 20–29. [Google Scholar]
- Vielstich, W.; Lamm, A.; Gasteiger, H. Handbook of Fuel Cells-Fundamentals, Technology and Applications. Fuel Cell Technol. Appl. 2003, 3, 597. [Google Scholar]
- Kumano, N.; Kudo, K.; Suda, A.; Akimoto, Y.; Ishii, M.; Nakamura, H. Controlling cracking formation in fuel cell catalyst layers. J. Power Sources 2019, 419, 219–228. [Google Scholar] [CrossRef]
- Takahashi, S.; Shimanuki, J.; Mashio, T.; Ohma, A.; Tohma, H.; Ishihara, A.; Ito, Y.; Nishino, Y.; Miyazawa, A. Observation of ionomer in catalyst ink of polymer electrolyte fuel cell using cryogenic transmission electron microscopy. Electrochim. Acta 2017, 224, 178–185. [Google Scholar] [CrossRef]
- Kundu, S.; Fowler, M.; Simon, L.; Grot, S. Morphological features (defects) in fuel cell membrane electrode assemblies. J. Power Sources 2006, 157, 650–656. [Google Scholar] [CrossRef]
- Swamy, T.; Kumbur, E.C.; Mench, M.M. Characterization of Interfacial Structure in PEFCs: Water Storage and Contact Resistance Model. J. Electrochem. Soc. 2010, 157, B77–B85. [Google Scholar] [CrossRef]
- Ahn, C.-Y.; Jang, S.; Cho, Y.-H.; Choi, J.; Kim, S.; Kim, S.M.; Sung, Y.-E.; Choi, M. Guided cracking of electrodes by stretching prism-patterned membrane electrode assemblies for high-performance fuel cells. Sci. Rep. 2018, 8, 1257. [Google Scholar] [CrossRef]
- Ramani, D.; Singh, Y.; Orfino, F.P.; Dutta, M.; Kjeang, E. Characterization of Membrane Degradation Growth in Fuel Cells Using X-ray Computed To-mography. J. Electrochem. Soc. 2018, 165, F3200–F3208. [Google Scholar] [CrossRef]
- Singh, Y.; Orfino, F.P.; Dutta, M.; Kjeang, E. 3D Failure Analysis of Pure Mechanical and Pure Chemical Degradation in Fuel Cell Membranes. J. Electrochem. Soc. 2017, 164, F1331–F1341. [Google Scholar] [CrossRef]
- White, R.T.; Wu, A.; Najm, M.; Orfino, F.P.; Dutta, M.; Kjeang, E. 4D in situ visualization of electrode morphology changes during accelerated degradation in fuel cells by X-ray computed tomography. J. Power Sources 2017, 350, 94–102. [Google Scholar] [CrossRef]




| Setting Values | |
|---|---|
| Applied voltage (V) | 30–40 |
| 1st pulse width (μs) | 100 |
| Pulse interval (μs) | 0 |
| 2nd pulse width (μs) | 0 |
| Frequency (Hz) | 100 |
| Droplet speed (m/s) | 6.5 |
| Setting Values | |
|---|---|
| Standard current density (A/cm2) | 0.4 |
| Quantity (H2/Air) (mL/min) | 38.4/160.2 |
| Gas temperature (°C) | 80 |
| Gas humidity (%Rh) | 100 |
| Cell temperature (°C) | 80 |
| Operating pressure of H2 gas (hPa) | 1500 |
| Operating pressure of Air gas(hPa) | 1500 |
| Back pressure (hPa) | 1013 |
| Range of impedance frequency (Hz) | 0.07–10,000 |
| Superimposed current (mA) | 16.5 |
| (wt.%) | Reference [25] | In This Study |
|---|---|---|
| Pt/Carbon | 6.6 | 4.2 |
| Nafion | 3.4 | 2.6 |
| Water | 60.3 | 63.8 |
| 1-propanol | 6.9 | 5.7 |
| Ethanol | 22.8 | 23.6 |
| Total | 100 | 99.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamaki, Y.; Sugiura, K. Influence of the Catalyst Layer Structure Formed by Inkjet Coating Printer on PEFC Performance. Polymers 2021, 13, 899. https://doi.org/10.3390/polym13060899
Tamaki Y, Sugiura K. Influence of the Catalyst Layer Structure Formed by Inkjet Coating Printer on PEFC Performance. Polymers. 2021; 13(6):899. https://doi.org/10.3390/polym13060899
Chicago/Turabian StyleTamaki, Yushi, and Kimihiko Sugiura. 2021. "Influence of the Catalyst Layer Structure Formed by Inkjet Coating Printer on PEFC Performance" Polymers 13, no. 6: 899. https://doi.org/10.3390/polym13060899
APA StyleTamaki, Y., & Sugiura, K. (2021). Influence of the Catalyst Layer Structure Formed by Inkjet Coating Printer on PEFC Performance. Polymers, 13(6), 899. https://doi.org/10.3390/polym13060899







