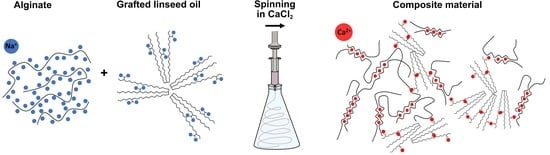
Hybrid Filaments from Saccaharina lattisima Biomass: Engineering of Alginate Properties with Maleic Anhydride Grafted Linseed Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
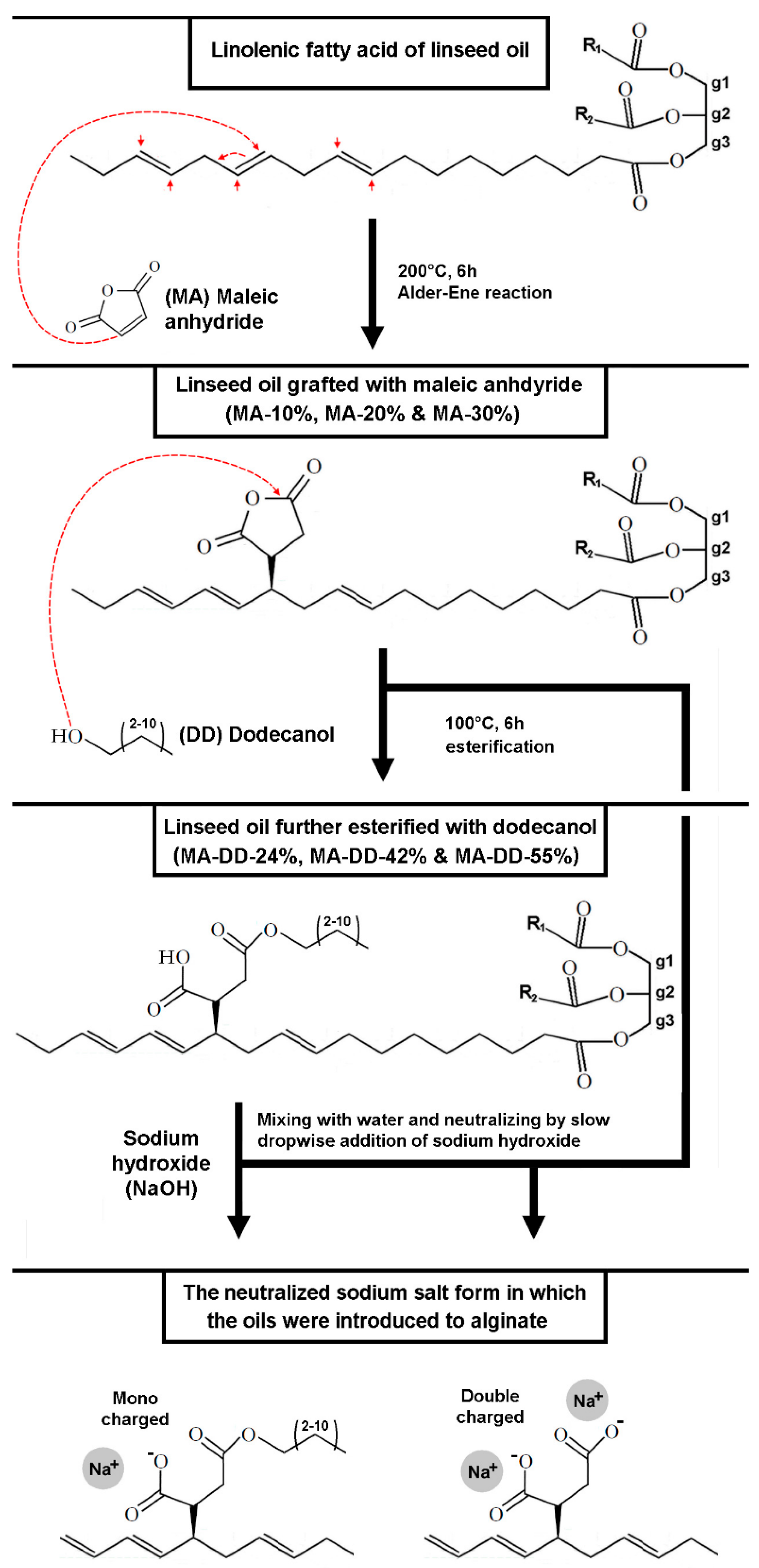
2.2. Grafting of Linseed Oil
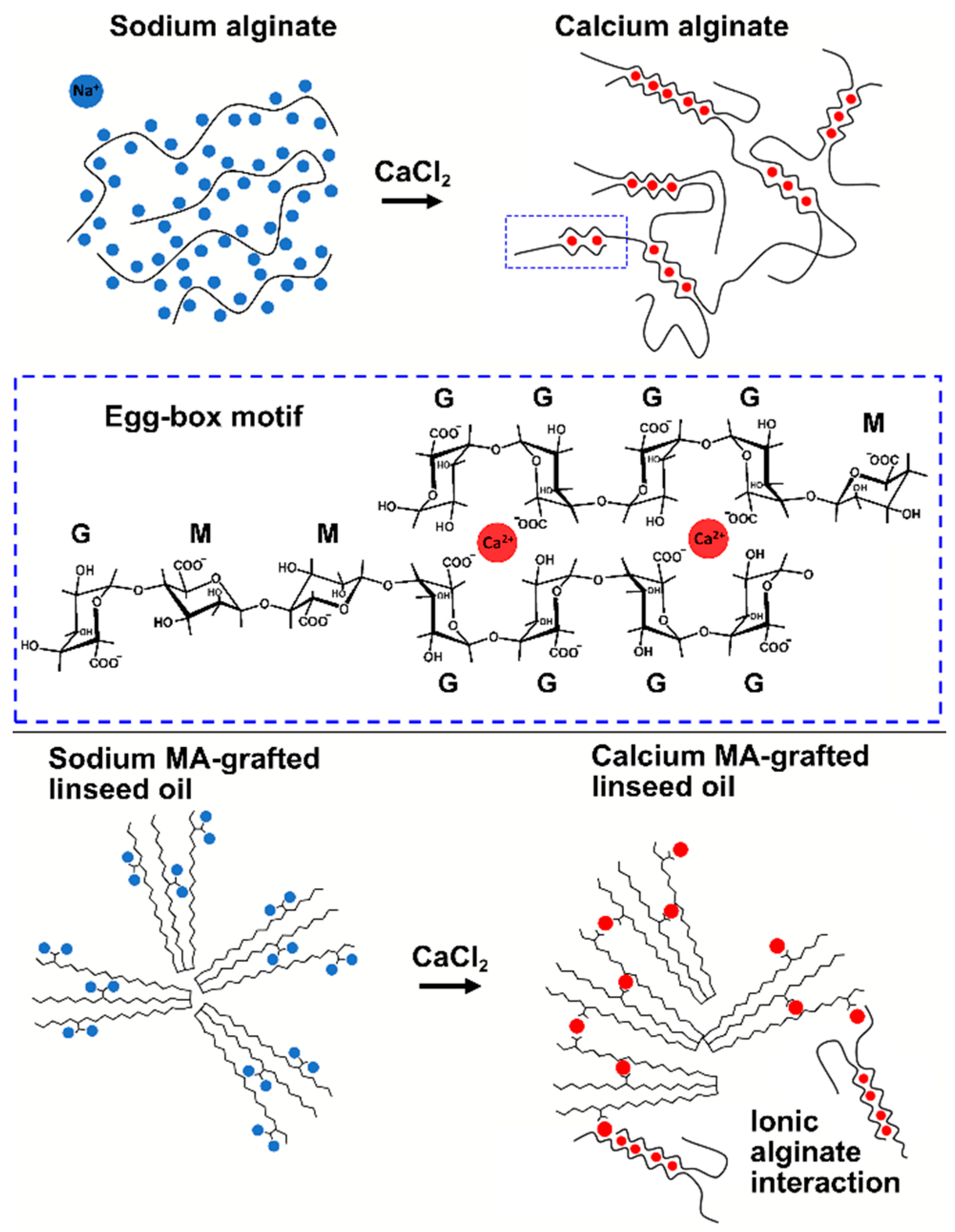
2.3. Filaments of Alginate, Salts, and Grafted Linseed Oil
2.4. Tensile Testing
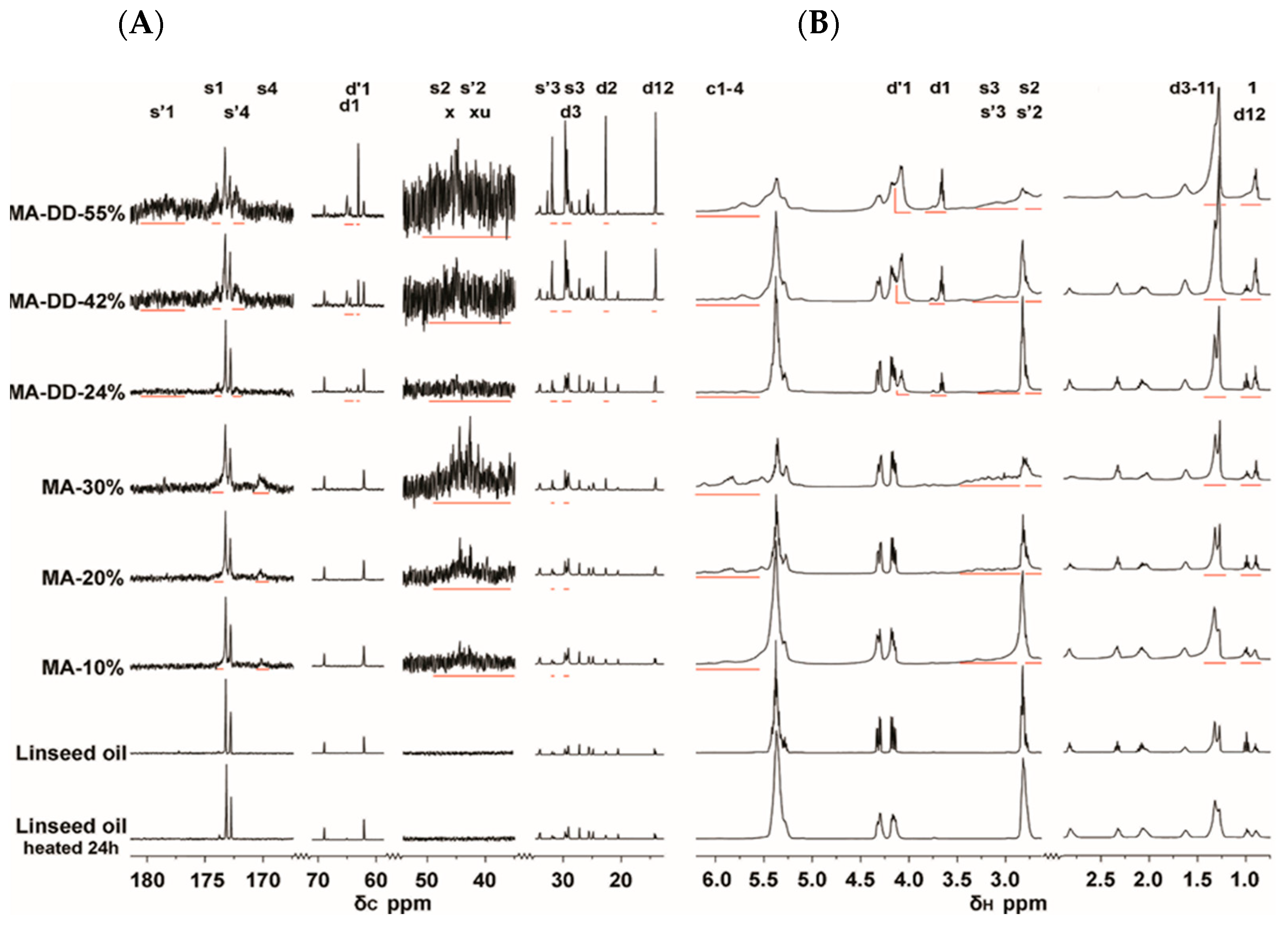
2.5. Nuclear Magnetic Resonance, NMR
2.6. Rheology Measurements
2.7. pH Measurements
3. Results and Discussion
3.1. Characterization of Alginate
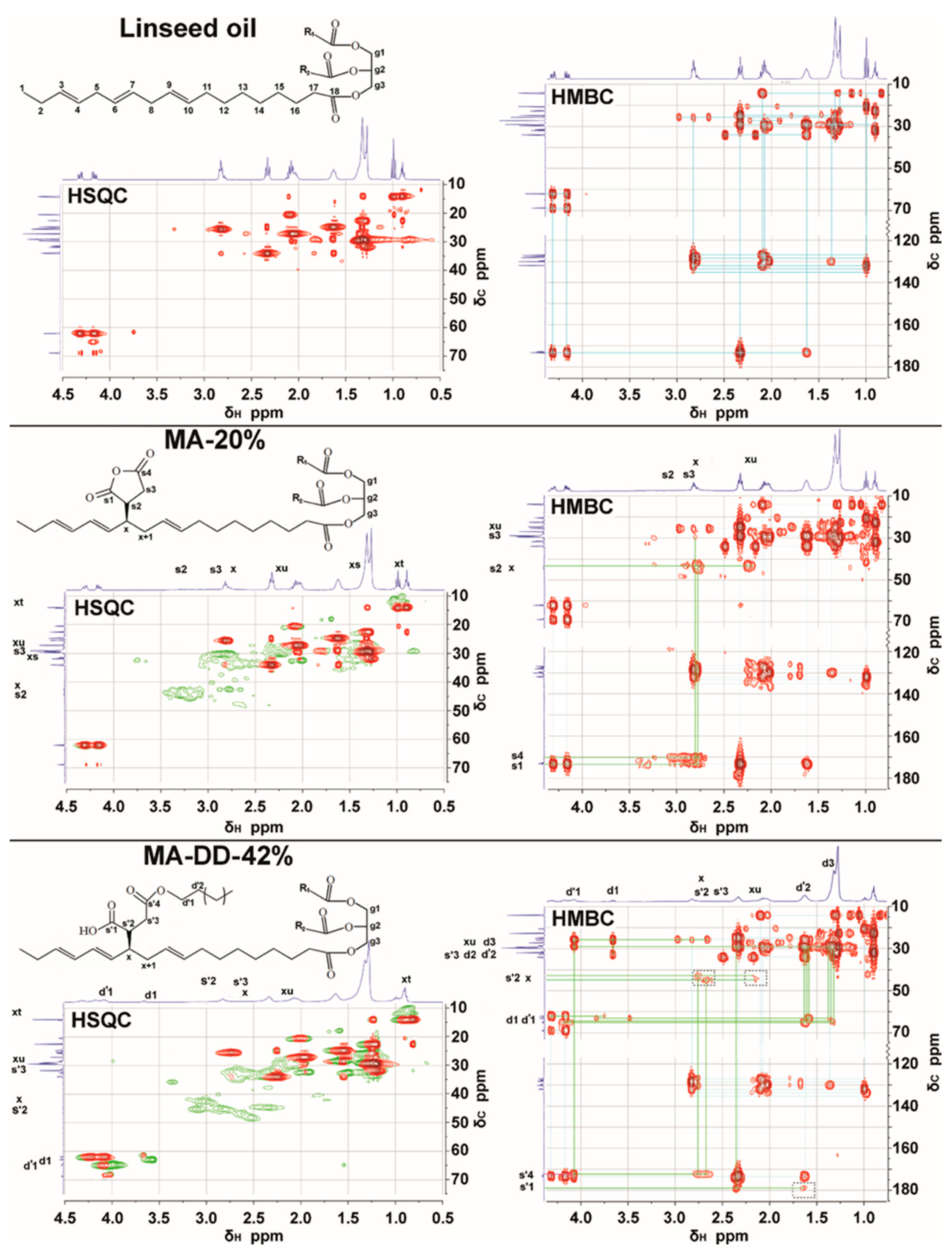
3.2. Characterization of Grafted Linseed Oil
3.3. Tensile Properties of Alginate with Modified Linseed Oil and Calcium Salts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jard, G.; Marfaing, H.; Carrère, H.; Delgenes, J.; Steyer, J.; Dumas, C. French Brittany macroalgae screening: Composition and methane potential for potential alternative sources of energy and products. Bioresour. Technol. 2013, 144, 492–498. [Google Scholar] [CrossRef]
- Rinaudo, M. Seaweed Polysaccharides. In Comprehensive Glycoscience: From Chemistry to Systems Biology; Kamerling, H., Ed.; Elsevier: Oxford, UK, 2007; pp. 691–735. [Google Scholar]
- Atalla, R.H.; Hackney, J.M. Structural Polysaccharides In Molecular Architecture of Plant Cell Walls from Algae to Hardwoods. In MRS Fall Meeting; Cambridge Univ Press: Cambridge, UK, 1991; Volume 255, pp. 387–397. [Google Scholar]
- Sterner, M.; Ribeiro, M.S.; Gröndahl, F.; Edlund, U. Cyclic fractionation process for Saccharina latissima using aqueous chelator and ion exchange resin. Environ. Boil. Fishes 2017, 29, 3175–3189. [Google Scholar] [CrossRef]
- Kohn, R. Ion binding on polyuronates - alginate and pectin. Pure Appl. Chem. 1975, 42, 371–397. [Google Scholar] [CrossRef]
- Rinaudo, M.; Jiilas, M.; Recherches, C.D. Interaction of Monovalent and Divalent Counterions with Some Carboxylic Polysaccharides. J. Polym. Sci. Part A Polym. Chem. 1974, 12, 2073–2081. [Google Scholar] [CrossRef]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from Algae. In Biopolymers Online; Wiley: Hoboken, NJ, USA, 2002; Volume 1, pp. 1–30. [Google Scholar]
- Sterner, M.; Edlund, U.M. High-Performance Filaments from Fractionated Alginate by Polyvalent Cross-Linking: A Theoretical and Practical Approach. Biomacromolecules 2018, 19, 3311–3330. [Google Scholar] [CrossRef]
- Goswami, B.C.; Elizabeth, P.; Africa, S.; Hall, D.M. Properties of Fibers and Yarns. In Textile Sizing; CRC Press: New York, NY, USA, 2004; pp. 24–152. [Google Scholar]
- Muri, J.M.; Brown, P.J. Biodegradable and Sustainable Fibres. In Alginate fibers; Blackburn, R.S., Ed.; Taylor & Francis: Abingdon, UK, 2005; Volume 47, pp. 89–110. [Google Scholar]
- Niekraszewicz, B.; Niekraszewicz, A. The structure of alginate, chitin and chitosan fibres. In Handbook of Textile Fibre Structure; Elsevier: Amsterdam, The Netherlands, 2009; pp. 266–304. [Google Scholar]
- Iwamoto, S.; Isogai, A.; Iwata, T. Structure and Mechanical Properties of Wet-Spun Fibers Made from Natural Cellulose Nanofibers. Biomacromolecules 2011, 12, 831–836. [Google Scholar] [CrossRef]
- Xu, W.; Shen, R.; Yan, Y.; Gao, J. Preparation and Characterization of Electrospun Alginate/PLA Nanofibers as Tissue Engineering Material by Emulsion Eletrospinning. J. Mech. Behav. Biomed. Mater. 2017, 65, 428–438. [Google Scholar] [CrossRef]
- Andersen, T.; Strand, B.L.; Formo, K.; Alsberg, E.; Christensen, B.E. Chapter 9. Alginates as biomaterials in tissue engineering. Carbohydr. Chem. 2011, 37, 227–258. [Google Scholar] [CrossRef]
- Venkatesan, J.; Nithya, R.; Sudha, P.N.; Kim, S.-K. Role of Alginate in Bone Tissue Engineering. In Marine Carbohydrates: Fundamentals and Applications, Part B; Elsevier BV: Amsterdam, The Netherlands, 2014; Volume 73, pp. 45–57. [Google Scholar]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Huq, T.; Saha, M.; Khan, R.A.; Khan, M.A.; Gafur, M.A. Effect of Silane Treatment on the Mechanical and Interfacial Properties of Calcium Alginate Fiber Reinforced Polypropylene Composite. J. Compos. Mater. 2010, 44, 2875–2886. [Google Scholar] [CrossRef]
- Keßler, M. Biodegradable Solvent Cast Films and Solution Electrospun Meshes for the Prevention of Postsurgical Adhesions. Ph.D. Thesis, Julius-Maximilians-Universität Würzburg, Würzburg, Germany, 2015. [Google Scholar]
- Clark, D.C.; Baker, W.E.; Russell, K.E.; Whitney, R.A. Dual monomer grafting of styrene and maleic anhydride onto model hydrocarbon substrates. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2456–2468. [Google Scholar] [CrossRef]
- Wahlstedt, G. Production of Organic and Non-Organic Farming 2017 Cereals, Dried Pulses, Oilseed Crops, Table Potatoes and Temporary Grasses - JO 14 SM 1801 - Jordbruksverket; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2018. [Google Scholar]
- TTrivedi, B.C.; Culbertson, B.M. Ene Reaction. In Maleic Anhydride; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1982; Volume 66, pp. 147–176. [Google Scholar]
- Alonso-Fagundez, N.; Granados, M.L.; Mariscal, R.; Ojeda, M. Selective Conversion of Furfural to Maleic Anhydride and Furan with VOx/Al2O3 Catalysts. ChemSusChem 2012, 5, 1984–1990. [Google Scholar] [CrossRef]
- Li, X.; Ko, J.; Zhang, Y. Highly Efficient Gas-Phase Oxidation of Renewable Furfural to Maleic Anhydride over Plate Vanadium Phosphorus Oxide Catalyst. ChemSusChem 2018, 11, 612–618. [Google Scholar] [CrossRef]
- Liang, S.; Liu, H.; Liu, J.; Wang, W.; Jiang, T.; Zhang, Z.; Han, B. Hydrogenation of methyl laurate to produce lauryl alcohol over Cu/ZnO/Al2O3 with methanol as the solvent and hydrogen source. Pure Appl. Chem. 2011, 84, 779–788. [Google Scholar] [CrossRef]
- Rinaudo, M.; Graebling, D. On the viscosity of sodium alginates in the presence of external salt. Polym. Bull. 1986, 15, 253–256. [Google Scholar] [CrossRef]
- Ozbek, H.; Fair, J.; Phillips, S.L. Viscosity of Aqueous Sodium Chloride Solution from 0–150 °C. In Proceedings of the American Chemical Society 29th Southeast Regional Meeting, Tapa, FL, USA, 9–11 November 1977; p. 38. [Google Scholar]
- Cox, W.P.; Merz, E.H. Correlation of dynamic and steady flow viscosities. J. Polym. Sci. 1958, 28, 619–622. [Google Scholar] [CrossRef]
- Lackinger, E.; Bacher, M.; Sartori, J.; Zweckmair, T.; Potthast, A. Synthesis and Characterization of 13C-Labeled Alkenyl Succinic Anhydride (ASA) with Defined Double Bond Location. Curr. Org. Chem. 2014, 18, 1208–1217. [Google Scholar] [CrossRef]
- Lie, M.S.F.; Jie, K.; Pasha, M.K.; Alam, M.S. Synthesis and Nuclear Magnetic Resonance Properties of All Geometrical Isomers of Conjugated Linoleic Acids. Lipids 1997, 32, 1041–1044. [Google Scholar]
- Lie, M.S.F.; Jie, K.; Lam, C.C. 1H-Nuclear Magnetic Resonance Spectroscopic Studies of Saturated, Acetylenic and Ethylenic Triacylglycerols. Chem. Phys. Lipids 1995, 77, 155–171. [Google Scholar]
- Vlahov, G.; Shaw, A.D.; Kell, D.B. Use of 13 C nuclear magnetic resonance distortionless enhancement by polarization transfer pulse sequence and multivariate analysis to discriminate olive oil cultivars. J. Am. Oil Chem. Soc. 1999, 76, 1223–1231. [Google Scholar] [CrossRef]
- Frost, D.; Gunstone, F. The PMR analysis of non-conjugated alkenoic and alkynoic acids and esters. Chem. Phys. Lipids 1975, 15, 53–85. [Google Scholar] [CrossRef]
- Takaki, T.; Yanagi, M.; Tanabe, M.; Oyanagi, S.; Itoh, H.; Watanabe, H.; Utsunomiya, A.; Ono, Y.; Tsuchihashi, M. Unsaturated Hemiesters of Alkyl Succinic Acid, Polymers Therefrom and Use of Said Polymers. EP0747342 A1, 1996. [Google Scholar]
- Holmberg, K.; Johansson, J.-A.; Bergman, A.; Brunk, U.; Dallner, G.; Berg, J.-E. Addition of Maleic Anhydride to Esters of Mono-unsaturated Fatty Acids. Acta Chem. Scand. 1982, 36b, 481–485. [Google Scholar] [CrossRef]
- Stefanoiu, F.; Candy, L.; Vaca-Garcia, C.; Borredon, E. Kinetics and mechanism of the reaction between maleic anhydride and fatty acid esters and the structure of the products. Eur. J. Lipid Sci. Technol. 2008, 110, 441–447. [Google Scholar] [CrossRef]
- Klemenz, A.; Schwinger, C. Investigation of elasto-mechanical properties of alginate microcapsules by scanning acoustic microscopy. J. Biomed. Mater. Res. 2003, 65, 237–243. [Google Scholar] [CrossRef] [PubMed]





| Grafted Oils | ||||||
|---|---|---|---|---|---|---|
| MA-10% | MA-20% | MA-30% | MA-DD-24% | MA-DD-42% | MA-DD-55% | |
| Linseed oil % (w/w) | 90.0 | 80.0 | 70.0 | 75.7 | 58.0 | 44.6 |
| Maleic anhydride (MA) % (w/w) | 10.0 | 20.0 | 30.0 | 8.4 | 14.5 | 19.1 |
| n-dodecanol (DD) % (w/w) | 0 | 0 | 0 | 15.9 | 27.5 | 36.3 |
| MA + DD % (w/w) | - | - | - | 24.3 | 42.0 | 55.4 |
| Charge density 1 (mol/kg) | 2.0 | 4.0 | 6.0 | 0.8 | 1.5 | 1.9 |
| Commercial Alginate | Extracted Alginate | Commercial Alginate | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grafted Linseed Oil | Calcium Chloride | Calcium Oxalate | Calcium Phytate | |||||||||||||
| MA-DD-24% | MA-DD-42% | MA-DD-55% | MA-10% | MA-20% | MA-30% | |||||||||||
| Initial alginate conc. (% w/w) | 3 | 3 | 3 | 3 | 3 | 3 | 1.83 | 2 | 2 | 2 | 3 | 5 | 2 | 5 | 2 | 2 |
| Drawing CaCl2 conc. (M) | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.25 | 0.02 | 0.02 | 0.09 | 0.09 | 0.25 | 0.02 | 0.02 |
| Final filament diameter (μm) | 191 | 203 | 2017 | 202 | 245 | 241 | 175 | 131 | 134 | 163 | 160 | 215 | 182 | 256 | 146 | 156 |
| Excess calcium salt (% w/w) | 33 | 33 | 33 | 33 | 33 | 33 | 33 | 0 | 39 | 0 | 0 | 7 | 22 | 30 | 6 | 9 |
| Sample denotation | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P |
| Commercial Alginate | Extracted Alginate | Commercial Alginate | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grafted Linseed Oil | Calcium Chloride | Calcium Oxalate | Calcium Phytate | |||||||||||||
| MA-DD-24% | MA-DD-42% | MA-DD-55% | MA-10% | MA-20% | MA-30% | |||||||||||
| Excess calcium salt (% w/w) | 33 | 33 | 33 | 33 | 33 | 33 | 33 | 0 | 39 | 0 | 0 | 7 | 22 | 30 | 6 | 9 |
| Dry filament drawing stress with/without excess salt (MPa) | 5.27 | 4.67 | 4.08 | 4.71 | 3.2 | 3.31 | 6.28 | 11.21 | 10.71 | 7.24 | 7.51 | 4.16 | 5.81 | 2.94 | 9.02 | 7.90 |
| 7.87 | 6.97 | 6.1 | 7.04 | 4.78 | 4.94 | 9.37 | 11.21 | 15.99 | 7.24 | 7.51 | 4.57 | 7.45 | 4.38 | 9.6 | 8.69 | |
| Measure stress-at-break higher average/average/STD (MPa) | 246 | 264 | 260 | 229 | 227 | 216 | 262 | 521 | 214 | 447 | 429 | 380 | 298 | 157 | 386 | 317 |
| 240 | 252 | 249 | 223 | 215 | 212 | 251 | 507 | 210 | 441 | 400 | 369 | 274 | 145 | 364 | 310 | |
| 5.5 | 7.7 | 6.9 | 5.1 | 7.1 | 3.6 | 6.3 | 14 | 4.9 | 8.6 | 29.3 | 7.1 | 24.7 | 2.8 | 14.5 | 8.3 | |
| Calculated stress-at-break with/without excess salt (Equations (6) and (7)) (MPa) | 388 | 367 | 3434 | 368 | 301 | 306 | 419 | 529 | 513 | 447 | 450 | 368 | 390 | 307 | 468 | 444 |
| 457 | 437 | 414 | 439 | 371 | 377 | 489 | n.s. | 600 | n.s. | n.s. | 381 | 434 | 370 | 479 | 461 | |
| Measured stress-at-break, excess salt adjusted (Equation (8)) (MPa) | 368 | 394 | 388 | 342 | 338 | 323 | 391 | n.s | 350 | n.s | n.s. | 409 | 382 | 224 | 411 | 348 |
| Δstress-at-break by the added excess salt (Equation (7) – Equation (8)) (MPa) | 91 | 43 | 26 | 96 | 33 | 54 | 99 | n.s. | 249 | n.s. | n.s. | −28 | 51 | 146 | 68 | 113 |
| Δstress-at-break by the added excess salt (Equation (7) – Equation (8)) (MPa/%) * | 2.7 | 1.3 | 0.8 | 2.9 | 1 | 1.6 | 3.9 | n.s. | 6.4 | n.s. | n.s. | −4 | 2.3 | 4.9 | 11.3 | 12.5 |
| Δstress-at-break by the added excess salt (Equation (7) – Equation (8)) (%/%) ** | 0.6 | 0.3 | 0.2 | 0.7 | 0.3 | 0.4 | 0.6 | n.s. | 1.1 | n.s. | n.s. | −1 | 0.5 | 1.3 | 2.4 | 2.7 |
| Moisture absorption after 3 days in 50% air humidity (% w/w) | 8.5 | 9.9 | 9.4 | 8.4 | 10.6 | 10.6 | n.m. | n.m. | 14 | n.m. | 11.8 | n.m. | n.m. | 10.3 | 11.8 | 12.8 |
| Sample denotation | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sterner, M.; Edlund, U. Hybrid Filaments from Saccaharina lattisima Biomass: Engineering of Alginate Properties with Maleic Anhydride Grafted Linseed Oil. Polymers 2021, 13, 836. https://doi.org/10.3390/polym13050836
Sterner M, Edlund U. Hybrid Filaments from Saccaharina lattisima Biomass: Engineering of Alginate Properties with Maleic Anhydride Grafted Linseed Oil. Polymers. 2021; 13(5):836. https://doi.org/10.3390/polym13050836
Chicago/Turabian StyleSterner, Martin, and Ulrica Edlund. 2021. "Hybrid Filaments from Saccaharina lattisima Biomass: Engineering of Alginate Properties with Maleic Anhydride Grafted Linseed Oil" Polymers 13, no. 5: 836. https://doi.org/10.3390/polym13050836
APA StyleSterner, M., & Edlund, U. (2021). Hybrid Filaments from Saccaharina lattisima Biomass: Engineering of Alginate Properties with Maleic Anhydride Grafted Linseed Oil. Polymers, 13(5), 836. https://doi.org/10.3390/polym13050836