Isolation of Chitin from Black Soldier Fly (Hermetia illucens) and Its Usage to Metal Sorption
Abstract
1. Introduction
- Dietetics—it is used in the production of supplements, food thickeners and pharmaceuticals [9];
2. Materials and Methods
2.1. Insect Material
2.2. Chemicals
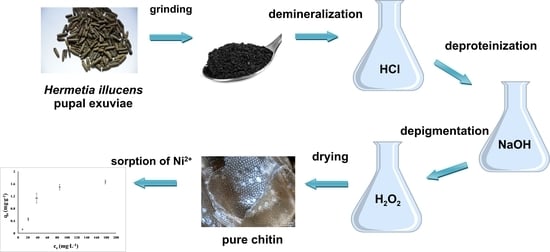
2.3. Chitin Isolation from Hermetia Illucens Exuviae
2.4. Nickel Sorption
2.5. Methods of Chitin Characterization and Sorption Effect
2.5.1. Optical and Confocal Microscopy
2.5.2. X-Ray Powder Diffraction (XRPD)
2.5.3. Thermogravimetric Analysis (TG, DTG, DTA)
2.5.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5.5. Energy Dispersive X-Ray Spectroscopy (EDX)
2.6. Statistical Analysis
3. Results
3.1. Chitin Isolation from H. illucens Pupal Exuviae
3.2. Optical and Confocal Microscopy
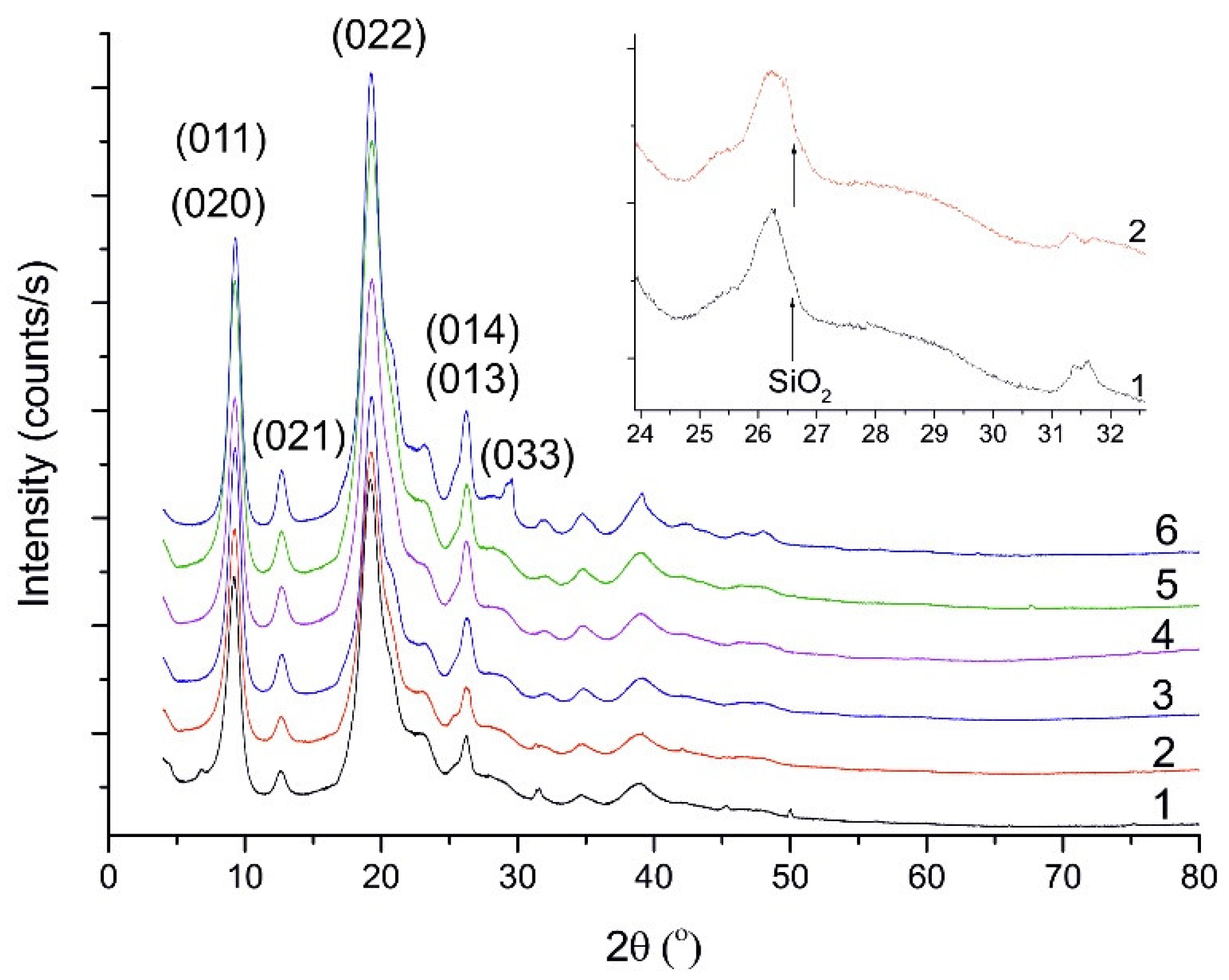
3.3. X-ray Powder Diffraction (XRPD)
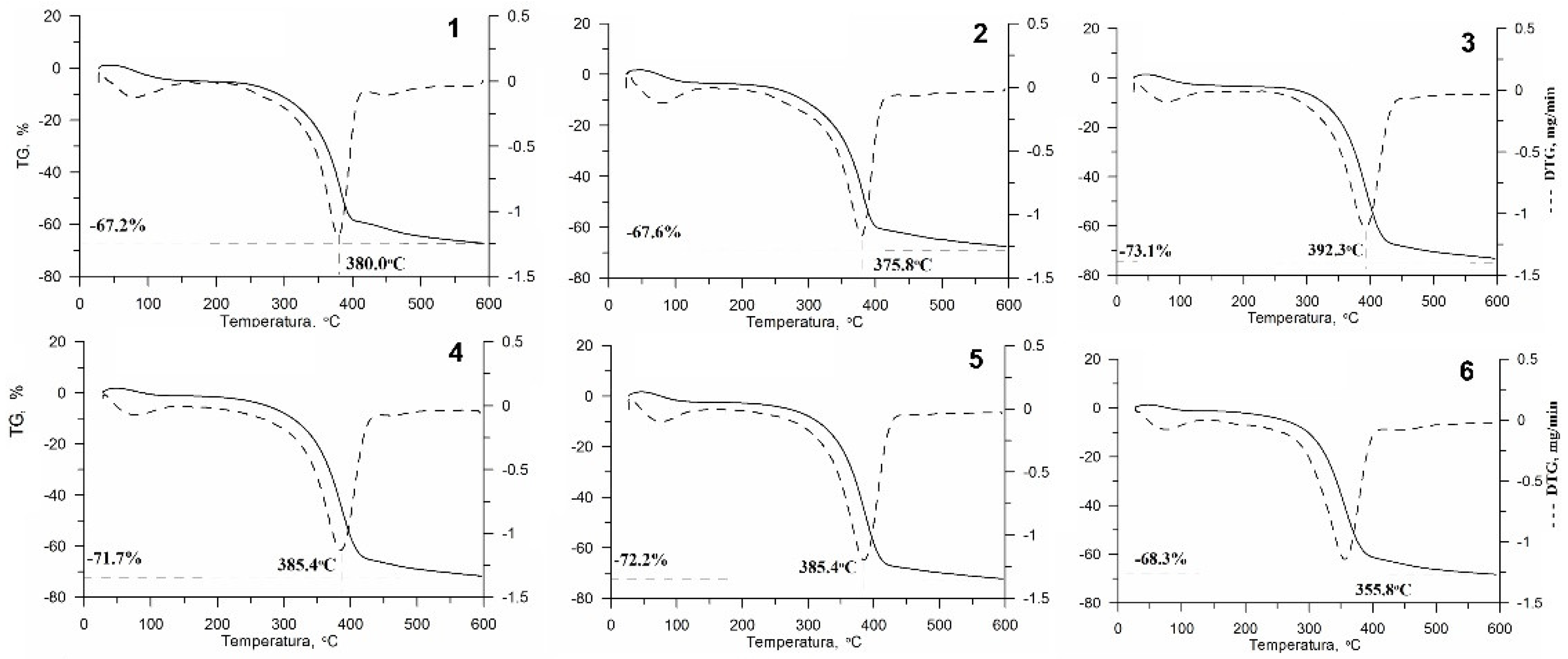
3.4. Thermogravimetric Analysis (TG, DTG, DTA)
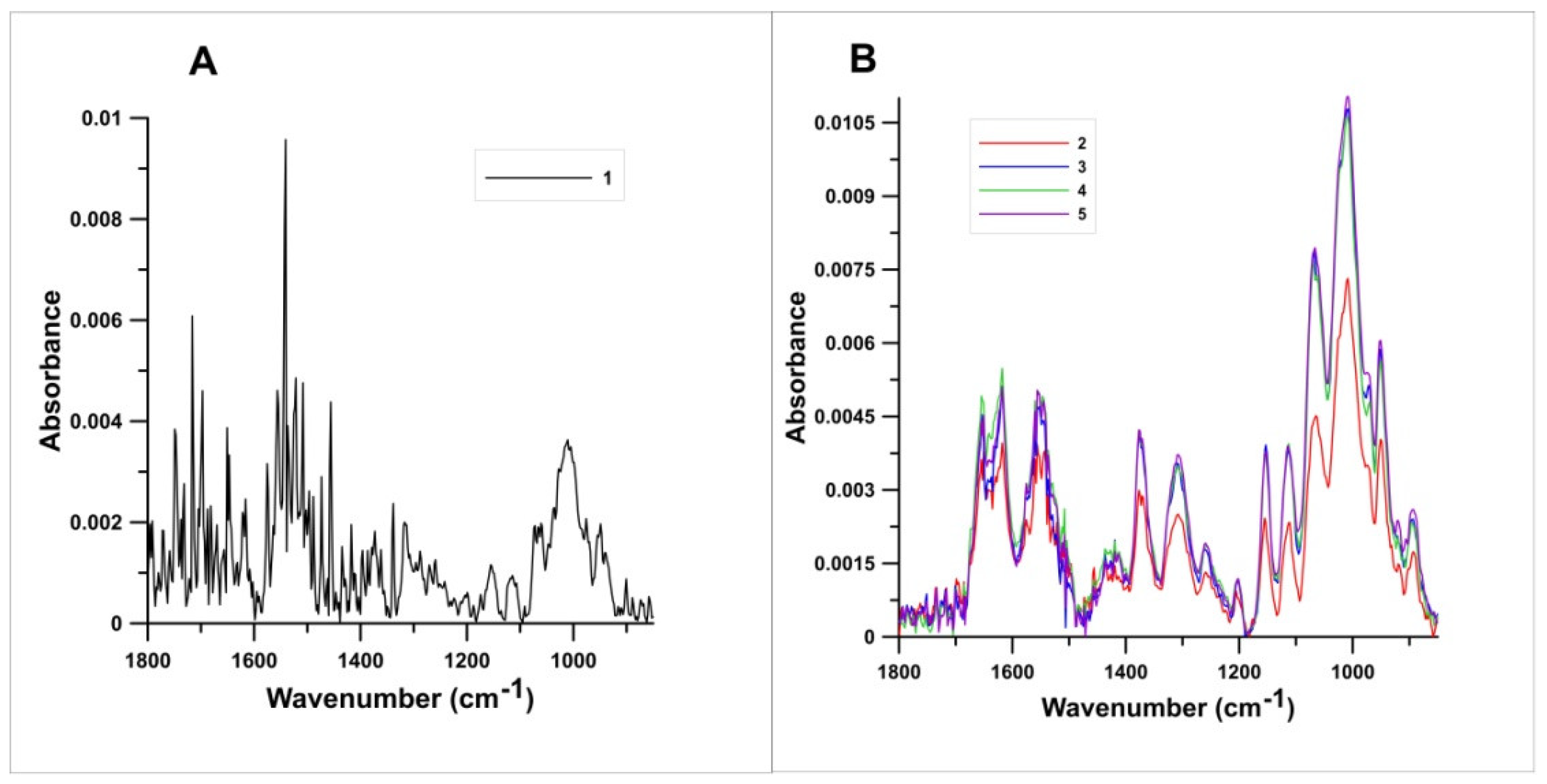
3.5. Fourier-Transform Infrared Spectroscopy (FTIR)
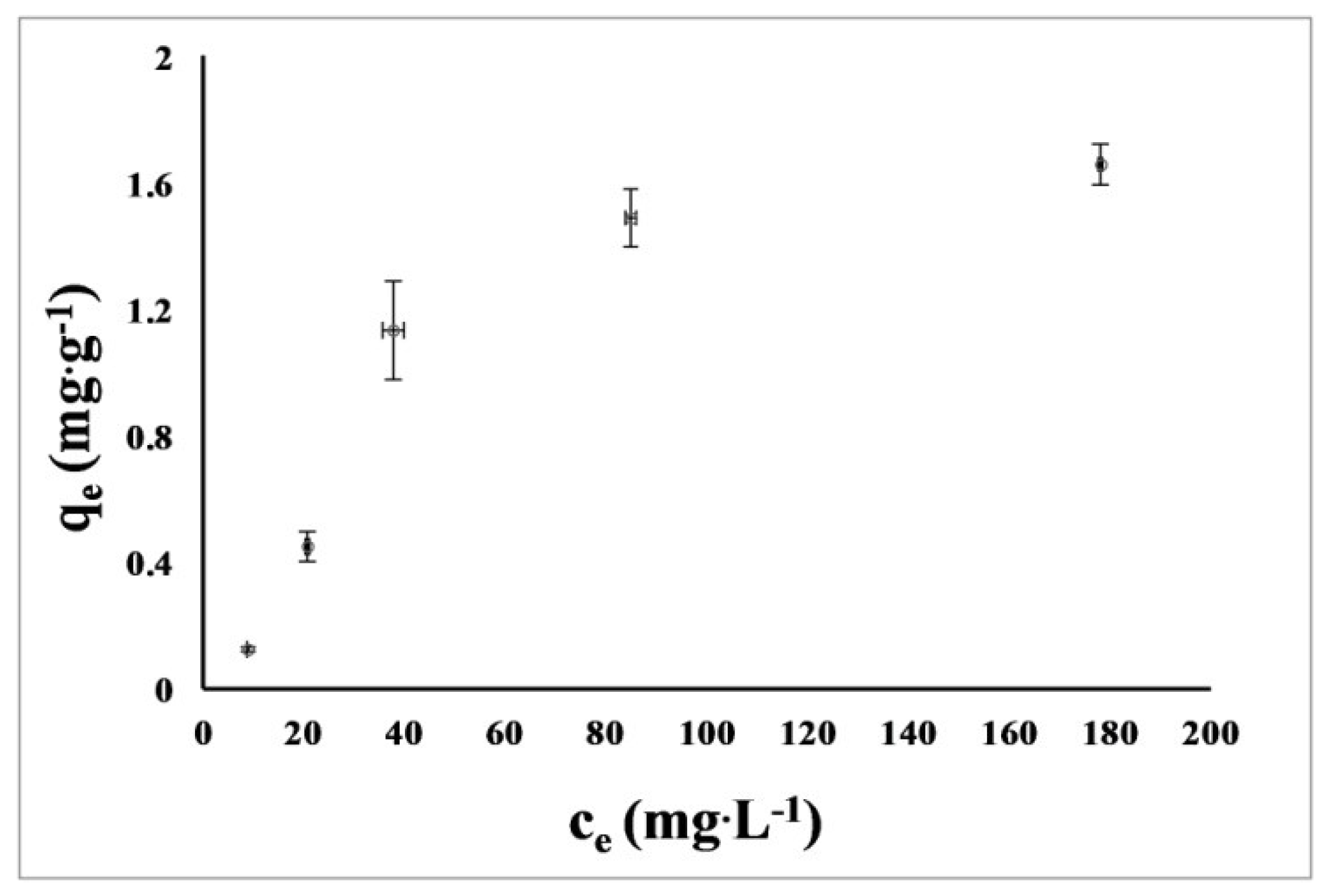
3.6. Sorption of Nickel Ions
3.7. Energy Dispersive X-Ray Spectroscopy (EDX)
4. Discussion
4.1. Chitin Isolation from H. illucens Pupal Exuviae
4.2. Optical and Confocal Microscopy
4.3. X-Ray Powder Diffraction (XRPD)
4.4. Thermogravimetric Analysis (TG, DTG, DTA)
4.5. Fourier-Transform Infrared Spectroscopy (FTIR)
4.6. Sorption of Nickel Ions
4.7. Energy Dispersive X-Ray Spectroscopy (EDX)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Huis, A.; van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security, 1st ed.; Fao Forestry Paper: Rome, Italy, 2013; pp. 67–88. [Google Scholar]
- Dossey, A.T.; Morales-Ramos, J.; Guadalupe Rojas, M. Insects as Sustainable Food Ingredients, Production, Processing and Food Application, 1st ed.; Elsevier: Cambridge, MA, USA, 2016; pp. 1–27. [Google Scholar] [CrossRef]
- Waśko, A.; Bulak, P.; Polak-Berecka, M.; Nowak, K.; Polakowski, C.; Bieganowski, A. The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int. J. Biol. Macromol. 2016, 92, 316–320. [Google Scholar] [CrossRef]
- Čičková, H.; Newton, L.; Lacy, R.C.; Kozánek, M. The use of fly larvae for organic waste treatment. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef]
- Bzowska-Bakalarz, M.; Bulak, P.; Bereś, P.K.; Czarnigowska, A.; Czarnigowski, J.; Karamon, B.; Pniak, M.; Bieganowski, A. Using gyroplane for application of Trichogramma spp. against the European corn borer in maize. Pest Manag. Sci. 2020, 76, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Draczyński, Z. Kopoliesterbutyrylo-acetylowy Chityny Jako Nowy Aktywny Składnik Nanokompozytów Polimerowo Włóknistych, 1st ed.; Zeszyty naukowe nr 1159, Rozprawy Naukowe; Z. 449 Politechnika Łódzka: Łódź, Poland, 2013; pp. 24–31. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and Application. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, M.M. Applications of chitin and its derivatives in biological medicine. Int. J. Mol. Sci. 2010, 11, 5152–5164. [Google Scholar] [CrossRef] [PubMed]
- Majeti, N.V.; Kumar, R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N. Production, Properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and chitosan: Chemistry, Properties and applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Heller, J.; Barr, J.; Ng, S.Y.; Schwach Abdellauoi, K.; Gurny, R. Poly(ortho esters): Synthesis, characterization, properties and uses. Adv. Drug. Deliv. Rev. 2002, 54, 1015–1039. [Google Scholar] [CrossRef]
- Cuong, H.N.; Minh, N.C.; Van Hoa, N.; Trung, T.S. Preparation and characterization of high purity β-chitin from squid pens (Loligo chenisis). Int. J. Biol. Macromol. 2016, 93, 442–447. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, O.; Xu, Y.; Xia, W. Recovery of Chitin from Antarctic Krill (Euphausia superba) Shell Waste by Microbial Deproteinization and Demineralization. J. Aquat. Food Product Techn. 2017, 26, 1210–1220. [Google Scholar] [CrossRef]
- Fadlaoui, S.; El Asri, O.; Mohammed, L.; Sihame, A.; Omari, A.; Melhaoui, M. Isolation and characterization of chitin from shells of the freshwater crab Potamon algeriense. Prog. Chem. Appl. Chitin. Its Deriv. 2019, 24, 23–35. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.-J. Chitin and chitosan production from shrimp shells using ammonium-based ionic liquids. Inter. J. Biol. Macromol. 2019, 130, 818–826. [Google Scholar] [CrossRef]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Inter. J. Biolog. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Abo Elsoud, M.M.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Abo Elsoud El Kady Bull. Natl. Res. Cent. 2019, 43, 1–12. [Google Scholar] [CrossRef]
- Badawy, R.M.; Mohamed, H.I. Chitin extration, Composition of Different Six Insect Species and Their Comparable Characteristics with That of the Shrimp. J. Am. Sci. 2015, 11, 127–134. [Google Scholar]
- Abidin, N.A.Z.; Kormin, F.; Abidin, N.A.Z.; Anuar, N.A.F.M.; Bakar, M.F.A. The Potential of Insects as Alternative Sources of Chitin: An Overview on the Chemical Method of Extraction from Various Sources. Int. J. Mol. Sci. 2020, 21, 4978. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Tech. Biotech. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Antonov, A.; Ivanov, G.; Pastukhova, N.; Bovykina, G. Production of chitin from dead Hermetia Illucens. Iop Conf. Ser. Earth Environ. Sci. 2019, 315, 042003. [Google Scholar] [CrossRef]
- Smets, R.; Verbinnen, B.; van de Voorde, I.; Aerts, G.; Claes, J.; Van Der Borght, M. Sequential Extraction and Characterisation of Lipids; Proteins; and Chitin from Black Soldier Fly (Hermetia illucens) Larvae; Prepupae; and Pupae. Waste Biomass Valorization 2020, 11, 6455–6466. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Brigode, C.; Hobbi, P.; Jafari, H.; Verwilghen, F.; Baeten, E.; Shavandi, A. Isolation and physicochemical properties of chitin polymer from insect farm side stream as a new source of renewable biopolymer. J. Clean. Prod. 2020, 275, 122924. [Google Scholar] [CrossRef]
- Kurchenko, V.P.; Kukulyanskaya, T.A.; Azarko, I.I.; Zueva, O.Y.; Khizmatullin, R.G.; Varlamov, V.P. Physicochemical Properties of Chitin–Melanin and Melanoprotein Complexes from Bee Corpses. Appl. Biochem. Microb. 2006, 42, 331–334. [Google Scholar] [CrossRef]
- Pestov, A.V.; Drachuk, S.V.; Koryakova, O.V.; Yatluk, Y.G. Isolation and Characterization of Chitin-Glucan Complexes from the Mycothallus of Fungi Belonging to Russula Genus. Chem. Sustain. Develop. 2009, 17, 281–287. [Google Scholar]
- Filipkowska, U.; Rodziewicz, J. Effectiveness of dye RB5 adsorption onto chitin and chitosan under static and dynamic conditions. Prog. Chem. App. Chitin Its Deriv. 2009, 14, 33–40. [Google Scholar]
- Szymczyk, P.; Filipkowska, U.; Jóźwiak, T.; Kuczajowska-Zadrożna, M. Phosphate removal from aqueous solutions by chitin and chitosan in flakes. Prog. Chem. App. Chitin Its Deriv. 2016, 21, 192–202. [Google Scholar] [CrossRef]
- Kousalyaa, G.N.; Gandhi, M.R.; Viswanathanc, N.; Meenakshi, S. Preparation and metal uptake studies of modified forms of chitin. Int. J. Biol. Macromol. 2010, 47, 583–589. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Bikiaris, D.N.; Kyzas, G.Z. Chitin Adsorbents for Toxic Metals: A Review. Int. J. Mol. Sci. 2017, 18, 114. [Google Scholar] [CrossRef]
- Boulaiche, W.; Hamdi, B.; Trari, M. Removal of heavy metals by chitin: Equilibrium, kinetic and thermodynamic studies. Appl. Water. Sci. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- de Souza, P.R.; do Carmo Ribeiro, T.M.; Lôbo, A.P.; Tokumoto, M.S.; de Jesus, R.M.; Lôbo, I.P. Removal of bromophenol blue anionic dye from water using a modified exuviae of Hermetia illucens larvae as biosorbent. Environ. Monit. Assess. 2020, 192, 1–16. [Google Scholar] [CrossRef]
- Draczyński, Z. Honeybee corpses as an available source of chitin. J. Appl. Polym. Sci. 2008, 109, 1974–1981. [Google Scholar] [CrossRef]
- Percot, A.; Viton, C.; Domard, A. Optimization of Chitin Extraction from Shrimp Shells. Biomacromolecules 2003, 4, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Nwe, N.; Furuike, T.; Tamura, H. Chapter One—Isolation and Characterization of Chitin and Chitosan from Marine Origin. In Advances in Food and Nutrition Research, 1st ed.; Ferreira, I.C.F.R., Barros, L., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 90, pp. 1–81. [Google Scholar] [CrossRef]
- WAXSFIT. Available online: http://www2.ath.bielsko.pl/~mrabiej/waxsfit/sub/main_en/ (accessed on 4 January 2021).
- ReX Powder diffraction. Available online: https://www.rexpd.org/ (accessed on 4 January 2021).
- Sikorski, P.; Hori, R.; Wada, M. Revisit of α-Chitin Crystal Structure Using High Resolution X-ray Diffraction Data. Biomacromolecules 2009, 10, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin producedfrom silkworm crysalides. Carbohyd. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Nemtsev, S.V.; Zueva, O.Y.; Khismatullin, M.R.; Albulov, A.I.; Varlamov, V.P. Isolation of Chitin and Chitosan from Honeybees. Appl. Biochem. Microb. 2004, 40, 39–43. [Google Scholar] [CrossRef]
- Hahn, T.; Roth, A.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Arsiwallab, T.; Zibeka, S. New methods for high-accuracy insect chitin measurement. J. Sci. Food Agric. 2018, 98, 5069–5073. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.-S.; Kim, D.-Y.; Shin, W.-S. Characterization of chitosan extracted from mealworm beetle (Tenebrio molitor, Zophobas morio) and rhinoceros beetle (Allomyrina dichotoma) and their antibacterial activities. Int. J. Biol. Macromol. 2019, 125, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Brown, R.L.; Monroe, W. Use of confocal laser scanning microscopy in systematics of insects with a comparison of fluorescence from different stains. Syst. Entomol. 2009, 34, 10–14. [Google Scholar] [CrossRef]
- Moussian, B.; Schwarz, H.; Bartoszewski, S.; Nüsslein-Volhard, C. Involvement of Chitin in Exoskeleton Morphogenesis in Drosophila melanogaster. J. Morphol. 2005, 264, 117–130. [Google Scholar] [CrossRef]
- Rabasović, M.D.; Pantelić, D.V.; Jelenković, B.M.; Ćurčić, S.B.; Rabasović, M.S.; Vrbica, M.D.; Lazović, V.M.; Ćurčić, B.P.M.; Krmpota, A.J. Nonlinear microscopy of chitin and chitinous structures: A case study of two cave-dwelling insects. J. Biomed. Opt. 2015, 20, 016010. [Google Scholar] [CrossRef]
- Tsurkan, M.V.; Voronkina, A.; Khrunyk, Y.; Wysokowski, M.; Petrenko, I.; Ehrlich, H. Progress in chitin analytics. Carbohydr. Polym. 2021, 252, 117204. [Google Scholar] [CrossRef]
- Kaya, M.; Erdogan, S.; Mol, A.; Baran, T. Comparison of chitin structures isolated from seven Orthoptera species. Int. J. Biol. Macromol. 2015, 72, 797–805. [Google Scholar] [CrossRef]
- Purkayastha, D.; Sarkar, S. Physicochemical Structure Analysis of Chitin Extracted from Pupa Exuviae and Dead Imago of Wild Black Soldier Fly (Hermetia illucens). J. Polym. Environ. 2020, 28, 445–457. [Google Scholar] [CrossRef]
- Zhou, P.; Li, J.; Yan, T.; Wang, X.; Huang, J.; Kuang, Z.; Ye, M.; Pan, M. Selectivity of deproteinization and demineralization using natural deep eutectic solvents for production of insect chitin (Hermetia illucens). Carbohydr. Polym. 2019, 225, 115255. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef]
- Ioelovich, M. Crystallinity and Hydrophility of Chitin and Chitosan. Res. Rev. J. Chem. 2014, 3, 7–14. [Google Scholar]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Tech. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Guibal, E.; Van Vooren, M.; Dempsey, B.A.; Roussy, J. A review of the use of chitosan for the removal of particulate and dissolved contaminants. Sep. Sci. Technol. 2006, 41, 2487–2514. [Google Scholar] [CrossRef]
- Roussy, J.; Van Vooren, M.; Dempsey, B.A.; Guibal, E. Influence of chitosan characteristics on the coagulation and the flocculation of bentonite suspensions. Water Res. 2005, 39, 3247–3258. [Google Scholar] [CrossRef]
- Georgieva, V.; Zvezdova, D.; Vlaev, L. Non-isothermal kinetics of thermal degradation of chitin. J. Therm. Anal. Calorim. 2013, 111, 763–771. [Google Scholar] [CrossRef]
- Stolarek, P.; Ledakowicz, S. Pyrolysis kinetics of chitin by non-isothermal thermogravimetry. Thermochim. Acta 2005, 433, 200–208. [Google Scholar] [CrossRef]
- Wanjun, T.; Cunxin, W.; Donghua, C. Kinetic studies on the pyrolysis of chitin and chitosan. Polym. Degrad. Stabil. 2005, 87, 389–394. [Google Scholar] [CrossRef]
- Arora, S.; Lal, S.; Kumar, S.; Kumar, M.; Kumar, M. Comparative degradation kinetic studies of three biopolymers: Chitin, chitosan and cellulose. Arch. Appl. Sci. Res. 2011, 3, 188–201. [Google Scholar]
- Kaya, M.; Lelešius, E.; Nagrockaitė, R.; Sargin, I.; Arslan, G.; Mol, A.; Baran, T.; Can, E.; Bitim, B. Differentiations of Chitin Content and Surface Morphologies of Chitins Extracted from Male and Female Grasshopper Species. PLoS ONE 2015, 10, e0115531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, Y.; Yu, L.; Zhang, C.; Xu, X.; Xue, Y.; Li, Z.; Xue, C. Crystalline structure and thermal property characterization of chitin from Antarctic krill (Euphausia superba). Carbohydr. Polym. 2013, 92, 90–97. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P. Preparation and characterization of α-chitin from cicada sloughs. Mater. Sci. Eng. C 2010, 30, 357–363. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Tozak, K.O.; Mol, A.; Sezen, G. Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). Biotech. Bioprocess Eng. 2015, 20, 168–179. [Google Scholar] [CrossRef]
- Poerio, A.; Petit, C.; Jehl, J.-P.; Arab-Tehrany, E.; Mano, J.-F.; Cleymand, F. Extraction and Physicochemical Characterization of Chitin from Cicada orni Sloughs of the South-Eastern French Mediterranean Basin. Molecules 2020, 25, 2543. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, G.; Andal, N.M.; Anbalagan, K. Adsorption studies of iron(III) on chitin. J. Chem. Sci. 2005, 117, 663–672. [Google Scholar] [CrossRef]
- Forutan, R.; Ehsandoost, E.; Hadipour, S.; Mobaraki, Z.; Saleki, M.; Mohebbi, G. Kinetic and equilibrium studies on the adsorption of lead by the chitin of pink shrimp (Solenocera melantho). Entomol. Appl. Sci. Lett. 2016, 3, 20–26. [Google Scholar]
- Karthik, R.; Meenakshi, S. Chemical modification of chitin with polypyrrole for the uptake of Pb(II) and Cd(II) ions. Intern. J. Biol. Macromol. 2015, 78, 157–164. [Google Scholar] [CrossRef]
- Xiong, C. Adsorption of cadmium (II) by chitin. J. Chem. Soc. Pak. 2010, 32, 429–435. [Google Scholar]
- Chui, V.W.D.; Mok, K.W.; Ng, C.Y.; Luong, B.P.; Ma, K.K. Removal and recovery of copper(II), chromium(III), and nickel(II) from solutions using crude shrimp chitin packed in small columns. Environ. Intern. 1996, 22, 463–468. [Google Scholar] [CrossRef]
- Yazidi, A.; Sellaoui, L.; Badawi, M.; Dotto, G.L.; Bonilla-Petriciolet, A.; Lamine, A.B.; Erto, A. Ternary adsorption of cobalt, nickel and methylene blue on a modified chitin: Phenomenological modeling and physical interpretation of the adsorption mechanism. Interj. Biol. Macromol. 2020, 158, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Hu, R.; Zhang, K.; Ma, S.; Zheng, L.; Yu, Z.; Zhang, J. Resistance of black soldier fly (Diptera: Stratiomyidae) larvae to combined heavy metals and potential application in municipal sewage sludge treatment. Environ. Sci. Pollut. Res. 2018, 25, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Kołodyńska, D.; Wnętrzak, R.; Leahy, J.J.; Hayes, M.H.B.; Kwapiński, W.; Hubicki, Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Acheampong, M.A.; Pereira, J.P.C.; Meulepas, R.J.W.; Lens, P.N.L. Biosorption of Cu(II) onto agricultural materials from tropical regions. J. Chem. Techn. Biotech. 2011, 86, 1184–1194. [Google Scholar] [CrossRef]


| Variant No. | Demineralization Stage | Deproteinization Stage | Depigmentation Stage | |
|---|---|---|---|---|
| chitin isolation | 1 | 1 M HCl to obtain acidic reaction at 22 °C for 1 h. Then NaOH to obtain neutral reaction. Rinsing by distilled water | 1 M NaOH at 80 °C to obtain alkaline reaction for 24 h. Then HCl to obtain neutral reaction. Rinsing by distilled water. | Without the stage of depigmentation |
| 2 | Water at 100 °C for 24 h | |||
| 3 | 9% H2O2 at 80 °C for 2.5 h | |||
| 4 | 9% H2O2 at 80 °C for 5 h | |||
| 5 | 1% KMnO4 at 80 °C for 20 min. Then oxalic acid (4% C2H2O4) to reduce the excess of KMnO4. Then NaOH to precipitate the manganese. Rinsing by distilled water | |||
| 6 | Sorption of Ni2+ on chitin isolated in variant 3 procedure | |||
| Variant Number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Yield (%) | 7.95 ± 0.20 b | 7.97 ± 0.10 b | 7.01 ± 0.12 c | 5.98 ± 0.08 a | 5.69 ± 0.28 a |
| Langmuir | qe exp [mg·g−1] | Freundlich | ||||
|---|---|---|---|---|---|---|
| KL | qm [mg·g−1] | R2 | K | 1/n | R2 | |
| 0.008 | 3.086 | 0.620 | 1.662 | 0.010 | 1.179 | 0.860 |
| Variant No. 3 | Variant No. 6 | |
|---|---|---|
| %C | 61.56 ± 7.40 | 62.71 ± 3.35 |
| %N | 11.60 ± 3.64 | 10.90 ± 1.69 |
| %O | 25.88 ± 4.93 | 25.28 ± 3.19 |
| %Na | 0.37 ± 0.19 | 0.18 ± 0.12 |
| %Al | 0.18 ± 0.15 | - |
| %Si | 0.11 ± 0.10 | - |
| %S | 0.33 | 0.07 ± 0.04 |
| %Cl | 0.33 ± 0.16 | 0.64 ± 0.19 |
| %Ca | 0.07 | - |
| %Zn | - | - |
| %Cd | - | - |
| %Ni | - | 0.23 ± 0.06 |
| %Pb | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Złotko, K.; Waśko, A.; Kamiński, D.M.; Budziak-Wieczorek, I.; Bulak, P.; Bieganowski, A. Isolation of Chitin from Black Soldier Fly (Hermetia illucens) and Its Usage to Metal Sorption. Polymers 2021, 13, 818. https://doi.org/10.3390/polym13050818
Złotko K, Waśko A, Kamiński DM, Budziak-Wieczorek I, Bulak P, Bieganowski A. Isolation of Chitin from Black Soldier Fly (Hermetia illucens) and Its Usage to Metal Sorption. Polymers. 2021; 13(5):818. https://doi.org/10.3390/polym13050818
Chicago/Turabian StyleZłotko, Katarzyna, Adam Waśko, Daniel M. Kamiński, Iwona Budziak-Wieczorek, Piotr Bulak, and Andrzej Bieganowski. 2021. "Isolation of Chitin from Black Soldier Fly (Hermetia illucens) and Its Usage to Metal Sorption" Polymers 13, no. 5: 818. https://doi.org/10.3390/polym13050818
APA StyleZłotko, K., Waśko, A., Kamiński, D. M., Budziak-Wieczorek, I., Bulak, P., & Bieganowski, A. (2021). Isolation of Chitin from Black Soldier Fly (Hermetia illucens) and Its Usage to Metal Sorption. Polymers, 13(5), 818. https://doi.org/10.3390/polym13050818