Oligoether/Zwitterion Diblock Copolymers: Synthesis and Application as Cathode-Coating Material for Li Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PPEGMA
2.3. Synthesis of PPEGMA-b-SPB
2.4. Preparation of Neat and Li Composite Films
2.5. Methods
3. Results and Discussion
3.1. Synthesis of Oligoetehr/Zwitterion Diblock Copolymers
3.2. Thermal Properties
3.3. Surface Morphology
3.4. Ionic Conductivity
3.5. Electrochemical Properties
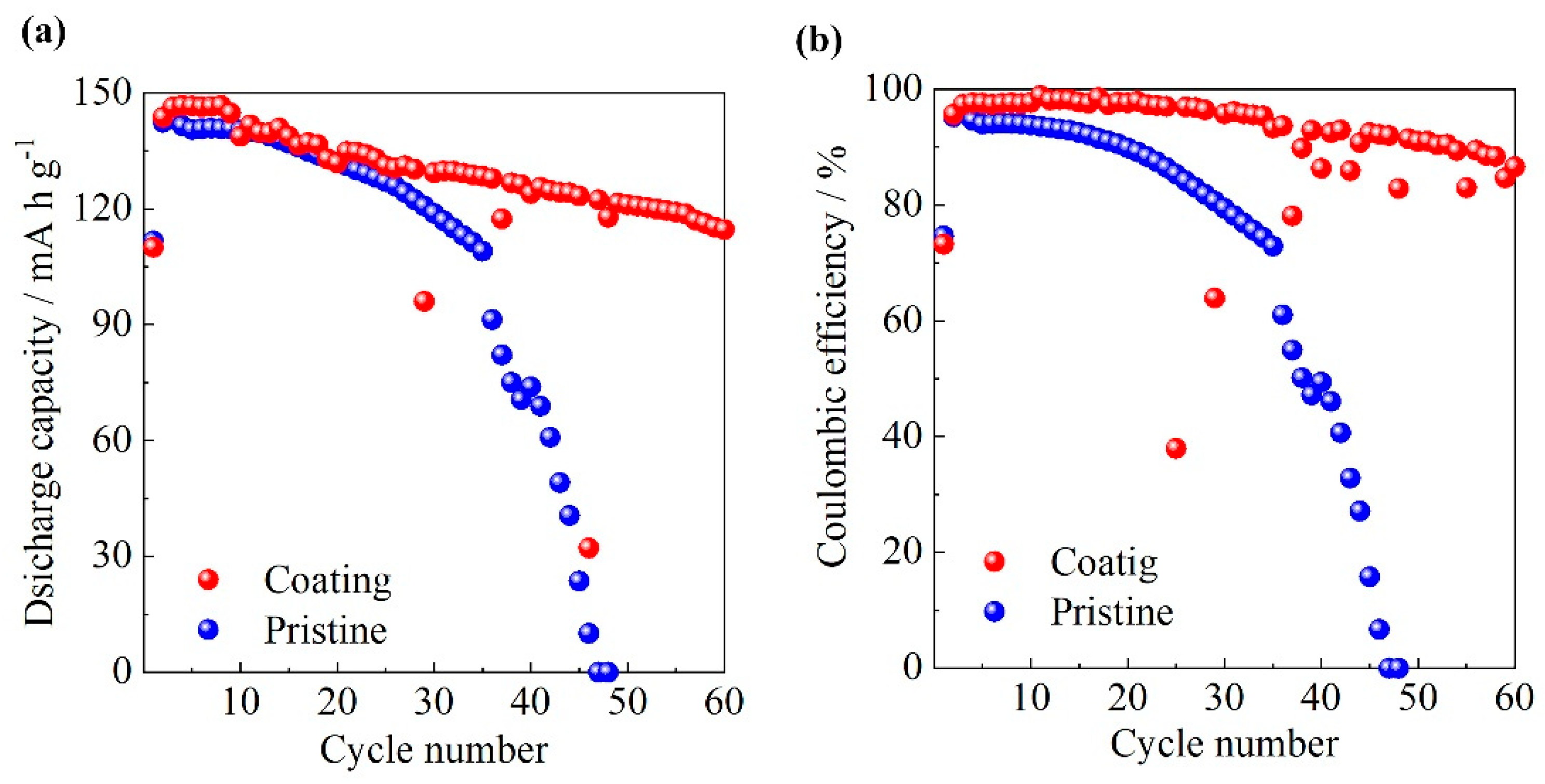
3.6. Charge/Discharge Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gray, F.M. Polymer Electrolyte; Royal Society of Chemistry: Cambridge, UK, 1997. [Google Scholar]
- Meyer, W.H. Polymer Electrolytes for Lithium-Ion Batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Tominaga, Y.; Yamazaki, K. Fast Li-ion conduction in poly(ethylene carbonate)-based electrolytes and composites filled with TiO2 nanoparticles. Chem. Commun. 2014, 50, 4448–4450. [Google Scholar] [CrossRef]
- Okumura, T.; Nishimura, S. Lithium ion conductive properties of aliphatic polycarbonate. Solid State Ionics 2014, 267, 68–73. [Google Scholar] [CrossRef]
- Sun, B.; Mindemark, J.; Edström, K.; Brandell, D. Polycarbonate-based solid polymer electrolytes for Li-ion batteries. Solid State Ionics 2014, 262, 738–742. [Google Scholar] [CrossRef]
- Mackanic, D.G.; Yan, X.; Zhang, Q.; Matsuhisa, N.; Yu, Z.; Jiang, Y.; Manika, T.; Lopez, J.; Yan, H.; Liu, K.; et al. Decoupling of mechanical properties and ionic conductivity in supramolecular lithium ion conductors. Nat. Commun. 2019, 10, 5384. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Croce, F.; Curini, R.; Martinelli, A.; Persi, L.; Ronci, F.; Scrosati, B.; Caminiti, R. Physical and Chemical Properties of Nanocomposite Polymer Electrolytes. J. Phys. Chem. B 1999, 103, 10632–10638. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Hirao, M.; Ito-Akita, K.; Ohno, H. Ion conduction in zwitterionic-type molten salts and their polymers. J. Mater. Chem. 2001, 11, 1057–1062. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Narita, A.; Ohno, H. Design of Ionic Liquids for Electrochemical Applications. Aust. J. Chem. 2004, 57, 139–144. [Google Scholar] [CrossRef]
- Ohno, H.; Yoshizawa-Fujita, M.; Kohno, Y. Design and properties of functional zwitterions derived from ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 10978–10991. [Google Scholar] [CrossRef] [PubMed]
- Tiyapiboonchaiya, C.; Pringle, J.M.; Sun, J.; Byrne, N.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. The zwitterion effect in high-conductivity polyelectrolyte materials. Nat. Mater. 2004, 3, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Byrne, N.; Pringle, J.M.; Tiyapiboonchaiya, C.; MacFarlane, D.R.; Forsyth, M. The additive effect of zwitterion and nano-particles on ion dissociation in polyelectrolytes. Electrochim. Acta 2005, 50, 2733–2738. [Google Scholar] [CrossRef]
- Byrne, N.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. The Zwitterion Effect in Ionic Liquids: Towards Practical Rechargeable Lithium-Metal Batteries. Adv. Mater. 2005, 17, 2497–2501. [Google Scholar] [CrossRef]
- Yoshizawa-Fujita, M.; Tamura, T.; Takeoka, Y.; Rikukawa, M. Low-melting zwitterion: Effect of oxyethylene units on thermal properties and conductivity. Chem. Commun. 2011, 47, 2345–2347. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yoshizawa-Fujita, M.; Zhu, H.; Forsyth, M.; Takeoka, Y.; Rikukawa, M. Improvement of charge/discharge properties of oligoether electrolytes by zwitterions with an attached cyano group for use in lithium-ion secondary batteries. Electrochim. Acta 2015, 186, 471–477. [Google Scholar] [CrossRef]
- Suematsu, M.; Yoshizawa-Fujita, M.; Zhu, H.; Forsyth, M.; Takeoka, Y.; Rikukawa, M. Effect of zwitterions on electrochemical properties of oligoether-based electrolytes. Electrochim. Acta 2015, 175, 209–213. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, F.; Lakuntza, O.; Oteo, U.; Qiao, L.; Martinez-Ibañez, M.; Zhu, H.; Carrasco, J.; Forsyth, M.; Armand, M. Suppressed Mobility of Negative Charges in Polymer Electrolytes with an Ether-Functionalized Anion. Angew. Chem. Int. Ed. 2019, 58, 12070–12075. [Google Scholar] [CrossRef]
- Yoshizawa-Fujita, M.; Shimada, K.; Takeoka, Y.; Rikukawa, M. Synthesis of Random Copolymers Containing Zwitterions and Their Evaluation as Electrolyte Materials. Kobunshi Ronbunshu 2015, 72, 624–629. [Google Scholar] [CrossRef]
- Galin, M.; Marchal, E.; Mathis, A.; Galin, J.-C. Poly(ammonioalkanesulfonate) Blends with Polar Organic Species and Alkali Metal Salts: Structure, Glass Transition and Ionic Conductivity. Polym. Adv. Technol. 1997, 8, 75–86. [Google Scholar] [CrossRef]
- Galin, M.; Mathis, A.; Galin, J.-C. Amorphous blends of poly(zwitterions) and zwitterionomers of the ammonioalkoxydicyanoethenolate type with some alkali metal salts. Polym. Adv. Technol. 2001, 12, 574–582. [Google Scholar] [CrossRef]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Niitani, T.; Shimada, M.; Kawamura, K.; Kanamura, K. Characteristics of new-type solid polymer electrolyte controlling nano-structure. J. Power Sources 2005, 146, 386–390. [Google Scholar] [CrossRef]
- Niitani, T.; Shimada, M.; Kawamura, K.; Dokko, K.; Rho, Y.-H.; Kanamura, K. Synthesis of Li[sup +] Ion Conductive PEO-PSt Block Copolymer Electrolyte with Microphase Separation Structure. Electrochem. Solid-State Lett. 2005, 8, A385. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ichikawa, T.; Ohno, H. Design of ionic liquid-based polyelectrolytes by combining ‘nanostructurisation’ and ‘zwitterionisation’. Polym. Chem. 2016, 7, 1230–1233. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Yoshizawa-Fujita, M.; Takeoka, Y.; Rikukawa, M. Effect of a pyrrolidinium zwitterion on charge/discharge cycle properties of Li/LiCoO2 and graphite/Li cells containing an ionic liquid electrolyte. J. Power Sources 2016, 331, 308–314. [Google Scholar] [CrossRef]
- Di Marco, G.; Lanza, M.; Pieruccini, M. Influence of poly(ethylene glycol)methacrylate on the morphology and conductivity of poly(ethylene oxide)-sodium thiocyanate complexes. Solid State Ionics 1996, 89, 117–125. [Google Scholar] [CrossRef]
- Ratner, M.A.; Shriver, D.F. Ion transport in solvent-free polymers. Chem. Rev. 1988, 88, 109–124. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, M.; Gao, X.; Bao, D.; Sun, Q.; Holmes, N.; Duan, H.; Mukherjee, S.; Adair, K.; Zhao, C.; et al. Determining the limiting factor of the electrochemical stability window for PEO-based solid polymer electrolytes: Main chain or terminal –OH group? Energy Environ. Sci. 2020, 13, 1318–1325. [Google Scholar] [CrossRef]
- Qi, C.; Iwahashi, T.; Zhou, W.; Kim, D.; Yamaguchi, S.; Yoshizawa-Fujita, M.; Ouchi, Y. Enhancement of the electrochemical stability of tetraglyme-Li[TFSA] electrolyte systems by adding [Bimps] zwitterion: An in-situ IV-SFG study. Electrochim. Acta 2020, 361, 137020. [Google Scholar] [CrossRef]
- Abraham, K.M.; Jiang, Z.; Carroll, B. Highly Conductive PEO-like Polymer Electrolytes. Chem. Mater. 1997, 9, 1978–1988. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Wei, F.; Zhang, J.-G.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Zhu, H.; Forsyth, M.; Takeoka, Y.; Rikukawa, M.; Yoshizawa-Fujita, M. Synthesis and evaluation of a novel pyrrolidinium-based zwitterionic additive with an ether side chain for ionic liquid electrolytes in high-voltage lithium-ion batteries. Electrochim. Acta 2017, 241, 272–280. [Google Scholar] [CrossRef]
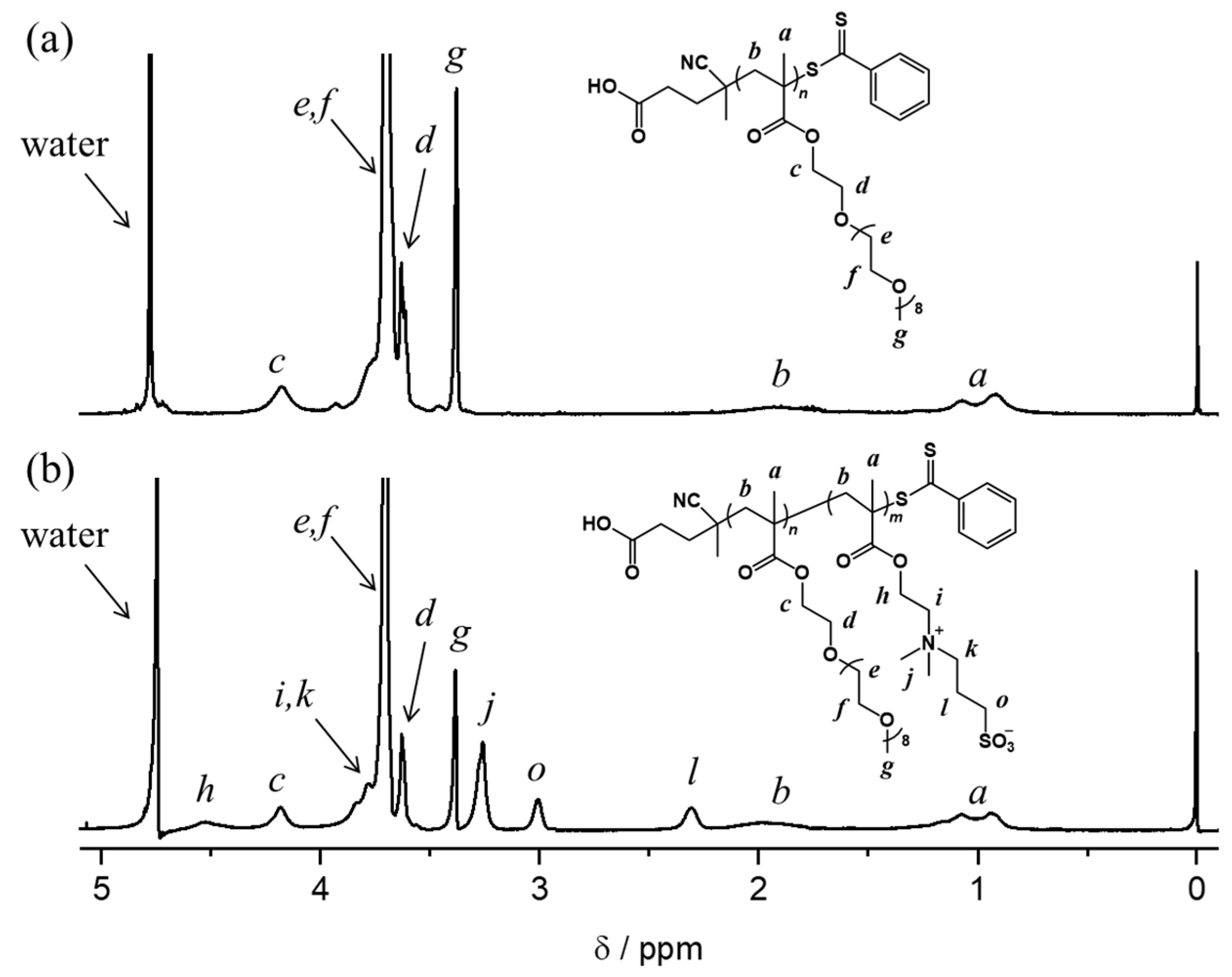
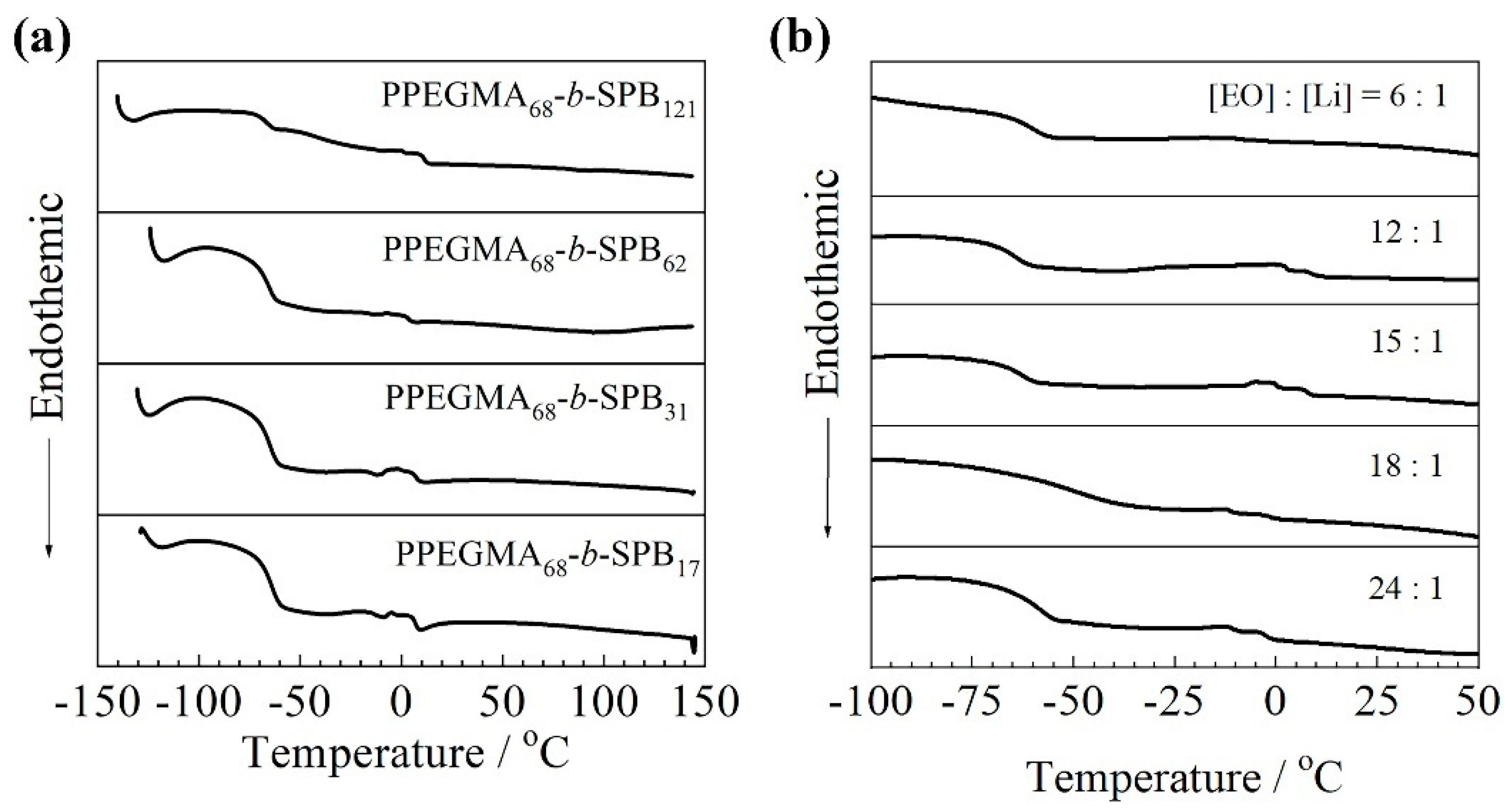
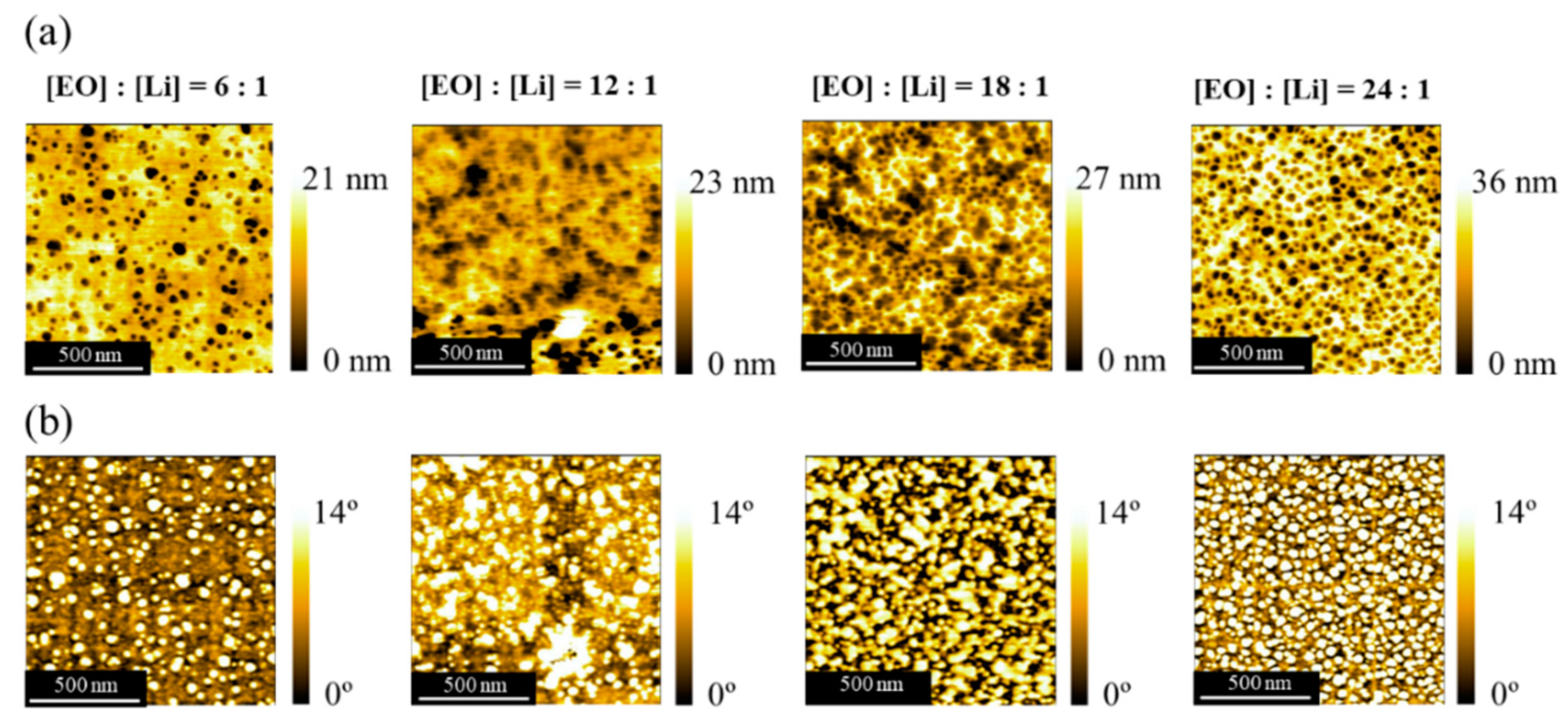
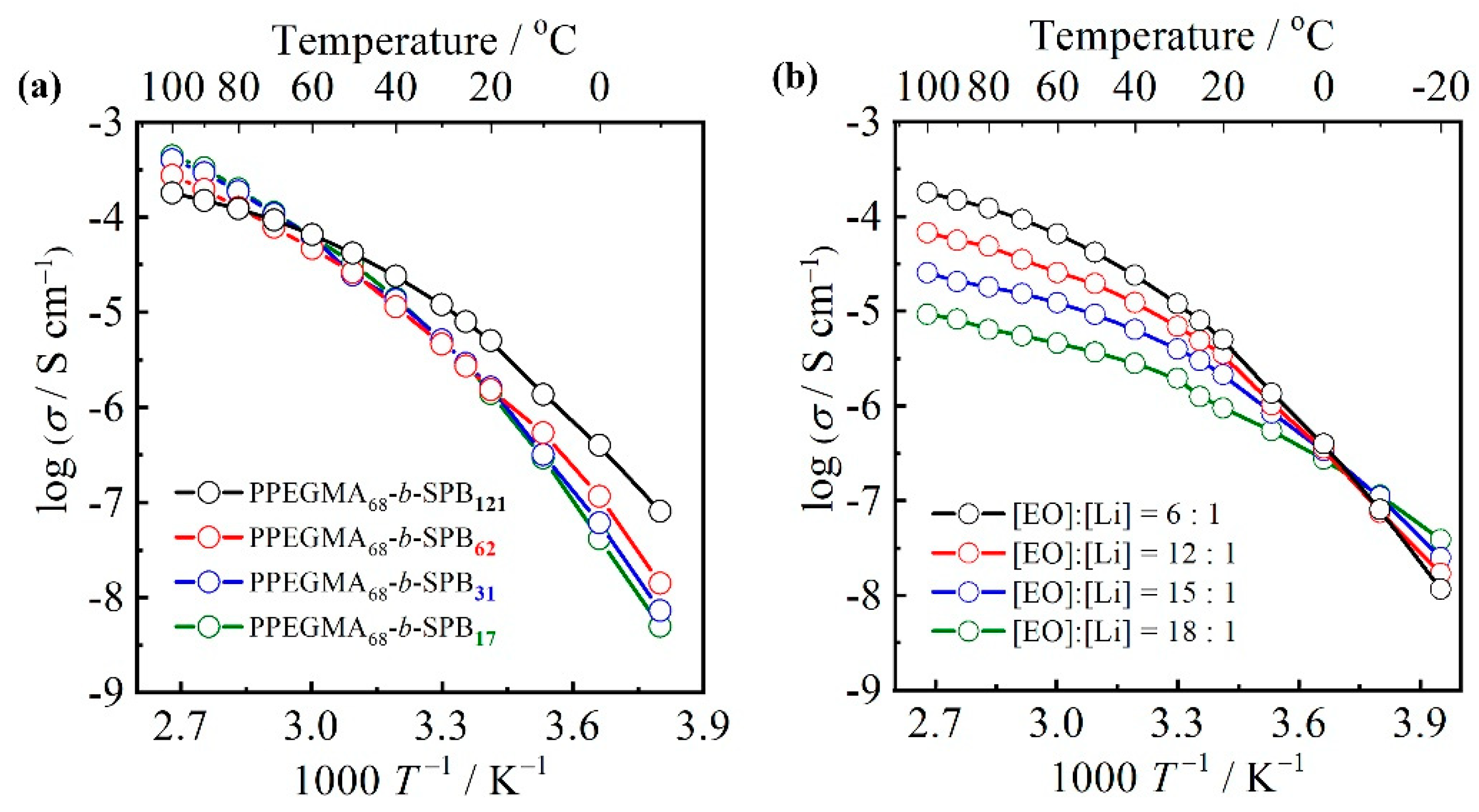

| Sample | Mna/g mol−1 | m b | m/n c | Tg,Ld/°C | Tg,He/°C |
|---|---|---|---|---|---|
| PPEGMA68-b-SPB121 | 67,000 | 121 | 1.8 | −78 | 10 |
| PPEGMA68-b-SPB62 | 50,500 | 62 | 0.91 | −76 | 2 |
| PPEGMA68-b-SPB31 | 42,000 | 31 | 0.46 | −70 | 7 |
| PPEGMA68-b-SPB17 | 37,800 | 17 | 0.25 | −70 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshizawa-Fujita, M.; Ishii, J.; Takeoka, Y.; Rikukawa, M. Oligoether/Zwitterion Diblock Copolymers: Synthesis and Application as Cathode-Coating Material for Li Batteries. Polymers 2021, 13, 800. https://doi.org/10.3390/polym13050800
Yoshizawa-Fujita M, Ishii J, Takeoka Y, Rikukawa M. Oligoether/Zwitterion Diblock Copolymers: Synthesis and Application as Cathode-Coating Material for Li Batteries. Polymers. 2021; 13(5):800. https://doi.org/10.3390/polym13050800
Chicago/Turabian StyleYoshizawa-Fujita, Masahiro, Jun Ishii, Yuko Takeoka, and Masahiro Rikukawa. 2021. "Oligoether/Zwitterion Diblock Copolymers: Synthesis and Application as Cathode-Coating Material for Li Batteries" Polymers 13, no. 5: 800. https://doi.org/10.3390/polym13050800
APA StyleYoshizawa-Fujita, M., Ishii, J., Takeoka, Y., & Rikukawa, M. (2021). Oligoether/Zwitterion Diblock Copolymers: Synthesis and Application as Cathode-Coating Material for Li Batteries. Polymers, 13(5), 800. https://doi.org/10.3390/polym13050800








