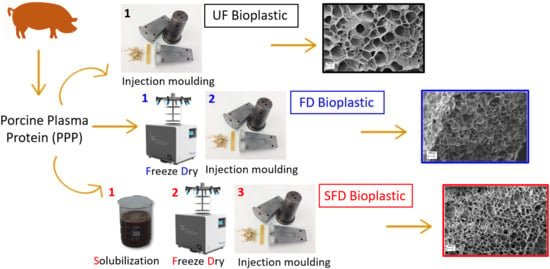
Strengthening of Porcine Plasma Protein Superabsorbent Materials through a Solubilization-Freeze-Drying Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Methods
2.2.1. Linear Viscoelastic Properties
2.2.2. Tensile Properties
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Water Uptake
2.2.5. Scanning Electron Microscopy
2.3. Statistical Analysis
3. Results and Discussion
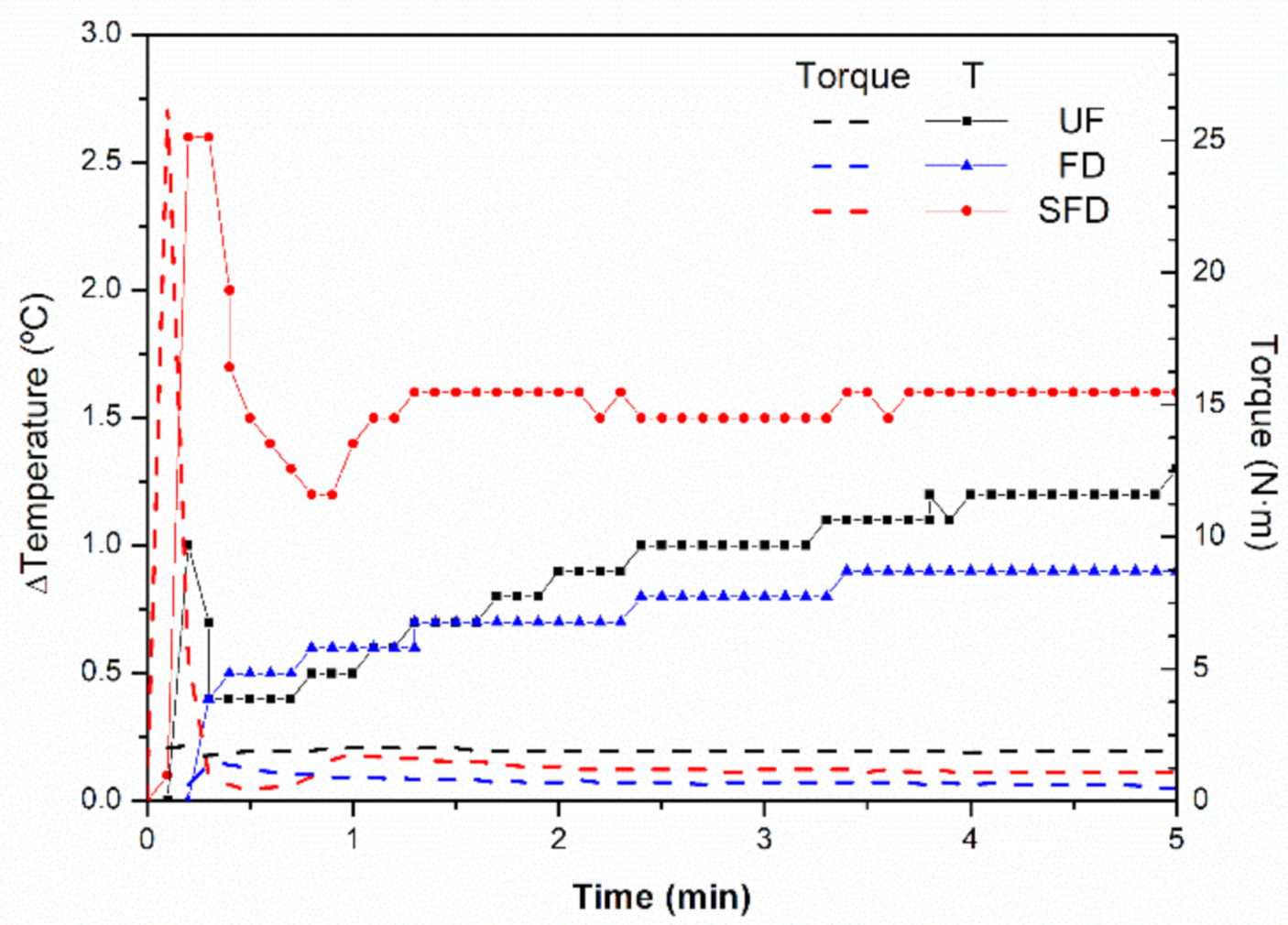
3.1. Mixing Stage
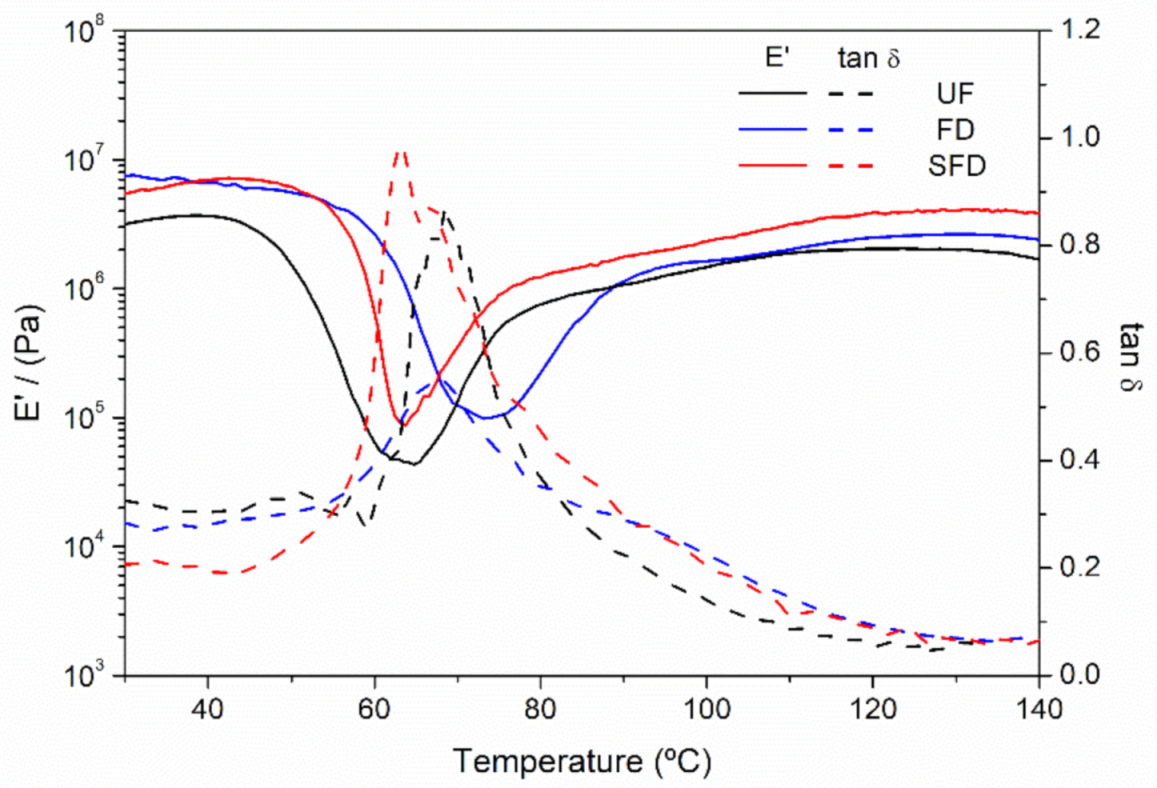
3.2. Thermal Characterization of the Systems
3.2.1. Evolution of the Rheological Properties of the Blends with Temperature
3.2.2. Differential Scanning Calorimetry (DSC)
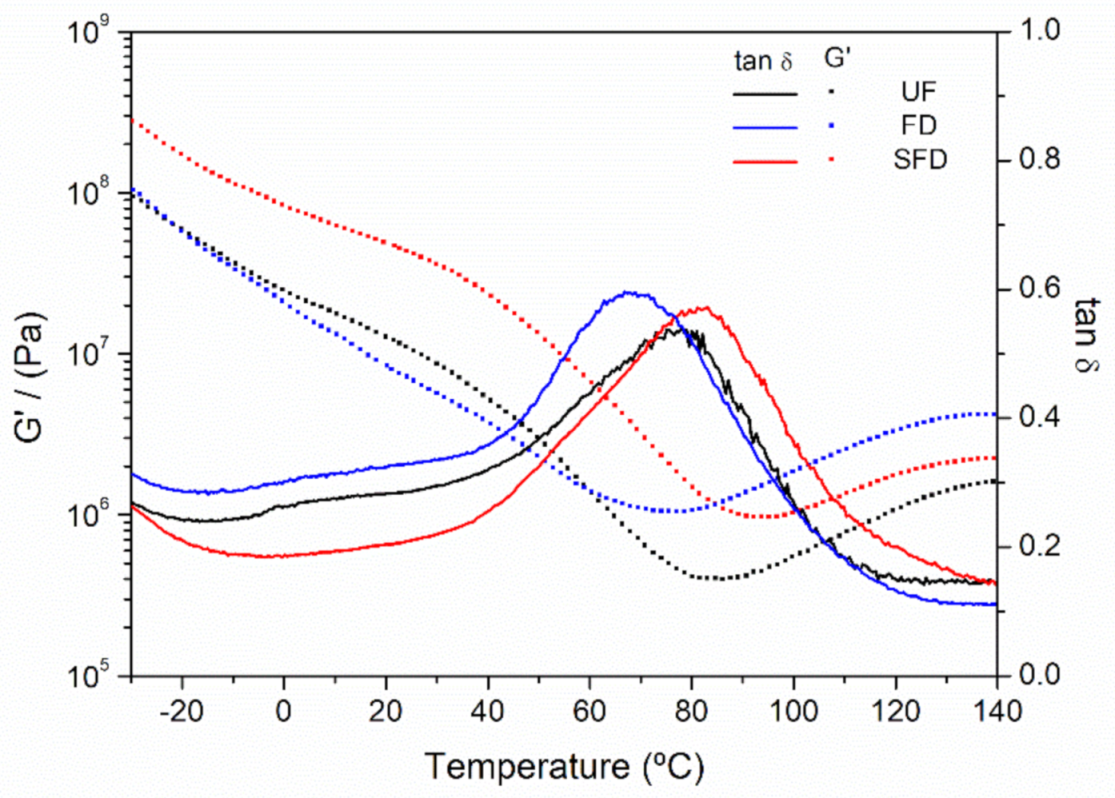
3.2.3. Evolution of the Rheological Properties of the PPP-Based Materials with Temperature
3.3. Mechanical Characterization of the PPP-Based Materials
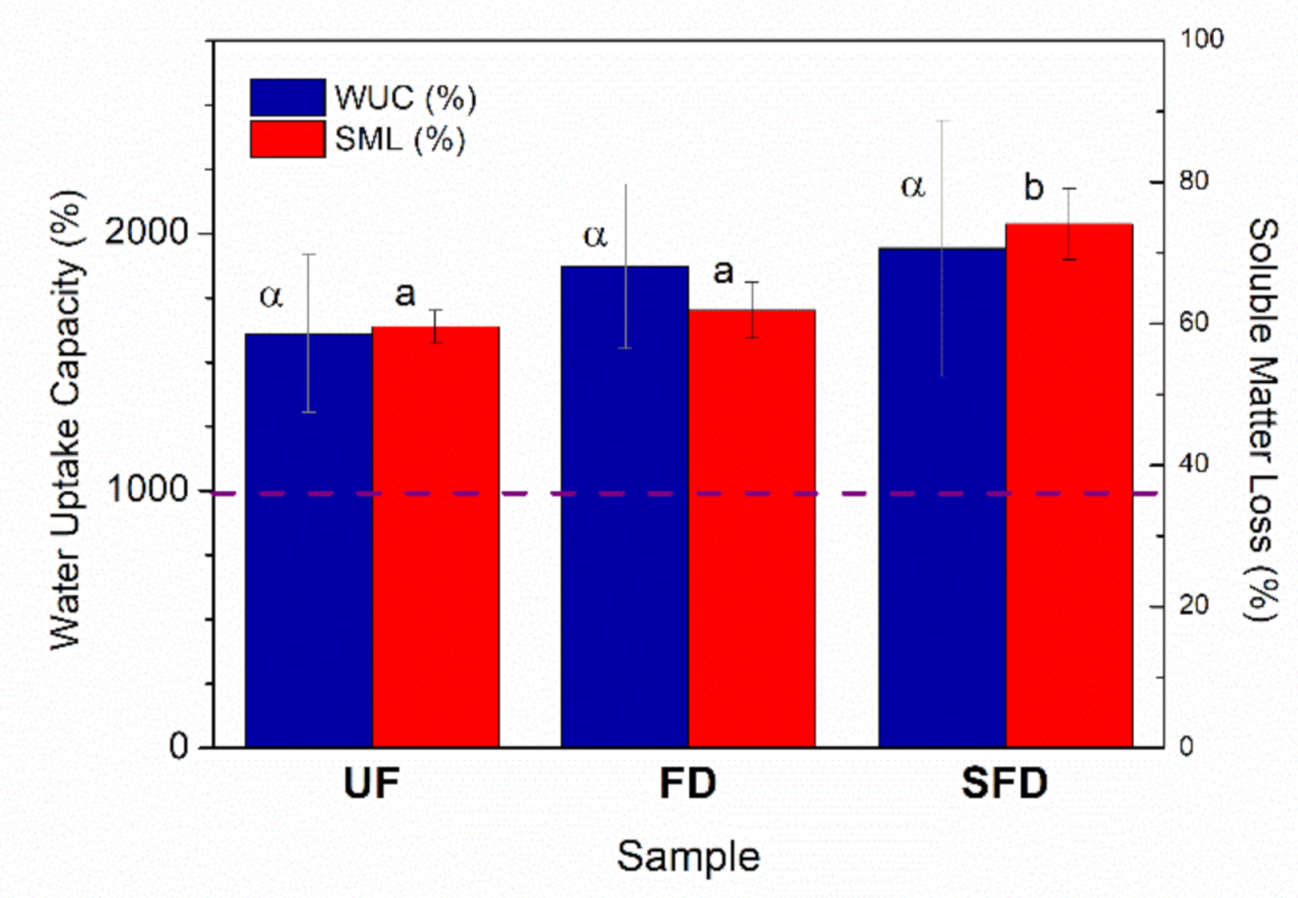
3.4. Water Uptake Capacity of PPP-Based Materials
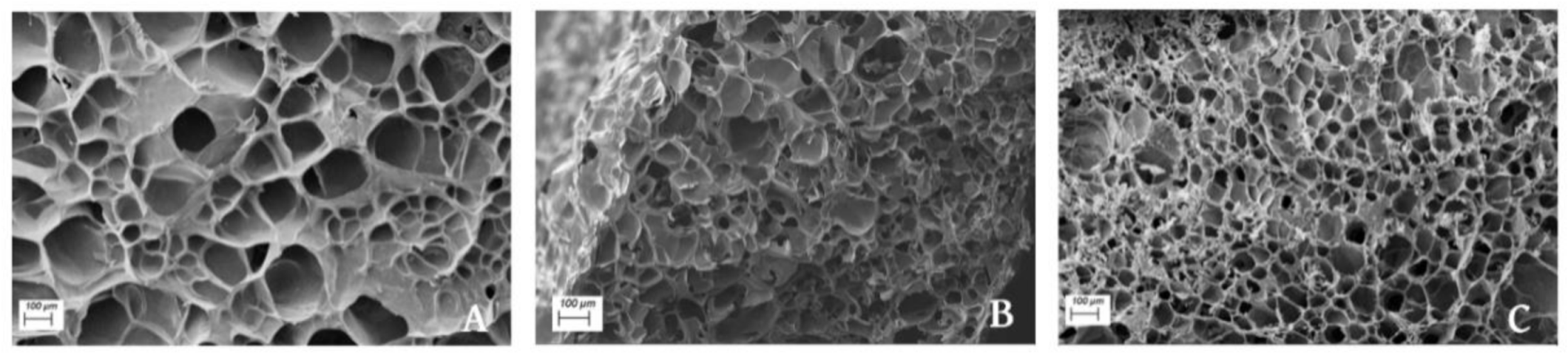
3.5. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuadri, A.A.A.; Romero, A.; Bengoechea, C.; Guerrero, A. The Effect of Carboxyl Group Content on Water Uptake Capacity and Tensile Properties of Functionalized Soy Protein-Based Superabsorbent Plastics. J. Polym. Environ. 2018, 26, 2934–2944. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Romero, A.; Bengoechea, C.; Guerrero, A. Natural superabsorbent plastic materials based on a functionalized soy protein. Polym. Test. 2017, 58, 126–134. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Fernandes, F.M.; Wicklein, B.; Alcântara, A.C.S.; Aranda, P. Fibrous clays based bionanocomposites. Prog. Polym. Sci. 2013, 38, 1392–1414. [Google Scholar] [CrossRef]
- Song, W.; Xin, J.; Zhang, J. One-pot synthesis of soy protein (SP)-poly(acrylic acid) (PAA) superabsorbent hydrogels via facile preparation of SP macromonomer. Ind. Crops Prod. 2017, 100, 117–125. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Bengoechea, C.; Rodríguez, N.; Guerrero, A. Development of green superabsorbent materials from a by-product of the meat industry. J. Clean. Prod. 2019, 223, 651–661. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Bengoechea, C.; Guerrero, A. Effect of pH on the properties of porcine plasma-based superabsorbent materials. Polym. Test. 2020, 85, 106453. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Bengoechea, C.; Guerrero, A. Composites from by-products of the food industry for the development of superabsorbent biomaterials. Food Bioprod. Process. 2020, 119, 296–305. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Pourjavadi, A.; Salimi, H.; Kurdtabar, M. Protein- and homo poly(amino acid)-based hydrogels with super-swelling properties. Polym. Adv. Technol. 2009, 20, 655–671. [Google Scholar] [CrossRef]
- Fernández-Espada, L.; Bengoechea, C.; Cordobés, F.; Guerrero, A. Protein/glycerol blends and injection-molded bioplastic matrices: Soybean versus egg albumen. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; del Toro, A.J.; Aguilar, J.M.; Bengoechea, C.; Guerrero, A.; Bengoechea, C.; Del Toro, A.; Aguilar, J.M.; Guerrero, A.; Bengoechea, C. Optimization of a thermal process for the production of superabsorbent materials based on a soy protein isolate. Ind. Crops Prod. 2018, 125, 573–581. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Del Toro, A.J.; Aguilar, J.M.; Guerrero, A.; Bengoechea, C. Formation of soy protein-based superabsorbent materials through optimization o f a thermal processing. Afinidad 2019, 76, 23–29. [Google Scholar]
- Capezza Villa, A.J. Novel Superabsorbent Materials Obtained from Plant Proteins; Deparment of Plant Breeding, Swedish University of Agricultural Sciences: Alnarp, Sweden, 2017. [Google Scholar]
- Jin, S.-K.; Choi, J.-S.; Kim, G.-D. Effect of porcine plasma hydrolysate on physicochemical, antioxidant, and antimicrobial properties of emulsion-type pork sausage during cold storage. Meat Sci. 2021, 171, 108293. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.; Rendueles, M.; Díaz, M. Production of porcine hemoglobin peptides at moderate temperature and medium pressure under a nitrogen stream. Functional and antioxidant properties. J. Agric. Food Chem. 2012, 60, 5636–5643. [Google Scholar] [CrossRef]
- Del Hoyo, P.; Rendueles, M.; Díaz, M. Effect of processing on functional properties of animal blood plasma. Meat Sci. 2008, 78, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, L.A.; Wiersema, D.; Hulshoff, P.; de Wit, J. Management of Waste from Animal Product Processing; FAO Corporate Document Repository: Wageningen, The Netherlands, 1996. [Google Scholar]
- Benitez, B.; Barboza, Y.; Bracho, M.; Izquierdo, P.; Archile, A.; Rangel, L.; Marquez, E. Efecto del pH y concentración de las proteínas sobre la propiedad de gelación de la sangre animal. Rev. Cient. Fac. Cienc. Vet. 1999, 9, 190–196. [Google Scholar]
- Parés, D.; Toldrà, M.; Saguer, E.; Carretero, C. Scale-up of the process to obtain functional ingredients based in plasma protein concentrates from porcine blood. Meat Sci. 2014, 96, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, S.; Saguer, E.; Toldrà, M.; Parés, D.; Carretero, C. Porcine plasma as polyphosphate and caseinate replacer in frankfurters. Meat Sci. 2012, 90, 624–628. [Google Scholar] [CrossRef]
- Hurtado, S.; Dagà, I.; Espigulé, E.; Parés, D.; Saguer, E.; Toldrà, M.; Carretero, C. Use of porcine blood plasma in “phosphate-free frankfurters”. Procedia Food Sci. 2011, 1, 477–482. [Google Scholar] [CrossRef]
- Ramos-Clamont, G.; Fernández-Michel, S.; Carrillo-Vargas, L.; Martinez-Calderón, E.; Vázquez-Moreno, L. Functional properties of protein fractions isolated from porcine blood. J. Food Sci. 2003, 68, 1196–1200. [Google Scholar] [CrossRef]
- Nuthong, P.; Benjakul, S.; Prodpran, T. Effect of some factors and pretreatment on the properties of porcine plasma protein-based films. LWT—Food Sci. Technol. 2009, 42, 1545–1552. [Google Scholar] [CrossRef]
- Samsalee, N.; Sothornvit, R. Development and characterization of porcine plasma protein-chitosan blended films. Food Packag. Shelf Life 2019, 22, 100406. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizers in edible films and coatings. In Innovations in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2005; pp. 403–433. ISBN 9780123116321. [Google Scholar]
- Nuthong, P.; Benjakul, S.; Prodpran, T. Characterization of porcine plasma protein-based films as affected by pretreatment and cross-linking agents. Int. J. Biol. Macromol. 2009, 44, 143–148. [Google Scholar] [CrossRef]
- García, M.C.; Torre, M.; Marina, M.L.L.; Laborda, F.; Rodriguez, A.R.; Garcia, M.C.; Torre, M.; Marina, M.L.L.; Laborda, F.; Rodriquez, A.R.; et al. Composition and characterization of soyabean and related products. Crit. Rev. Food Sci. Nutr. 1997, 37, 361–391. [Google Scholar] [CrossRef] [PubMed]
- Bourny, V.; Perez-Puyana, V.; Felix, M.; Romero, A.; Guerrero, A. Evaluation of the injection moulding conditions in soy/nanoclay based composites. Eur. Polym. J. 2017, 95, 539–546. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Bouroudian, E.; Perez-Puyana, V.; Guerrero, A.; Romero, A. Evaluation of different strengthening methods in the mechanical and functional properties of soy protein-based bioplastics. J. Clean. Prod. 2020, 262, 121517. [Google Scholar] [CrossRef]
- Gómez-Heincke, D.; Martínez, I.; Stading, M.; Gallegos, C.; Partal, P. Improvement of mechanical and water absorption properties of plant protein based bioplastics. Food Hydrocoll. 2017, 73, 21–29. [Google Scholar] [CrossRef]
- Rathna, G.V.N.; Damodaran, S. Swelling behavior of protein-based superabsorbent hydrogels treated with ethanol. J. Appl. Polym. Sci. 2001, 81, 2190–2196. [Google Scholar] [CrossRef]
- Gong, K.-J.; Shi, A.-M.; Liu, H.-Z.; Liu, L.; Hu, H.; Adhikari, B.; Wang, Q. Emulsifying properties and structure changes of spray and freeze-dried peanut protein isolate. J. Food Eng. 2016, 170, 33–40. [Google Scholar] [CrossRef]
- Stärtzel, P.; Gieseler, H.; Gieseler, M.; Abdul-Fattah, A.M.; Adler, M.; Mahler, H.-C.; Goldbach, P. Mannitol/l-Arginine-Based Formulation Systems for Freeze Drying of Protein Pharmaceuticals: Effect of the l-Arginine Counter Ion and Formulation Composition on the Formulation Properties and the Physical State of Mannitol. J. Pharm. Sci. 2016, 105, 3123–3135. [Google Scholar] [CrossRef] [PubMed]
- Costantino, H.R.; Firouzabadian, L.; Wu, C.; Carrasquillo, K.G.; Griebenow, K.; Zale, S.E.; Tracy, M.A. Protein spray freeze drying. 2. Effect of formulation variables on particle size and stability. J. Pharm. Sci. 2002, 91, 388–395. [Google Scholar] [CrossRef]
- Schersch, K.; Betz, O.; Garidel, P.; Muehlau, S.; Bassarab, S.; Winter, G. Systematic investigation of the effect of lyophilizate collapse on pharmaceutically relevant proteins I: Stability after freeze-drying. J. Pharm. Sci. 2010, 99, 2256–2278. [Google Scholar] [CrossRef] [PubMed]
- Apichartsrangkoon, A.; Ledward, D. Dynamic viscoelastic behaviour of high pressure treated gluten–soy mixtures. Food Chem. 2002, 77, 317–323. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Felix, M.; Romero, A.; Guerrero, A. Characterization of pea protein-based bioplastics processed by injection moulding. Food Bioprod. Process. 2016, 97, 100–108. [Google Scholar] [CrossRef]
- Fang, R.; Bogner, R.H.; Nail, S.L.; Pikal, M.J. Stability of Freeze-Dried Protein Formulations: Contributions of Ice Nucleation Temperature and Residence Time in the Freeze-Concentrate. J. Pharm. Sci. 2020, 109, 1896–1904. [Google Scholar] [CrossRef]
- Aguilar, J.M.; Jaramillo, A.; Cordobés, F.; Guerrerro, A. Influencia del procesado térmico sobre la reología de geles de albumen de huevo. Afinidad 2010, 545, 28–32. [Google Scholar]
- Aguilar, J.M.; Cordobes, F.; Jerez, A.; Guerrero, A. Influence of high pressure processing on the linear viscoelastic properties of egg yolk dispersions. Rheol. Acta 2007, 46, 731–740. [Google Scholar] [CrossRef]
- Zárate-Ramírez, L.S.; Romero, A.; Martínez, I.; Bengoechea, C.; Partal, P.; Guerrero, A. Effect of aldehydes on thermomechanical properties of gluten-based bioplastics. Food Bioprod. Process. 2014, 92, 20–29. [Google Scholar] [CrossRef]
- Bengoechea, C.; Arrachid, A.; Guerrero, A.; Hill, S.E.; Mitchell, J.R. Relationship between the glass transition temperature and the melt flow behavior for gluten, casein and soya. J. Cereal Sci. 2007, 45, 275–284. [Google Scholar] [CrossRef]
- Sedov, I.; Nikiforova, A.; Khaibrakhmanova, D. Evaluation of the binding properties of drugs to albumin from DSC thermograms. Int. J. Pharm. 2020, 583, 119362. [Google Scholar] [CrossRef]
- Johnson, C.M. Differential scanning calorimetry as a tool for protein folding and stability. Arch. Biochem. Biophys. 2013, 531, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Adebisi, A.O.; Kaialy, W.; Hussain, T.; Al-Hamidi, H.; Nokhodchi, A.; Conway, B.R.; Asare-Addo, K. Freeze-dried crystalline dispersions: Solid-state, triboelectrification and simultaneous dissolution improvements. J. Drug Deliv. Sci. Technol. 2020, 61, 102173. [Google Scholar] [CrossRef]
- Pierson, N.A.; Makarov, A.A.; Strulson, C.A.; Mao, Y.; Mao, B. Semi-automated screen for global protein conformational changes in solution by ion mobility spectrometry-massspectrometry combined with size-exclusion chromatography and differential hydrogen-deuterium exchange. J. Chromatogr. A 2017, 1496, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.L.; Foguel, D.; Da Poian, A.T.; Prevelige, P.E. The use of hydrostatic pressure as a tool to study viruses and other macromolecular assemblages. Curr. Opin. Struct. Biol. 1996, 6, 166–175. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Oliveira, S.; Bengoechea, C.; Sousa, I.; Raymundo, A.; Guerrero, A. A rheological approach to 3D printing of plasma protein based doughs. J. Food Eng. 2020, 288, 110255. [Google Scholar] [CrossRef]
- Felix, M.; Romero, A.; Cordobes, F.; Guerrero, A. Development of crayfish bio-based plastic materials processed by small-scale injection moulding. J. Sci. Food Agric. 2015, 95, 679–687. [Google Scholar] [CrossRef]
- Arsiccio, A.; Giorsello, P.; Marenco, L.; Pisano, R. Considerations on Protein Stability during Freezing and Its Impact on the Freeze-Drying Cycle: A Design Space Approach. J. Pharm. Sci. 2020, 109, 464–475. [Google Scholar] [CrossRef]
- Zhan, F.; Shi, M.; Wang, Y.; Li, B.; Chen, Y. Effect of freeze-drying on interaction and functional properties of pea protein isolate/soy soluble polysaccharides complexes. J. Mol. Liq. 2019, 285, 658–667. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Castillo, E.; Bengoechea, C.; Guerrero, A. Strengthening of Porcine Plasma Protein Superabsorbent Materials through a Solubilization-Freeze-Drying Process. Polymers 2021, 13, 772. https://doi.org/10.3390/polym13050772
Álvarez-Castillo E, Bengoechea C, Guerrero A. Strengthening of Porcine Plasma Protein Superabsorbent Materials through a Solubilization-Freeze-Drying Process. Polymers. 2021; 13(5):772. https://doi.org/10.3390/polym13050772
Chicago/Turabian StyleÁlvarez-Castillo, Estefanía, Carlos Bengoechea, and Antonio Guerrero. 2021. "Strengthening of Porcine Plasma Protein Superabsorbent Materials through a Solubilization-Freeze-Drying Process" Polymers 13, no. 5: 772. https://doi.org/10.3390/polym13050772
APA StyleÁlvarez-Castillo, E., Bengoechea, C., & Guerrero, A. (2021). Strengthening of Porcine Plasma Protein Superabsorbent Materials through a Solubilization-Freeze-Drying Process. Polymers, 13(5), 772. https://doi.org/10.3390/polym13050772