Fabrication and Properties of Electrospun Collagen Tubular Scaffold Crosslinked by Physical and Chemical Treatments
Abstract
:1. Introduction
2. Experiments
2.1. Materials
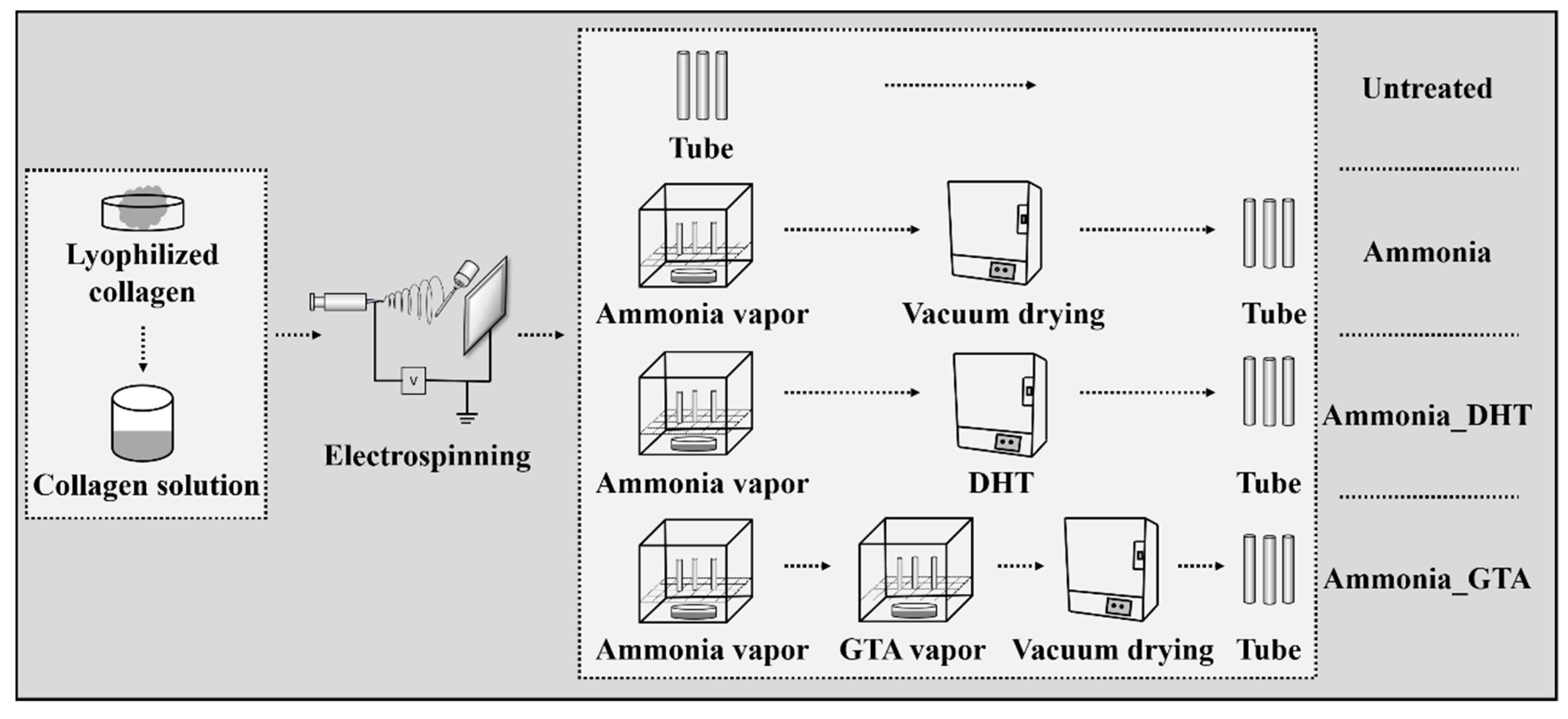
2.2. Fabrication of Electrospun Collagen Tube
2.3. Ammonia Treatment
2.4. Ammonia-Dehydrothermal (DHT) Treatment
2.5. Ammonia-Glutaraldehyde (GTA) Treatment
2.6. Characterizations
2.6.1. Morphological Observations
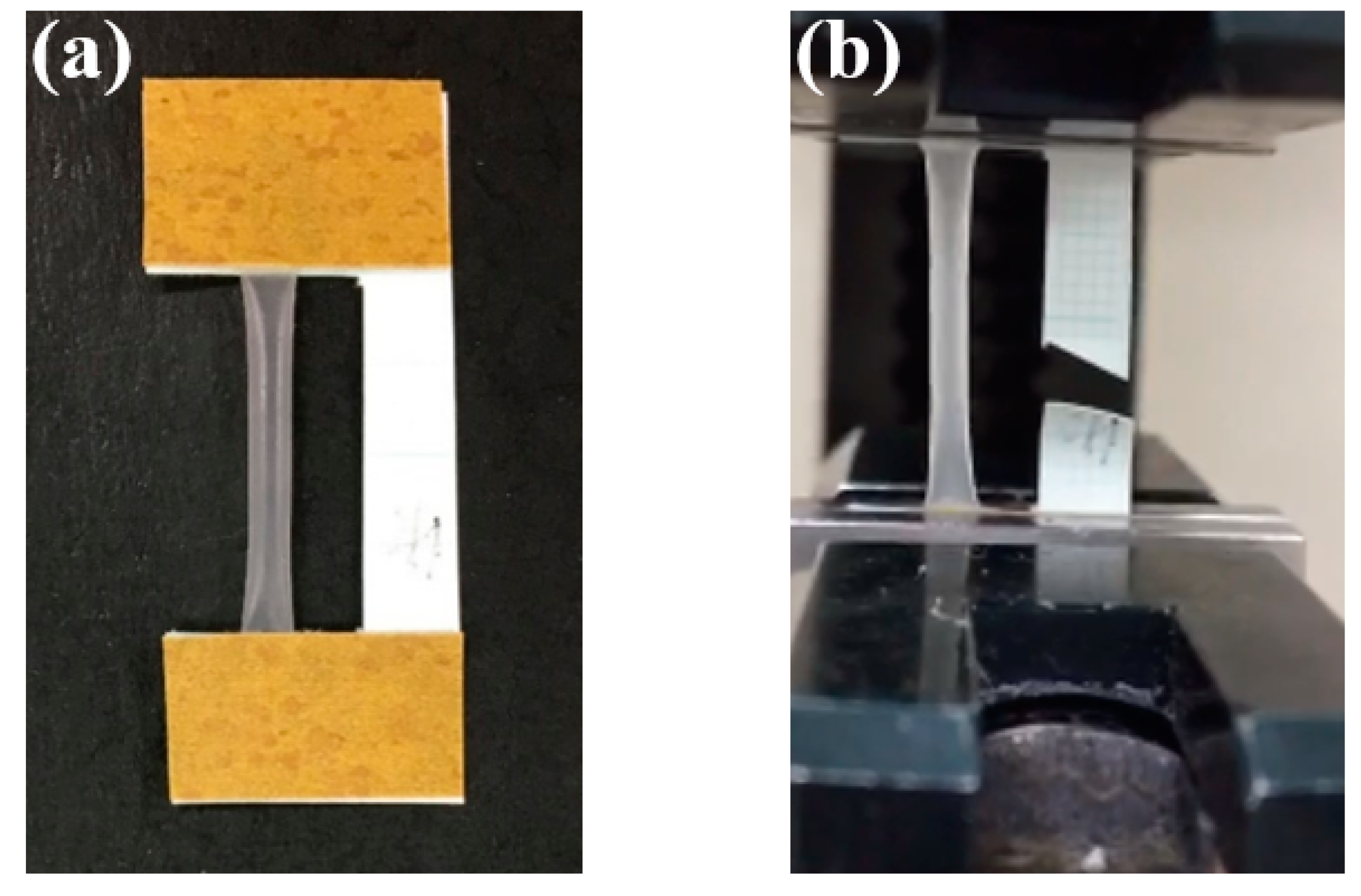
2.6.2. Mechanical Properties
2.6.3. Swelling Ratio and Weight Loss
2.6.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.6.5. Water Contact Angle
2.6.6. Statistical Analysis
3. Results and Discussion
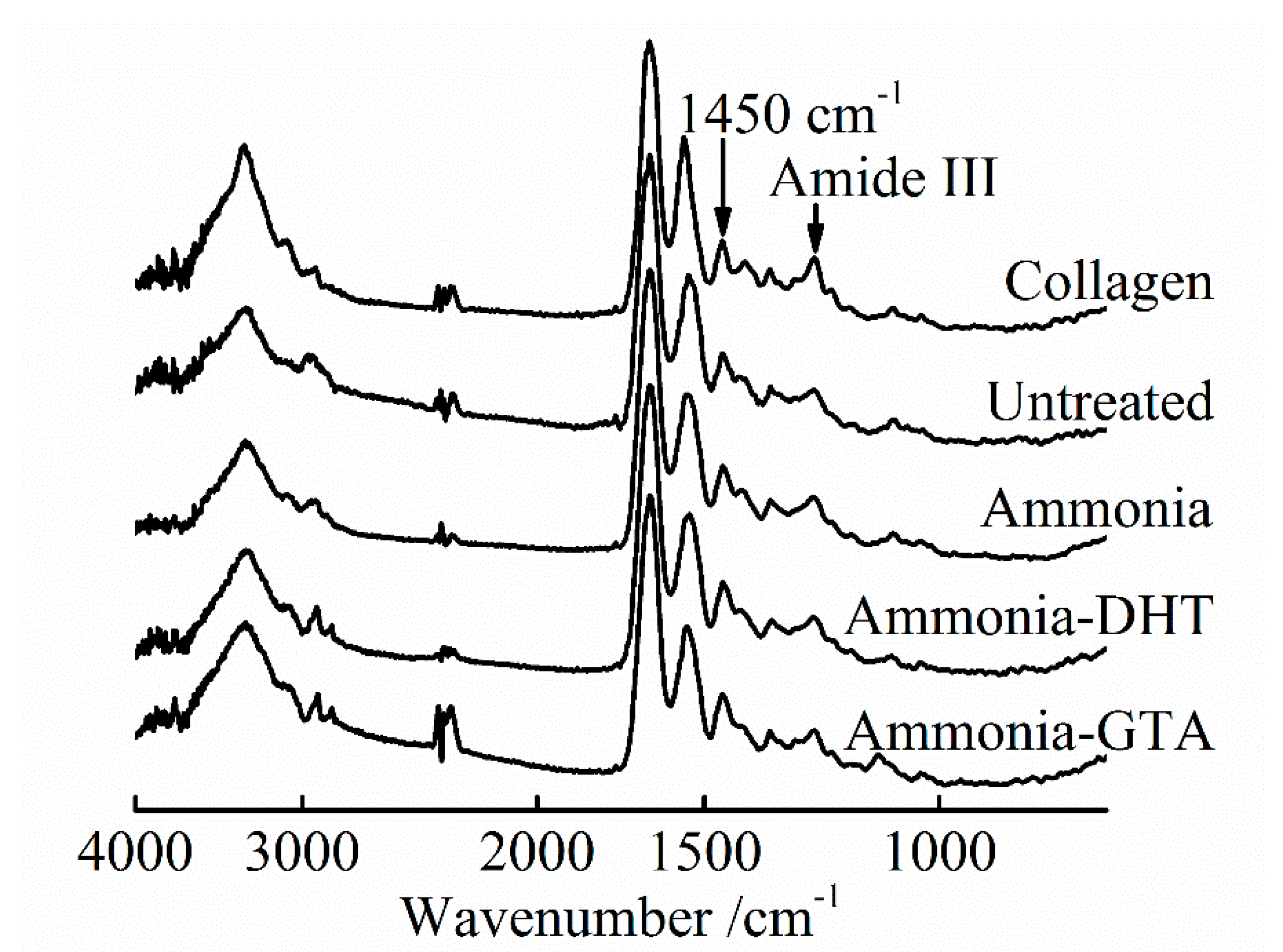
3.1. FTIR Spectroscopy Analysis
3.1.1. FTIR Spectroscopy Analysis of the Collagen Tubes as Electrospun
3.1.2. FTIR Spectroscopy Analysis of the Collagen Tubes after Treatments
3.2. Morphological Analyses
3.2.1. Morphology of the Collagen Tubes as Electrospun
3.2.2. Morphology of the Collagen Tubes after Treatments
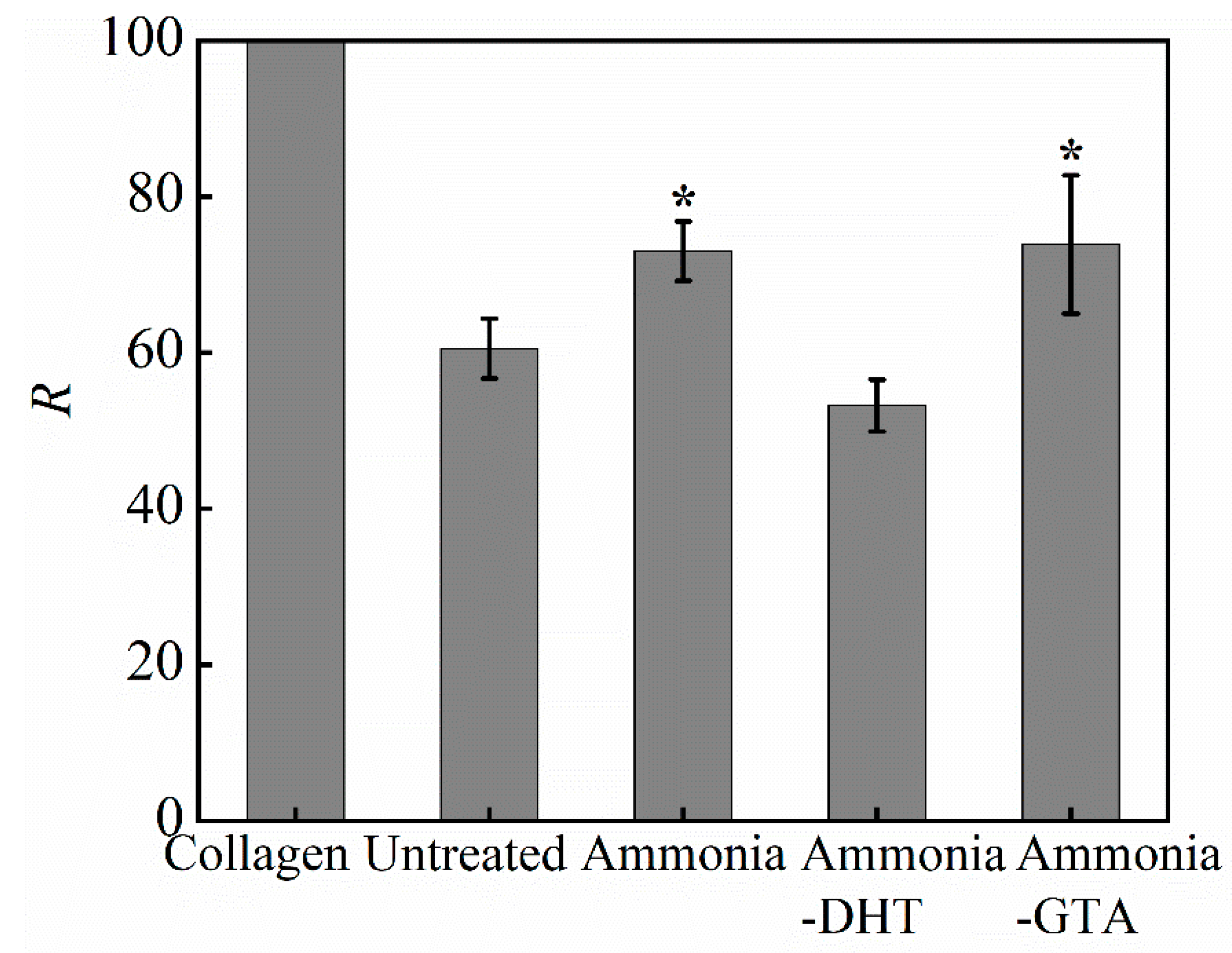
3.3. Swelling Ratio and Weight Loss
3.4. Mechanical Properties
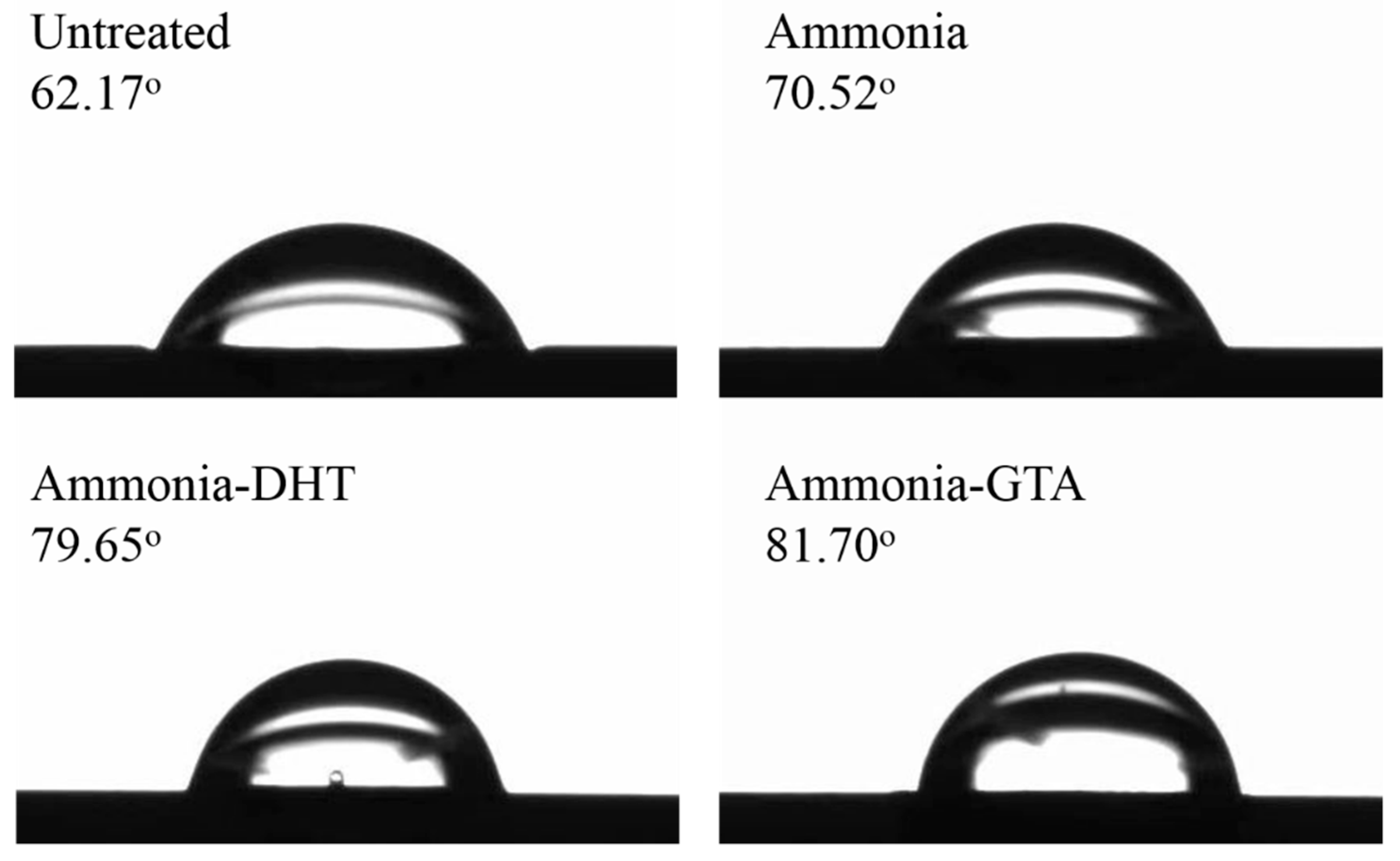
3.5. Water Contact Angle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef]
- Dehnavi, N.; Parivar, K.; Goodarzi, V.; Salimi, A.; Nourani, M.R. Systematically engineered electrospun conduit based on PGA/collagen/bioglass nanocomposites: The evaluation of morphological, mechanical, and bio-properties. Polym. Adv. Technol. 2019, 30, 2192–2206. [Google Scholar] [CrossRef]
- Stegemann, J.P.; Kaszuba, S.N.; Rowe, S.L. Review: Advances in Vascular Tissue Engineering Using Protein-Based Biomaterials. Tissue Eng. 2007, 13, 2601–2613. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.-K.; Ju, Y.M.; Cho, J.-G.; Jackson, J.D.; Lee, S.J.; Atala, A.; Yoo, J.J. End-to-side neurorrhaphy using an electrospun PCL/collagen nerve conduit for complex peripheral motor nerve regeneration. Biomaterials 2012, 33, 9027–9036. [Google Scholar] [CrossRef]
- Horng, I.; King, K.; Patel, J.; Pejavara, K.; Tallam, K.; Emge, T.; Murthy, N.S. Fabrication and Characterization of Polymeric Nerve Conduits. Tsinghua Sci. Technol. 2019, 10, 435–438. [Google Scholar]
- Seifu, D.G.; Purnama, A.; Mequanint, K.; Mantovani, D. Small-diameter vascular tissue engineering. Nat. Rev. Cardiol. 2013, 10, 410–421. [Google Scholar] [CrossRef]
- Verreck, G.; Chun, I.; Li, Y.; Kataria, R.; Zhang, Q.; Rosenblatt, J.; Decorte, A.; Heymans, K.; Adriaensen, J.; Bruining, M. Preparation and physicochemical characterization of biodegradable nerve guides containing the nerve growth agent sabeluzole. Biomaterials 2005, 26, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Inai, R.; Kotaki, M.; Ramakrishna, S. Aligned biodegradable nanofibrous structure: A potential scaffold for blood vessel engineering. Biomaterials 2004, 25, 877–886. [Google Scholar] [CrossRef]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun collagen nanofibers and their applications in skin tissue engi-neering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, R.; Ke, Q.; Morsi, Y.; Zhang, K.; Mo, X. Electrospun collagen–chitosan–TPU nanofibrous scaffolds for tissue engineered tubular grafts. Colloids Surf. B Biointerfaces 2011, 82, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymers 2008, 49, 2387–2425. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, Z.; Shariq, N.; Zohaib, K.; Masoud, V.; Sana, Z.; Bilal, N.; Farshid, S. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar]
- Saad, Q.; Muhammad, Z.; Shariq, N.; Zohaib, K.; Altaf, S.; Shehriar, H.; Ihtesham, R. Electrospinning of Chitosan-Based So-lutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar]
- Jing, X.; Li, H.; Mi, H.Y.; Liu, Y.J.; Tan, Y.M. Fabrication of Three-Dimensional Fluffy Nanofibrous Scaffolds for Tissue Engi-neering via Electrospinning and CO 2 Escaping Foaming. Ind. Eng. Chem. Res. 2019, 58, 9412–9421. [Google Scholar] [CrossRef]
- Kim, G.J.; Kim, K.O. Novel glucose-responsive of the transparent nanofiber hydrogel patches as a wearable biosensor via electrospinning. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, S.; Rinoldi, C.; Nakielski, P.; Ziai, Y.; Urbanek, O.; Li, X.; Kowalewski, T.A.; Ding, B.; Pierini, F. Ultraviolet Light-Assisted Electrospinning of Core–Shell Fully Cross-Linked P(NIPAAm-co-NIPMAAm) Hydrogel-Based Nanofibers for Ther-mally Induced Drug Delivery Self-Regulation. Adv. Mater. Interfaces 2020, 7, 2000247. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Wan, M.; Wang, Z.; Zou, X.; Zhao, Y.; Sun, L. A Facile Method for the Fabrication of Silver Nanoparticles Surface Decorated Polyvinyl Alcohol Electrospun Nanofibers and Controllable Antibacterial Activities. Polymer 2020, 12, 2486. [Google Scholar] [CrossRef] [PubMed]
- De Sio, L.; Ding, B.; Focsan, M.; Kogermann, K.; Pascoal-Faria, P.; Petronella, F.; Mitchell, G.; Zussman, E.; Pierini, F. Personalized Reusable Face Masks with Smart Nano-Assisted Destruction of Pathogens for COVID-19: A Visionary Road. Chem. A Eur. J. 2020. [Google Scholar] [CrossRef]
- Buluş, E.; Buluş, G.S.; Yakuphanoglu, F. Production of polylactic acid-activated charcoal nanofiber membranes for COVID-19 pandemic by electrospinning technique and determination of filtration efficiency. J. Mater. Electron. Dev. 2020, 4, 21–26. [Google Scholar]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Pabari, A.; Lloyd-Hughes, H.; Seifalian, A.M.; Mosahebi, A. Nerve Conduits for Peripheral Nerve Surgery. Plast. Reconstr. Surg. 2014, 133, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.; Yoo, M.K.; Park, I.K.; Kim, T.H.; Lee, H.C.; Lee, H.-S.; Oh, J.-S.; Akaike, T.; Cho, C.-S. A novel degradable poly-caprolactone networks for tissue engineering. Biomaterials 2003, 24, 801–808. [Google Scholar] [CrossRef]
- Lopes, M.S.; Jardini, A.L.; Filho, R.M. Poly (Lactic Acid) Production for Tissue Engineering Applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazrafshan, Z.; Stylios, G.K. Spinnability of collagen as a biomimetic material: A review. Int. J. Biol. Macromol. 2019, 129, 693–705. [Google Scholar] [CrossRef]
- Regev, O.; Vandebril, S.; Zussman, E.; Clasen, C. The role of interfacial viscoelasticity in the stabilization of an electrospun jet. Polymer 2010, 51, 2611–2620. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.-Y.L.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-spinning of pure collagen nano-fibres—Just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Figueiro, S.; Goes, J.; Moreira, R.; Sombra, A. On the physico-chemical and dielectric properties of glutaraldehyde crosslinked galactomannan–collagen films. Carbohydr. Polym. 2004, 56, 313–320. [Google Scholar] [CrossRef]
- Cheung, D.T.; Nimni, M.E. Mechanism of Crosslinking of Proteins by Glutaraldehyde I: Reaction with Model Compounds. Connect. Tissue Res. 1982, 10, 187–199. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, L.; Xu, H.; Yamamoto, M.; Shinoda, M.; Kishimoto, M.; Tanaka, T.; Yamane, H. Effect of the Application of a Dehydrothermal Treatment on the Structure and the Mechanical Properties of Collagen Film. Materials 2020, 13, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Zhou, L.; Xu, H.; Yamamoto, M.; Shinoda, M.; Tada, I.; Minami, S.; Urayama, K.; Yamane, H. The structure and properties of natural sheep casing and artificial films prepared from natural collagen with various crosslinking treatments. Int. J. Biol. Macromol. 2019, 135, 959–968. [Google Scholar] [CrossRef]
- Ho, H.-O.; Lin, C.-W.; Sheu, M.-T. Diffusion characteristics of collagen film. J. Control. Release 2001, 77, 97–105. [Google Scholar] [CrossRef]
- Le Corre-Bordes, D.; Hofman, K.; Hall, B. Guide to electrospinning denatured whole chain collagen from hoki fish using benign solvents. Int. J. Biol. Macromol. 2018, 112, 1289–1299. [Google Scholar] [CrossRef]
- Lai, G.; Li, Y.; Li, G. Effect of concentration and temperature on the rheological behavior of collagen solution. Int. J. Biol. Macromol. 2008, 42, 285–291. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Muyonga, J.; Cole, C.; Duodu, K. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Gordon, P.L.; Huang, C.; Lord, R.C.; Yannas, I.V. The Far-Infrared Spectrum of Collagen. Macromolecules 1974, 7, 954–956. [Google Scholar] [CrossRef]
- He, L.; Mu, C.; Shi, J.; Zhang, Q.; Shi, B.; Lin, W. Modification of collagen with a natural cross-linker, procyanidin. Int. J. Biol. Macromol. 2011, 48, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, Z.; Zhang, Y.; Chen, F.; Zhou, Y.; An, Q. Dehydrothermally crosslinked collagen/hydroxyapatite composite for enhanced in vivo bone repair. Colloids Surf. B Biointerfaces 2018, 163, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Tobolsky, A.V. Cross-linking of Gelatine by Dehydration. Nat. Cell Biol. 1967, 215, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.T.; Nimni, M.E. Mechanism of Crosslinking of Proteins by Glutaraldehyde II. Reaction with Monomeric and Polymeric Collagen. Connect. Tissue Res. 1982, 10, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Venugopal, J.; Huang, Z.-M.; Lim, C.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymers 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Angele, P.; Abke, J.; Kujat, R.; Faltermeier, H.; Schumann, D.; Nerlich, M.; Kinner, B.; Englert, C.; Ruszczak, Z.; Mehrl, R.; et al. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials 2004, 25, 2831–2841. [Google Scholar] [CrossRef]
- Tillman, B.W.; Yazdani, S.K.; Lee, S.J.; Geary, R.L.; Atala, A.; Yoo, J.J. The in vivo stability of electrospun polycaprolac-tone-collagen scaffolds in vascular reconstruction. Biomaterials 2009, 30, 583–588. [Google Scholar] [CrossRef]
- Fraser, R.; Macrae, T. Conformation in Fibrous Proteins and Related Synthetic Polypeptides; Elsevier: Amsterdam, The Netherlands, 1973. [Google Scholar]
- Elliott, J.T.; Woodward, J.T.; Umarji, A.; Mei, Y.; Tona, A. The effect of surface chemistry on the formation of thin films of native fibrillar collagen. Biomaterials 2007, 28, 576–585. [Google Scholar] [CrossRef]
- Silver, F.H.; Yannas, I.V.; Salzman, E.W. In vitro blood compatibility of glycosaminoglycan-precipitated collagens. J. Biomed. Mater. Res. 1979, 13, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Guo, Z.; He, P.; Chen, T.; Li, L.; Ding, S.; Li, H. Study on structure, mechanical property and cell cytocompatibility of electrospun collagen nanofibers crosslinked by common agents. Int. J. Biol. Macromol. 2018, 113, 476–486. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, P.; Wei, B.; Mo, X.; Cui, F. Electrospun collagen–chitosan nanofiber: A biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomater. 2010, 6, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Chang, G.Y.; Chen, J.K. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 183–188. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Feng, F.; Zhang, H. Study on wettability, mechanical property and biocompatibility of electrospun gelatin/zein nanofibers cross-linked by glucose. Food Hydrocoll. 2019, 87, 1–10. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Ponzini, S.; De Stefano, P.; Ortoleva, G.; Vignati, L.; Draghi, L. Cross-Linking Optimization for Electrospun Gelatin: Challenge of Preserving Fiber Topography. Polymers 2020, 12, 2472. [Google Scholar] [CrossRef] [PubMed]
- Drexler, J.W.; Powell, H.M. Dehydrothermal Crosslinking of Electrospun Collagen. Tissue Eng. Part C Methods 2011, 17, 9–17. [Google Scholar] [CrossRef] [PubMed]

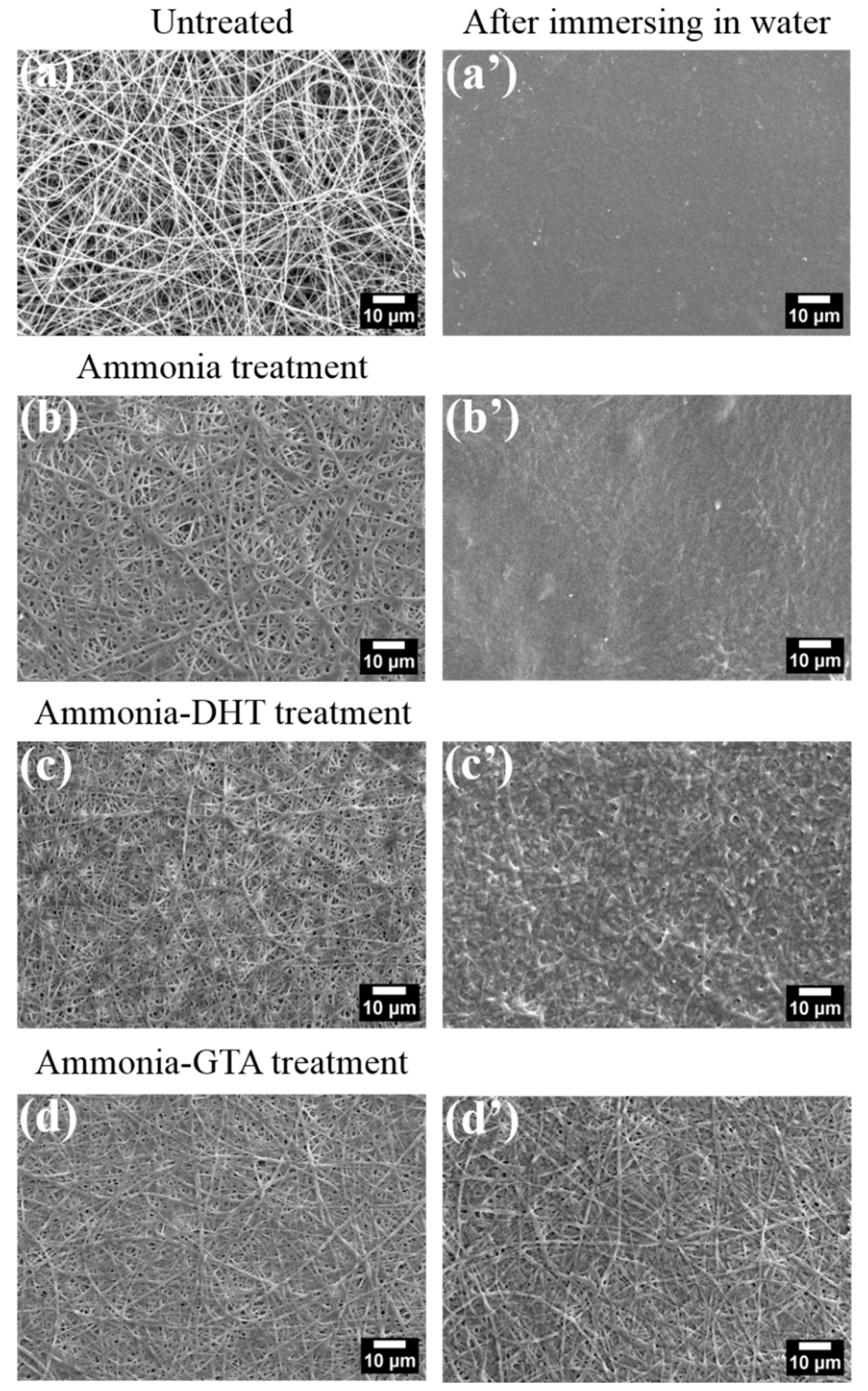



| Treatments | Untreated | Ammonia | Ammonia-DHT | Ammonia-GTA |
|---|---|---|---|---|
| Fiber diameter/nm | 336 ± 149 | 452 ± 174 | 439 ± 242 | 430 ± 227 |
| Increasing ratio/% | - | 34.5 | 30.7 | 28.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Meng, J.; Xu, H.; Shinoda, M.; Kishimoto, M.; Sakurai, S.; Yamane, H. Fabrication and Properties of Electrospun Collagen Tubular Scaffold Crosslinked by Physical and Chemical Treatments. Polymers 2021, 13, 755. https://doi.org/10.3390/polym13050755
Chen X, Meng J, Xu H, Shinoda M, Kishimoto M, Sakurai S, Yamane H. Fabrication and Properties of Electrospun Collagen Tubular Scaffold Crosslinked by Physical and Chemical Treatments. Polymers. 2021; 13(5):755. https://doi.org/10.3390/polym13050755
Chicago/Turabian StyleChen, Xuefei, Jie Meng, Huaizhong Xu, Masaya Shinoda, Masanori Kishimoto, Shinichi Sakurai, and Hideki Yamane. 2021. "Fabrication and Properties of Electrospun Collagen Tubular Scaffold Crosslinked by Physical and Chemical Treatments" Polymers 13, no. 5: 755. https://doi.org/10.3390/polym13050755
APA StyleChen, X., Meng, J., Xu, H., Shinoda, M., Kishimoto, M., Sakurai, S., & Yamane, H. (2021). Fabrication and Properties of Electrospun Collagen Tubular Scaffold Crosslinked by Physical and Chemical Treatments. Polymers, 13(5), 755. https://doi.org/10.3390/polym13050755








