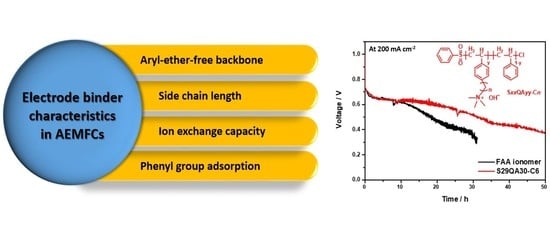
Polystyrene-Based Hydroxide-Ion-Conducting Ionomer: Binder Characteristics and Performance in Anion-Exchange Membrane Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
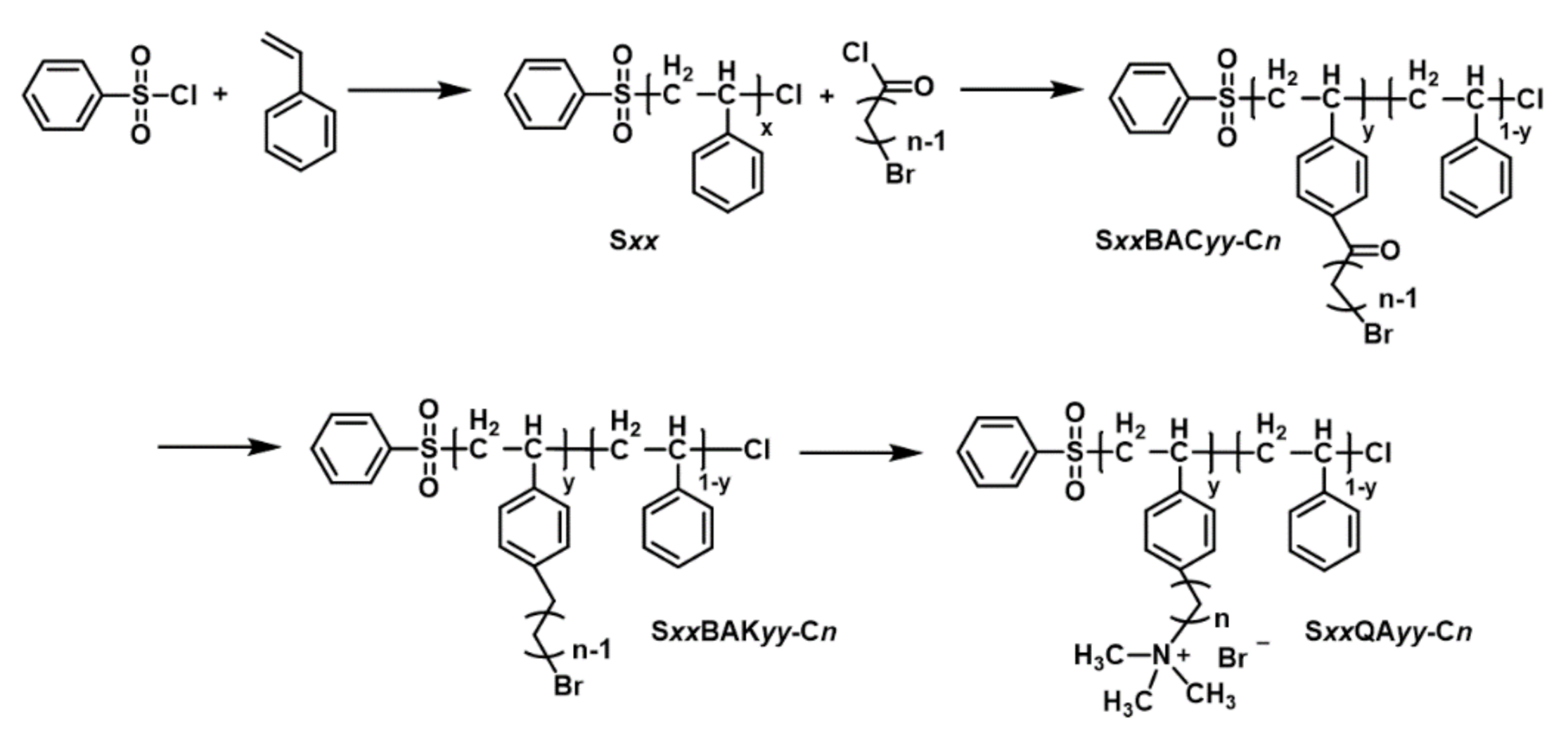
2.2. Synthesis of PS-Based Ionomers
2.2.1. Synthesis of the PS Backbone
2.2.2. Synthesis of Bromoacylated PS (SxxBACyy-Cn)
2.2.3. Synthesis of Bromoalkylated PS (SxxBAKyy-Cn)
2.2.4. Functionalization of Bromoalkylated PS (SxxQAyy-Cn)
2.3. Polymer Characterization
2.4. Ionomer Preparation
2.5. Analysis of Ionomer
2.6. MEA Fabrication
2.7. Single-Cell Performance Test
3. Results and Discussion
3.1. Polymer Synthesis and Structural Analysis
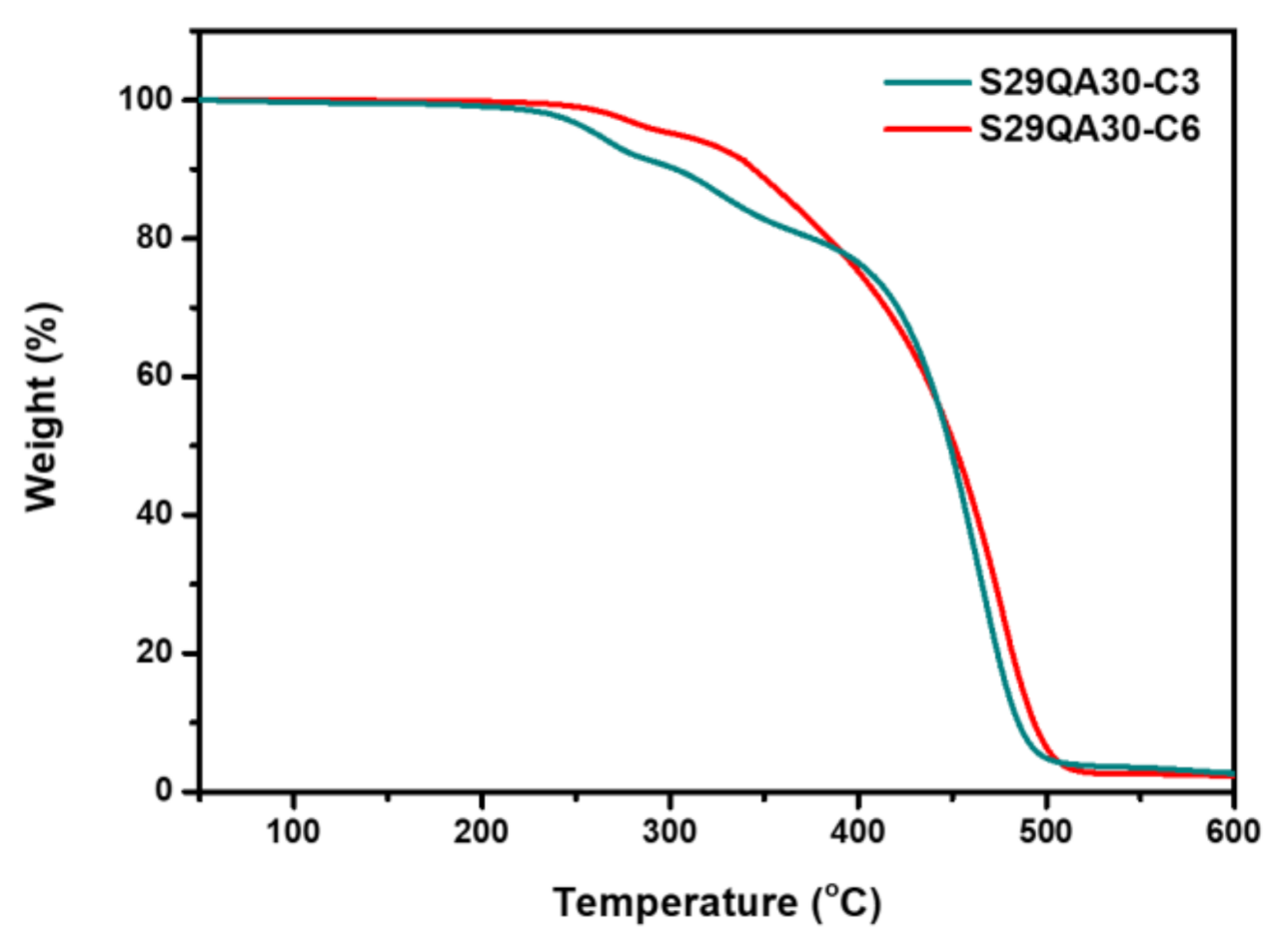
3.2. IEC and Thermal Decomposition Behavior
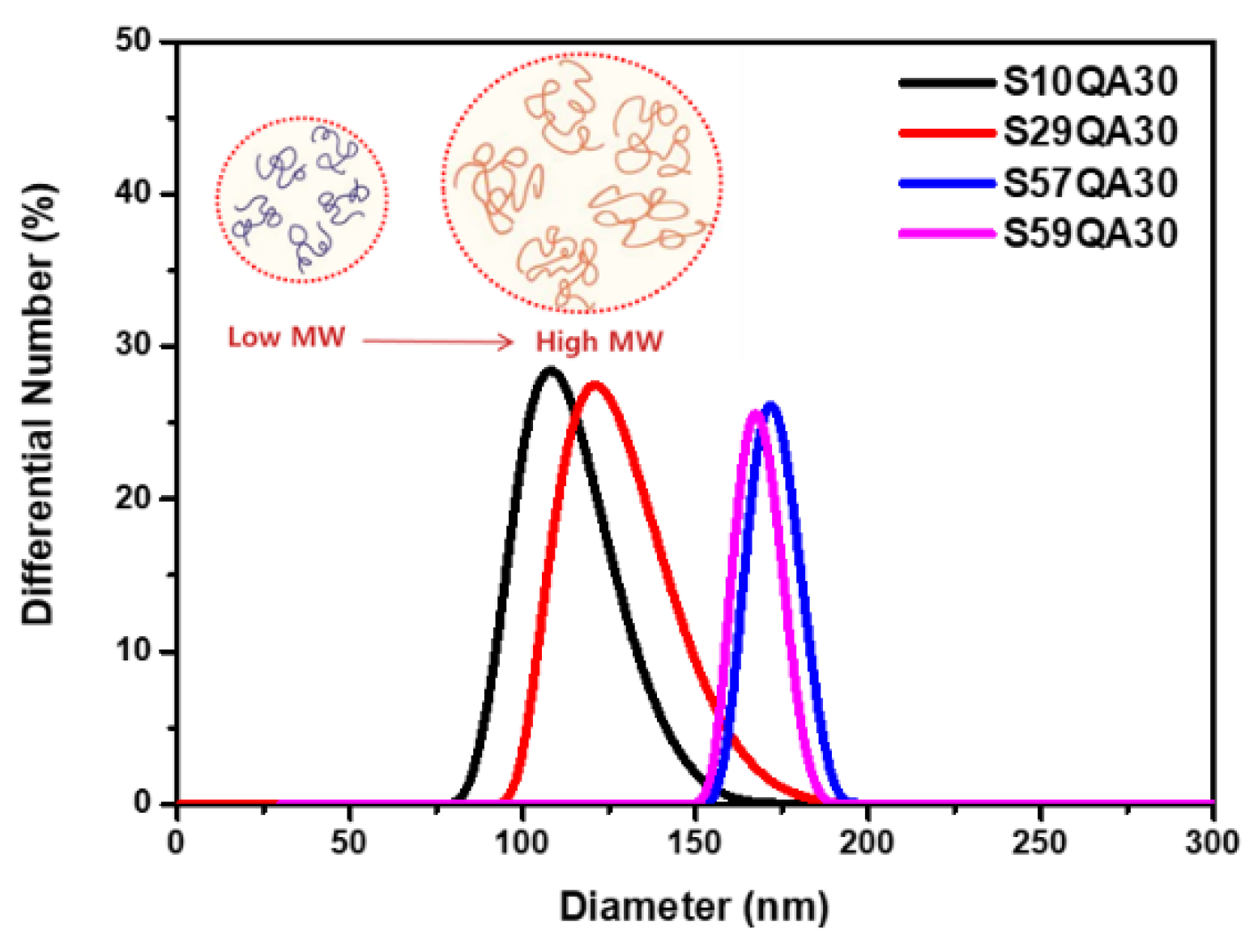
3.3. Morphology
3.4. Dispersion Property
3.5. AEMFC Performance
3.5.1. Effect of PS Backbone Length of the Sxxqa30-C6 Ionomer
3.5.2. Effect of Side Chain Length
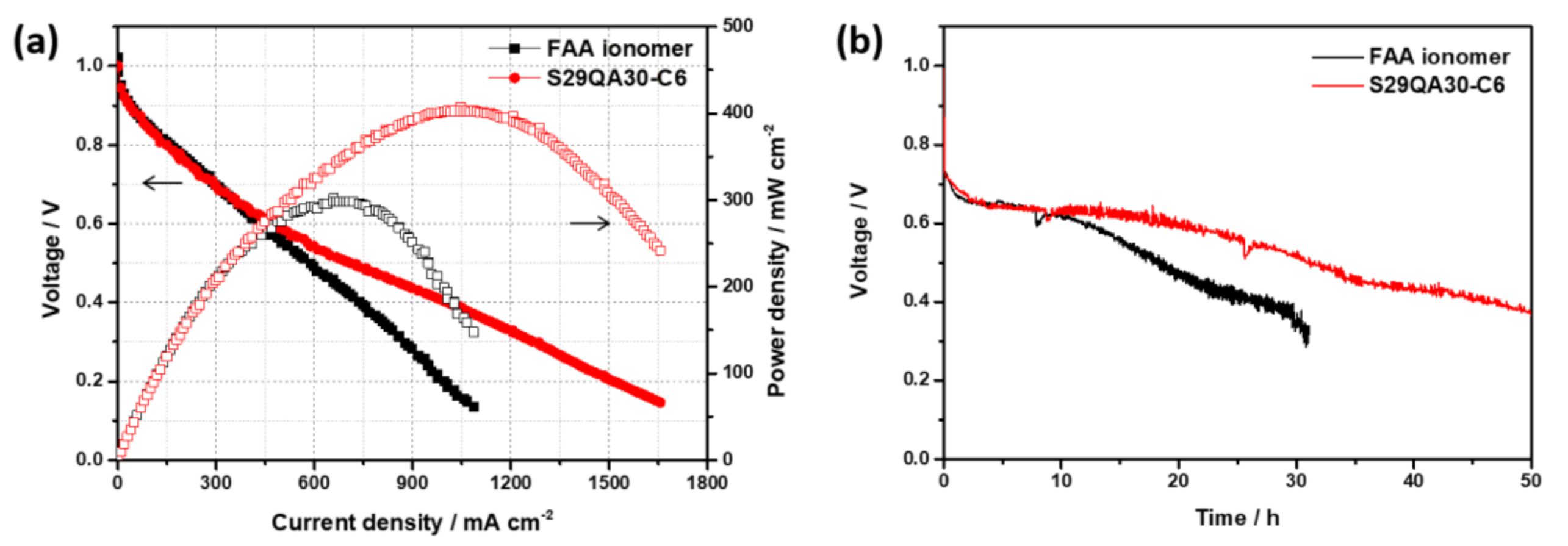
3.5.3. Short-Term Durability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Shen, P.K. Advances in the high performance polymer electrolyte membranes for fuel cells. Chem. Soc. Rev. 2012, 41, 2382–2394. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.; Zhang, X.; Dong, H.; Xue, Q. A comprehensive review of energy management optimization strategies for fuel cell passenger vehicle. Int. J. Hydrog. Energy 2020, 45, 20293–20303. [Google Scholar] [CrossRef]
- Liang, M.; Liu, Y.; Xiao, B.; Yang, S.; Wang, Z.; Han, H. An analytical model for the transverse permeability of gas diffusion layer with electrical double layer effects in proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2018, 43, 17880–17888. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent advances in anodes for microbial fuel cells: An overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rodríguez-Couto, S. Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): An overview. Biochem. Eng. J. 2020, 164, 107779. [Google Scholar] [CrossRef]
- Arges, C.G.; Zhang, L. Anion exchange membranes’ evolution toward high hydroxide ion conductivity and alkaline resiliency. ACS Appl. Energy Mater. 2018, 1, 2991–3012. [Google Scholar] [CrossRef]
- Firouzjaie, H.A.; Mustain, W.E. Catalytic advantages, challenges, and priorities in alkaline membrane fuel cells. ACS Catal. 2020, 10, 225–234. [Google Scholar] [CrossRef]
- Serov, A.; Zenyuk, I.V.; Arges, C.G.; Chatenet, M. Hot topics in alkaline exchange membrane fuel cells. J. Power Sources 2018, 375, 149–157. [Google Scholar] [CrossRef]
- Goenaga, G.A.; Roy, A.L.; Cantillo, N.M.; Foister, S.; Zawodzinski Jr., T.A. A family of platinum group metal-free catalysts for oxygen reduction in alkaline media. J. Power Sources 2018, 395, 148–157. [Google Scholar] [CrossRef]
- Hagesteijn, K.F.; Jiang, S.; Ladewig, B.P. A review of the synthesis and characterization of anion exchange membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef]
- You, W.; Noonan, K.J.; Coates, G.W. Alkaline-stable anion exchange membranes: A review of synthetic approaches. Prog. Polym. Sci. 2020, 100, 101177. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Nam, S.Y. Recent advancements in applications of alkaline anion exchange membranes for polymer electrolyte fuel cells. J. Ind. Eng. Chem. 2019, 70, 70–86. [Google Scholar] [CrossRef]
- Umar, K.; Yaqoob, A.A.; Ibrahim, M.; Parveen, T.; Safian, M. Environmental applications of smart polymer composites. Smart Polym. Nanocompos. Biomed. Environ. Appl. 2020, 15, 295–320. [Google Scholar]
- Lim, H.; Lee, B.; Yun, D.; Al Munsur, A.Z.; Chae, J.E.; Lee, S.Y.; Kim, H.-J.; Nam, S.Y.; Park, C.H.; Kim, T.-H. Poly (2,6-dimethyl-1,4-phenylene oxide) s with various head groups: Effect of head groups on the properties of anion exchange membranes. ACS Appl. Mater. Interfaces 2018, 10, 41279–41292. [Google Scholar] [CrossRef]
- Wang, Z.; Parrondo, J.; Ramani, V. Alkaline stability of poly (phenylene oxide) based anion exchange membranes containing imidazolium cations. J. Electrochem. Soc. 2016, 163, F824. [Google Scholar] [CrossRef]
- Biancolli, A.L.G.; Herranz, D.; Wang, L.; Stehlíková, G.; Bance-Soualhi, R.; Ponce-González, J.; Ocón, P.; Ticianelli, E.A.; Whelligan, D.K.; Varcoe, J.R. ETFE-based anion-exchange membrane ionomer powders for alkaline membrane fuel cells: A first performance comparison of head-group chemistry. J. Mater. Chem. A 2018, 6, 24330–24341. [Google Scholar] [CrossRef]
- Chu, X.; Shi, Y.; Liu, L.; Huang, Y.; Li, N. Piperidinium-functionalized anion exchange membranes and their application in alkaline fuel cells and water electrolysis. J. Mater. Chem. A 2019, 7, 7717–7727. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, W.; Shen, Y.; Liu, L.; Dai, S.; Li, N. N-cyclic quaternary ammonium-functionalized anion exchange membrane with improved alkaline stability enabled by aryl-ether free polymer backbones for alkaline fuel cells. J. Membr. Sci. 2019, 587, 117135. [Google Scholar] [CrossRef]
- Kang, D.H.; Das, G.; Yoon, H.H.; Kim, I.T. A composite anion conducting membrane based on quaternized cellulose and poly (phenylene oxide) for alkaline fuel cell applications. Polymers 2020, 12, 2676. [Google Scholar] [CrossRef]
- Choi, J.; Kim, M.-H.; Han, J.Y.; Chae, J.E.; Lee, W.H.; Lee, Y.M.; Lee, S.Y.; Jang, J.H.; Kim, J.Y.; Henkensmeier, D. Application of spirobiindane-based microporous poly (ether sulfone) s as polymeric binder on solid alkaline exchange membrane fuel cells. J. Membr. Sci. 2018, 568, 67–75. [Google Scholar] [CrossRef]
- Liang, X.; Shehzad, M.A.; Zhu, Y.; Wang, L.; Ge, X.; Zhang, J.; Yang, Z.; Wu, L.; Varcoe, J.R.; Xu, T. Ionomer cross-linking immobilization of catalyst nanoparticles for high performance alkaline membrane fuel cells. Chem. Mater. 2019, 31, 7812–7820. [Google Scholar] [CrossRef]
- Matanovic, I.; Chung, H.T.; Kim, Y.S. Benzene adsorption: A significant inhibitor for the hydrogen oxidation reaction in alkaline conditions. J. Phys. Chem. Lett. 2017, 8, 4918–4924. [Google Scholar] [CrossRef] [PubMed]
- Maurya, S.; Dumont, J.H.; Villarrubia, C.N.; Matanovic, I.; Li, D.; Kim, Y.S.; Noh, S.; Han, J.; Bae, C.; Miller, H.A. Surface adsorption affects the performance of alkaline anion-exchange membrane fuel cells. ACS Catal. 2018, 8, 9429–9439. [Google Scholar] [CrossRef]
- Maurya, S.; Fujimoto, C.H.; Hibbs, M.R.; Narvaez Villarrubia, C.; Kim, Y.S. Toward improved alkaline membrane fuel cell performance using quaternized aryl-ether free polyaromatics. Chem. Mater. 2018, 30, 2188–2192. [Google Scholar] [CrossRef]
- Hibbs, M.R. Alkaline stability of poly (phenylene)-based anion exchange membranes with various cations. J. Polym. Sci. Part B: Polym. Physics 2013, 51, 1736–1742. [Google Scholar] [CrossRef]
- Lin, C.X.; Wang, X.Q.; Hu, E.N.; Yang, Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Quaternized triblock polymer anion exchange membranes with enhanced alkaline stability. J. Membr. Sci. 2017, 541, 358–366. [Google Scholar]
- Son, T.Y.; Kim, T.-H.; Nam, S.Y. Crosslinked pore-filling anion exchange membrane using the cylindrical centrifugal force for anion exchange membrane fuel cell system. Polymers 2020, 12, 2758. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.X.; Wang, X.Q.; Li, L.; Liu, F.H.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Triblock copolymer anion exchange membranes bearing alkyl-tethered cycloaliphatic quaternary ammonium-head-groups for fuel cells. J. Power Sources 2017, 365, 282–292. [Google Scholar] [CrossRef]
- Wang, X.Q.; Lin, C.X.; Liu, F.H.; Li, L.; Yang, Q.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Alkali-stable partially fluorinated poly (arylene ether) anion exchange membranes with a claw-type head for fuel cells. J. Mater. Chem. A 2018, 6, 12455–12465. [Google Scholar] [CrossRef]
- Chu, X.; Liu, L.; Huang, Y.; Guiver, M.D.; Li, N. Practical implementation of bis-six-membered N-cyclic quaternary ammonium cations in advanced anion exchange membranes for fuel cells: Synthesis and durability. J. Membr. Sci. 2019, 578, 239–250. [Google Scholar] [CrossRef]
- Li, C.; Fu, Z.; Shi, Y. Investigation on chain transfer reaction of benzene sulfonyl chloride in styrene radical polymerization. Macromol. Res. 2009, 17, 557–562. [Google Scholar] [CrossRef]
- Lee, B.; Lim, H.; Chae, J.E.; Kim, H.-J.; Kim, T.-H. Physically-crosslinked anion exchange membranes by blending ionic additive into alkyl-substituted quaternized PPO. J. Membr. Sci. 2019, 574, 33–43. [Google Scholar] [CrossRef]
- Hird, B.; Eisenberg, A. P-carboxylation of linear high molecular-mass polystyrene. J. Polym. Sci. Part A: Polym. Chem. 1993, 31, 1377–1381. [Google Scholar] [CrossRef]
- Yan, J.; Hickner, M.A. Anion exchange membranes by bromination of benzylmethyl-containing poly (sulfone) s. Macromolecules 2010, 43, 2349–2356. [Google Scholar] [CrossRef]
- Fang, M.; Liu, D.; Neelakandan, S.; Xu, M.; Liu, D.; Wang, L. Side-chain effects on the properties of highly branched imidazolium-functionalized copolymer anion exchange membranes. Appl. Surf. Sci. 2019, 493, 1306–1316. [Google Scholar] [CrossRef]
- Chae, J.E.; Yoo, S.J.; Kim, J.Y.; Jang, J.H.; Lee, S.Y.; Song, K.H.; Kim, H.-J. Hydrocarbon-based electrode ionomer for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2020, 45, 32856–32864. [Google Scholar] [CrossRef]
- Gupta, G.; Scott, K.; Mamlouk, M. Soluble polystyrene-b-poly (ethylene/butylene)-b-polystyrene based ionomer for anion exchange membrane fuel cells operating at 70 °C. Fuel Cells 2018, 18, 137–147. [Google Scholar] [CrossRef]
- Omasta, T.; Wang, L.; Peng, X.; Lewis, C.; Varcoe, J.; Mustain, W.E. Importance of balancing membrane and electrode water in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 205–213. [Google Scholar] [CrossRef]
- Dekel, D.R.; Rasin, I.G.; Page, M.; Brandon, S. Steady state and transient simulation of anion exchange membrane fuel cells. J. Power Sources 2018, 375, 191–204. [Google Scholar] [CrossRef]
- Truong, V.M.; Duong, N.B.; Wang, C.-L.; Yang, H. Effects of cell temperature and reactant humidification on anion exchange membrane fuel cells. Materials 2019, 12, 2048. [Google Scholar] [CrossRef]
- Omasta, T.J.; Park, A.M.; LaManna, J.M.; Zhang, Y.; Peng, X.; Wang, L.; Jacobson, D.L.; Varcoe, J.R.; Hussey, D.S.; Pivovar, B.S. Beyond catalysis and membranes: Visualizing and solving the challenge of electrode water accumulation and flooding in AEMFCs. Energy Environ. Sci. 2018, 11, 551–558. [Google Scholar] [CrossRef]
- Omasta, T.J.; Peng, X.; Lewis, C.A.; Varcoe, J.; Mustain, W.E. Improving performance in alkaline membrane fuel cells through enhanced water management. ECS Trans. 2016, 75, 949. [Google Scholar] [CrossRef]
- Matanovic, I.; Maurya, S.; Park, E.J.; Jeon, J.Y.; Bae, C.; Kim, Y.S. Adsorption of polyaromatic backbone impacts the performance of anion exchange membrane fuel cells. Chem. Mater. 2019, 31, 4195–4204. [Google Scholar] [CrossRef]
- Park, H.J.; Chu, X.; Kim, S.P.; Choi, D.; Jung, J.W.; Woo, J.; Baek, S.Y.; Yoo, S.J.; Chung, Y.-C.; Seong, J.G. Effect of N-cyclic cationic groups in poly (phenylene oxide)-based catalyst ionomer membranes for anion exchange membrane fuel cells. J. Membr. Sci. 2020, 608, 118183. [Google Scholar] [CrossRef]
- Kim, Y.S.; Pivovar, B.S. The membrane—Electrode interface in PEFCs: IV. The origin and implications of interfacial resistance. J. Electrochem. Soc. 2010, 157, B1616. [Google Scholar] [CrossRef]



| Sample | Reaction Parameter for PS Backbone | Molecular Weight of PS Backbone | IEC e (mmol g−1) | ||||
|---|---|---|---|---|---|---|---|
| Ratio a | Reaction Time (h) | Yield (%) | Mn (g mol−1) b | Mn (g mol−1) c | PDI d | ||
| S10QA30-C6 | 65 | 24 | 22.6 | 10,576 | 9735 | 1.56 | – |
| S29QA30-C3 | 300 | 24 | 18.1 | – | 28,893 | 1.83 | 1.74 ± 0.06 |
| S29QA30-C6 | 300 | 24 | 18.1 | – | 28,893 | 1.83 | 1.47 ± 0.05 |
| S57QA30-C6 | 700 | 24 | 13.8 | – | 56,950 | 2.01 | 1.45 ± 0.08 |
| S59QA30-C6 | 700 | 24 f | 13.6 | – | 58,931 | 2.69 | 1.45 ± 0.05 |
| S63QA30-C6 | 1000 | 24 | 14.9 | – | 63,067 | 1.96 | 1.44 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, J.E.; Lee, S.Y.; Yoo, S.J.; Kim, J.Y.; Jang, J.H.; Park, H.-Y.; Park, H.S.; Seo, B.; Henkensmeier, D.; Song, K.H.; et al. Polystyrene-Based Hydroxide-Ion-Conducting Ionomer: Binder Characteristics and Performance in Anion-Exchange Membrane Fuel Cells. Polymers 2021, 13, 690. https://doi.org/10.3390/polym13050690
Chae JE, Lee SY, Yoo SJ, Kim JY, Jang JH, Park H-Y, Park HS, Seo B, Henkensmeier D, Song KH, et al. Polystyrene-Based Hydroxide-Ion-Conducting Ionomer: Binder Characteristics and Performance in Anion-Exchange Membrane Fuel Cells. Polymers. 2021; 13(5):690. https://doi.org/10.3390/polym13050690
Chicago/Turabian StyleChae, Ji Eon, So Young Lee, Sung Jong Yoo, Jin Young Kim, Jong Hyun Jang, Hee-Young Park, Hyun Seo Park, Bora Seo, Dirk Henkensmeier, Kwang Ho Song, and et al. 2021. "Polystyrene-Based Hydroxide-Ion-Conducting Ionomer: Binder Characteristics and Performance in Anion-Exchange Membrane Fuel Cells" Polymers 13, no. 5: 690. https://doi.org/10.3390/polym13050690
APA StyleChae, J. E., Lee, S. Y., Yoo, S. J., Kim, J. Y., Jang, J. H., Park, H.-Y., Park, H. S., Seo, B., Henkensmeier, D., Song, K. H., & Kim, H.-J. (2021). Polystyrene-Based Hydroxide-Ion-Conducting Ionomer: Binder Characteristics and Performance in Anion-Exchange Membrane Fuel Cells. Polymers, 13(5), 690. https://doi.org/10.3390/polym13050690