Bacterial Cellulose Network from Kombucha Fermentation Impregnated with Emulsion-Polymerized Poly(methyl methacrylate) to Form Nanocomposite
Abstract
1. Introduction
2. Materials and Methods
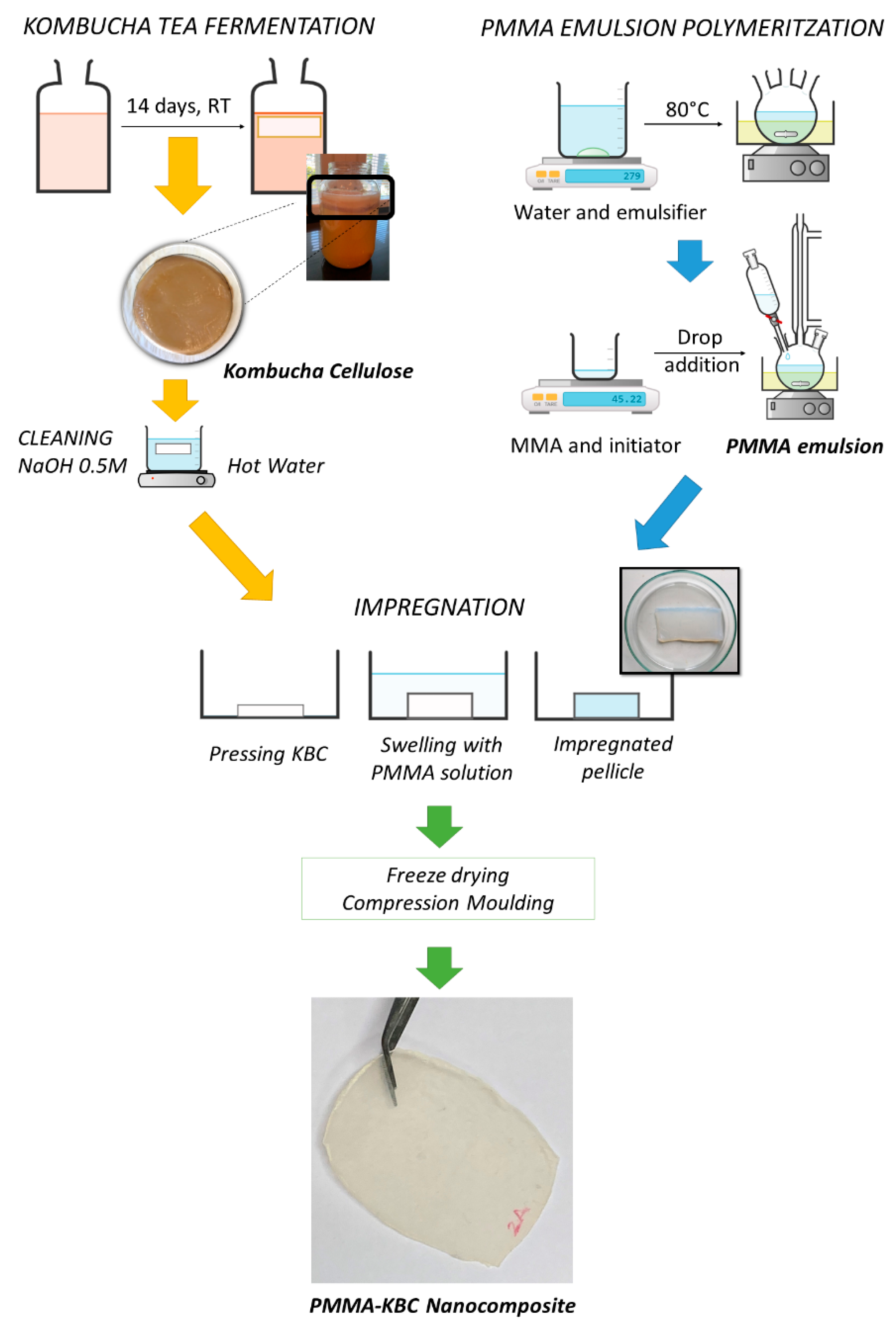
2.1. Bacterial Cellulose Production from Kombucha Tea
2.2. PMMA Emulsion Polymerisation
2.3. Preparation of PMMA-KBC Nanocomposites
2.4. Characterization Methods
3. Results
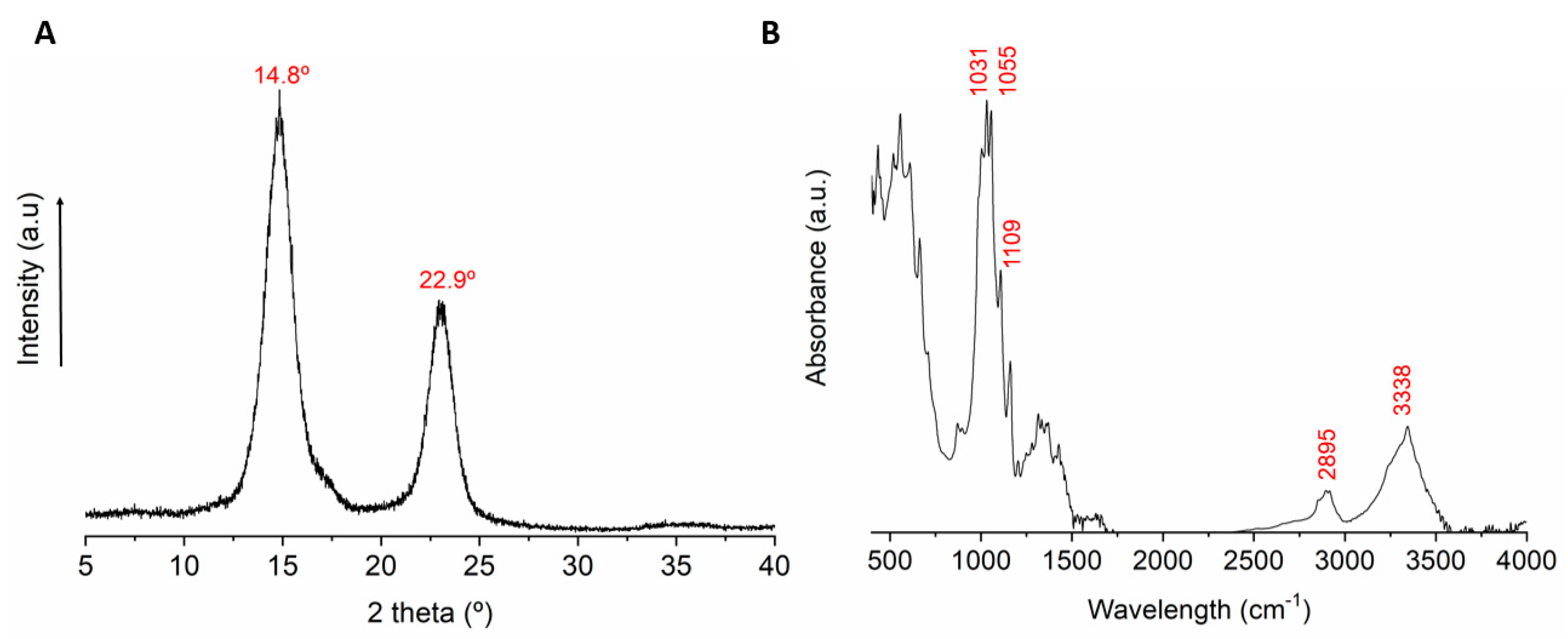
3.1. Structure and Properties of Kombucha Bacterial Cellulose
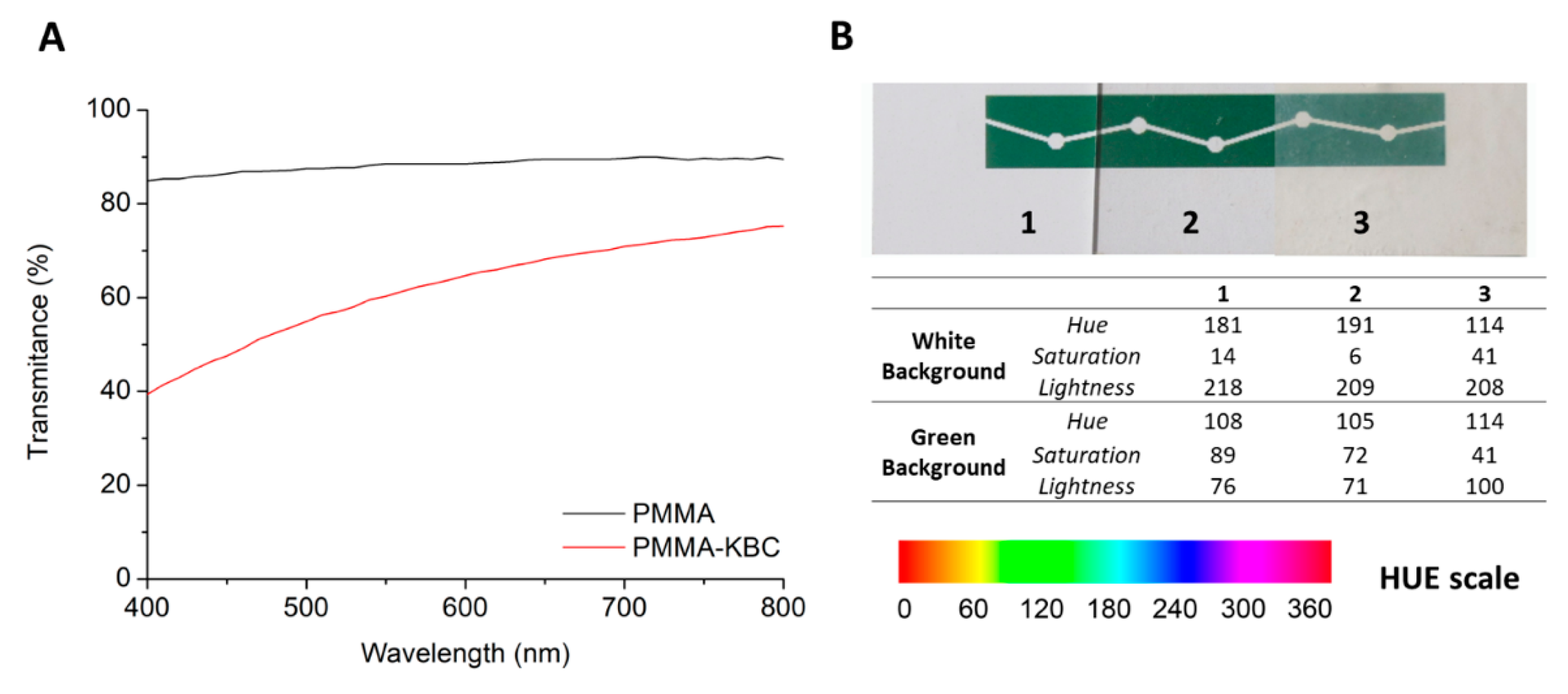
3.2. Optical Properties of PMMA and PMMA-KBC Nanocomposites
3.3. Mechanical Properties of the PMMA-KBC Nanocomposites
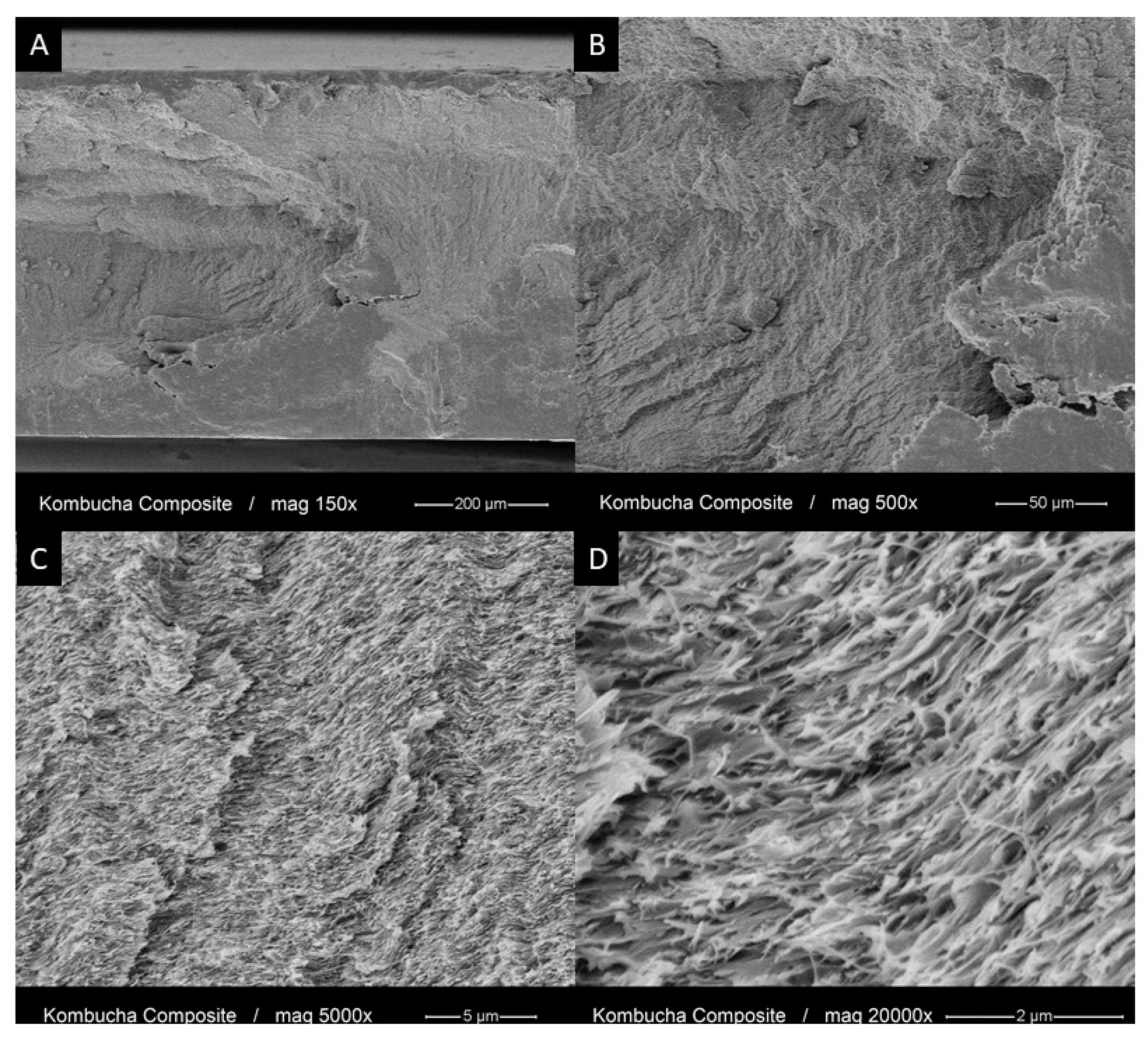
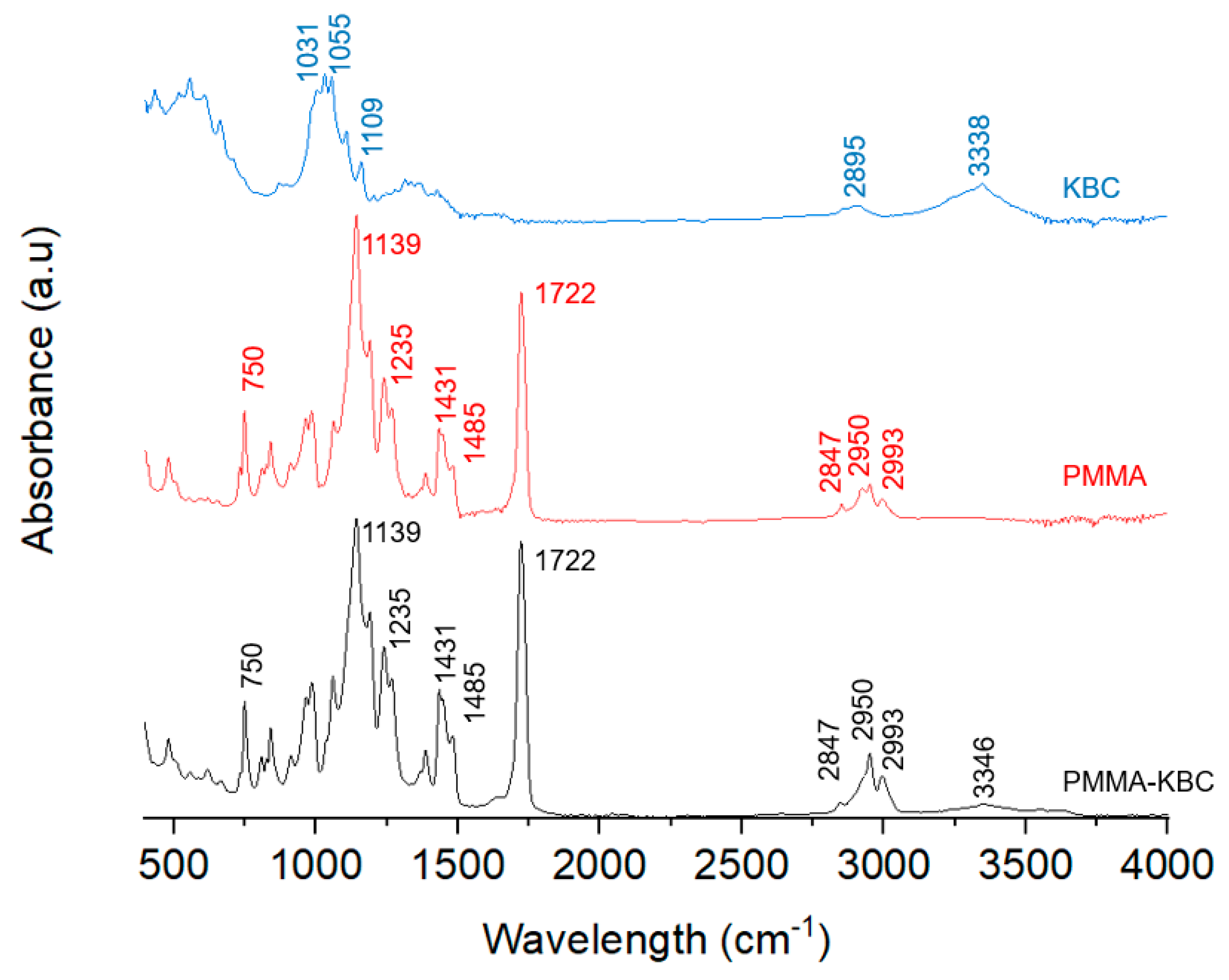
3.4. Morphology and Chemical Structure of the PMMA-KBC Nanocomposites
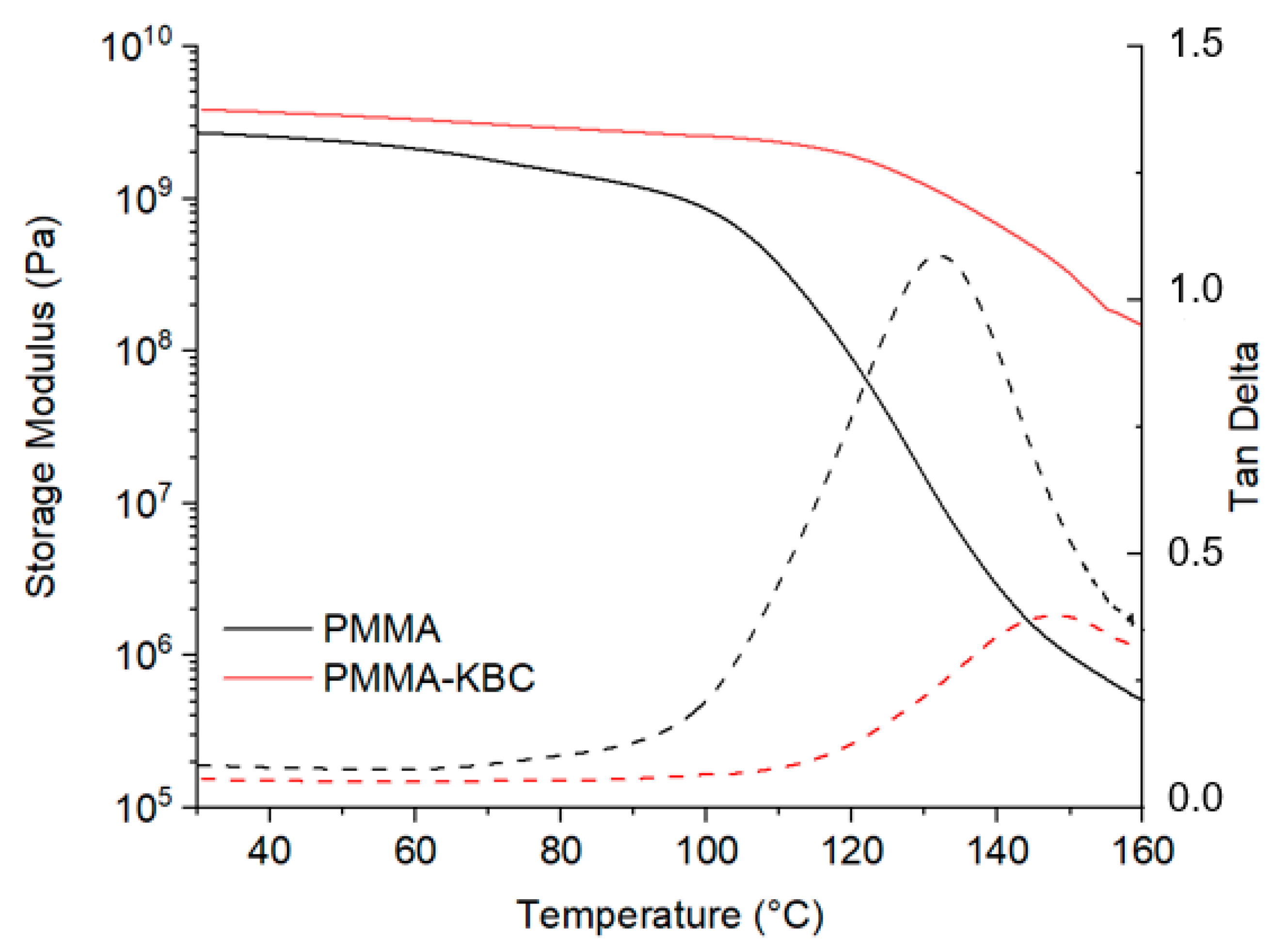
3.5. Dynamical Mechanical Thermal Properties of Nanocomposites
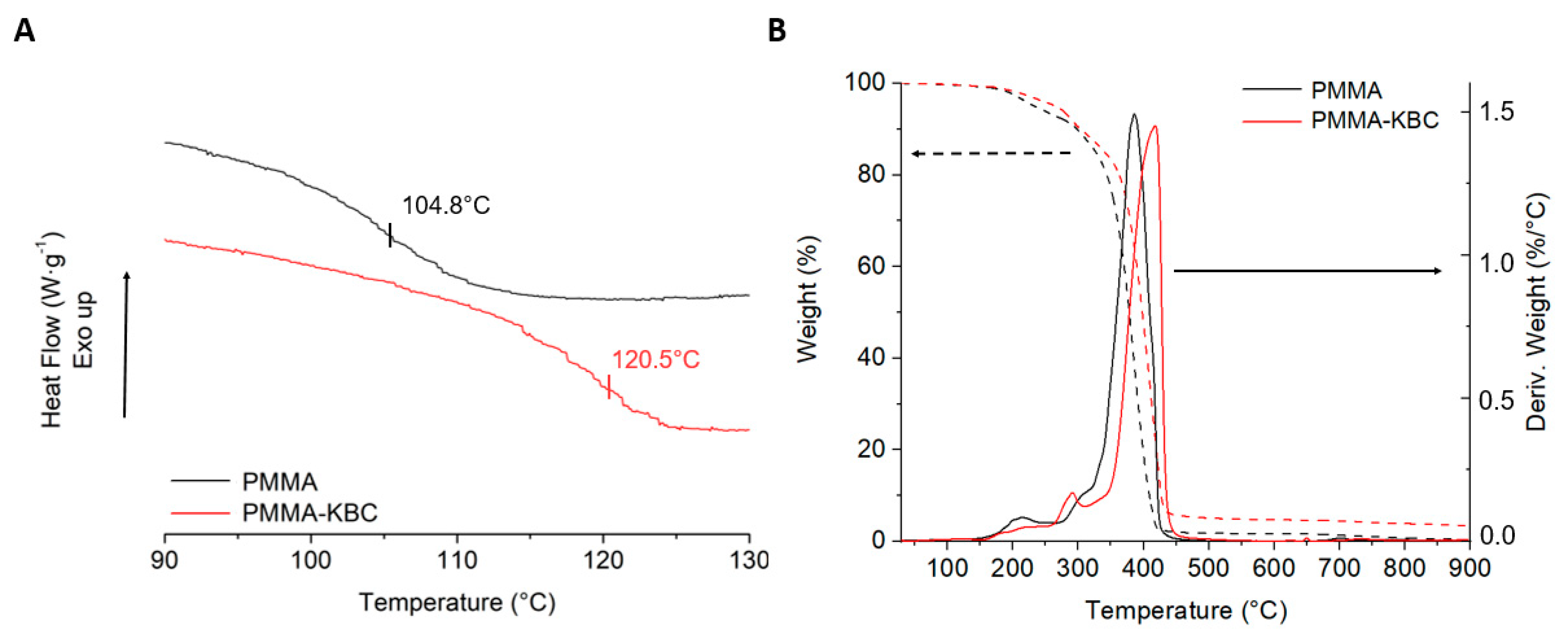
3.6. Thermal Properties of Nanocomposites
3.7. Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakagaito, A.N.; Iwamoto, S.; Yano, H. Bacterial cellulose: The ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl. Phys. A Mater. Sci. Process. 2005, 80, 93–97. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Wang, S.S.; Han, Y.H.; Chen, J.L.; Zhang, D.C.; Shi, X.X.; Ye, Y.X.; Chen, D.L.; Li, M. Insights into bacterial cellulose biosynthesis from different carbon sources and the associated biochemical transformation pathways in Komagataeibacter sp. W1. Polymers 2018, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, A.M.A.; Carrera, S.H.; Parra, R.; Keshavarz, T.; Iqbal, H.M.N. Bacterial cellulose: A sustainable source to develop value-added products—A review. BioResources 2016, 11, 5641–5655. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Azin, M.; Ashori, A. Production of bacterial cellulose using different carbon sources and culture media. Carbohydr. Polym. 2015, 117, 518–523. [Google Scholar] [CrossRef]
- Rajwade, J.M.; Paknikar, K.M.; Kumbhar, J.V. Applications of bacterial cellulose and its composites in biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef] [PubMed]
- Goh, W.N.; Rosma, A.; Kaur, B.; Fazilah, A.; Karim, A.A.; Bhat, R. Fermentation of black tea broth (kombucha): I. effects of sucrose concentration and fermentation time on the yield of microbial cellulose. Int. Food Res. J. 2012, 19, 109–117. [Google Scholar]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Mayser, P.; Fromme, S.; Leitzmann, G.; Gründer, K. The yeast spectrum of the ‘tea fungus Kombucha’. Mycoses 2009, 38, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Greenwalt, C.J.; Steinkraus, K.H.; Ledford, R.A. Kombucha, the Fermented Tea: Microbiology, Composition, and Claimed Health Effects. J. Food Prot. 2016, 63, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, C.; Farnworth, E. Tea, Kombucha, and health: A review. Food Res. Int. 2000, 33, 409–421. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Sederavičiūtė, F.; Bekampienė, P.; Domskienė, J. Effect of pretreatment procedure on properties of Kombucha fermented bacterial cellulose membrane. Polym. Test. 2019, 78, 105941. [Google Scholar] [CrossRef]
- Pircher, N.; Veigel, S.; Aigner, N.; Nedelec, J.M.; Rosenau, T.; Liebner, F. Reinforcement of bacterial cellulose aerogels with biocompatible polymers. Carbohydr. Polym. 2014, 111, 505–513. [Google Scholar] [CrossRef]
- Lacerda, P.S.S.; Barros-Timmons, A.M.M.V.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Nanostructured composites obtained by ATRP sleeving of bacterial cellulose nanofibers with acrylate polymers. Biomacromolecules 2013, 14, 2063–2073. [Google Scholar] [CrossRef]
- Huang, T.; Kuboyama, K.; Fukuzumi, H.; Ougizawa, T. PMMA/TEMPO-oxidized cellulose nanofiber nanocomposite with improved mechanical properties, high transparency and tunable birefringence. Cellulose 2018, 25, 2393–2403. [Google Scholar] [CrossRef]
- Fahma, F.; Hori, N.; Iwata, T.; Takemura, A. The morphology and properties of poly(methyl methacrylate)-cellulose nanocomposites prepared by immersion precipitation method. J. Appl. Polym. Sci. 2013, 128, 1563–1568. [Google Scholar] [CrossRef]
- Erbas Kiziltas, E.; Kiziltas, A.; Bollin, S.C.; Gardner, D.J. Preparation and characterization of transparent PMMA-cellulose-based nanocomposites. Carbohydr. Polym. 2015, 127, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Haque, M.M.U.; Oksman, K. Crosslinked poly(vinyl acetate) (PVAc) reinforced with cellulose nanocrystals (CNC): Structure and mechanical properties. Compos. Sci. Technol. 2016, 126, 35–42. [Google Scholar] [CrossRef]
- Geng, S.; Wei, J.; Aitomäki, Y.; Noël, M.; Oksman, K. Well-dispersed cellulose nanocrystals in hydrophobic polymers by: In situ polymerization for synthesizing highly reinforced bio-nanocomposites. Nanoscale 2018, 10, 11797–11807. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Aulin, C.; Netrval, J.; Wågberg, L.; Lindström, T. Aerogels from nanofibrillated cellulose with tunable oleophobicity. Soft Matter 2010, 6, 3298–3305. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef]
- Ali, Z.M.; Gibson, L.J. The structure and mechanics of nanofibrillar cellulose foams. Soft Matter 2013, 9, 1580–1588. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Yano, H.; Nogi, M.; Eichhorn, S.J. An estimation of the Young’s modulus of bacterial cellulose filaments. Cellulose 2008, 15, 507–513. [Google Scholar] [CrossRef]
- Tarrés, Q.; Boufi, S.; Mutjé, P.; Delgado-Aguilar, M. Enzymatically hydrolyzed and TEMPO-oxidized cellulose nanofibers for the production of nanopapers: Morphological, optical, thermal and mechanical properties. Cellulose 2017, 24, 3943–3954. [Google Scholar] [CrossRef]
- Zhu, C.; Li, F.; Zhou, X.; Lin, L.; Zhang, T. Kombucha-synthesized bacterial cellulose: Preparation, characterization, and biocompatibility evaluation. J. Biomed. Mater. Res. Part A 2014, 102, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Tomé, L.C.; Brandão, L.; Mendes, A.M.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Freire, C.S.R.; Marrucho, I.M. Preparation and characterization of bacterial cellulose membranes with tailored surface and barrier properties. Cellulose 2010, 17, 1203–1211. [Google Scholar] [CrossRef]
- Lee, K.Y.; Quero, F.; Blaker, J.J.; Hill, C.A.S.; Eichhorn, S.J.; Bismarck, A. Surface only modification of bacterial cellulose nanofibres with organic acids. Cellulose 2011, 18, 595–605. [Google Scholar] [CrossRef]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef] [PubMed]
- Dima, S.O.; Panaitescu, D.M.; Orban, C.; Ghiurea, M.; Doncea, S.M.; Fierascu, R.C.; Nistor, C.L.; Alexandrescu, E.; Nicolae, C.A.; Trica, B.; et al. Bacterial nanocellulose from side-streams of kombucha beverages production: Preparation and physical-chemical properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Garside, P.; Wyeth, P. Identification of Cellulosic Fibres by FTIR Spectroscopy. Stud. Conserv. 2003, 48, 269–275. [Google Scholar] [CrossRef]
- Nada, A.A.M.A.; Kamel, S.; El-Sakhawy, M. Thermal behaviour and infrared spectroscopy of cellulose carbamates. Polym. Degrad. Stab. 2000, 70, 347–355. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Yang, H.; Gong, M.; Hu, J.; Liu, B.; Chen, Y.; Xiao, J.; Li, S.; Dong, Z.; Chen, H. Cellulose Pyrolysis Mechanism Based on Functional Group Evolutions by Two-Dimensional Perturbation Correlation Infrared Spectroscopy. Energy Fuels 2020, 34, 3412–3421. [Google Scholar] [CrossRef]
- Gea, S.; Torres, F.G.; Troncoso, O.P.; Reynolds, C.T.; Vilasecca, F.; Iguchi, M.; Peijs, T. Biocomposites based on bacterial cellulose and apple and radish pulp. Int. Polym. Process. 2007, 22, 497–501. [Google Scholar] [CrossRef]
- Salem, S.; Oliver-Ortega, H.; Espinach, F.X.; Hamed, K.B.; Nasri, N.; Alcalà, M.; Mutjé, P. Study on the Tensile Strength and Micromechanical Analysis of Alfa Fibers Reinforced High Density Polyethylene Composites. Fibers Polym. 2019, 20, 602–610. [Google Scholar] [CrossRef]
- Oliver-Ortega, H.; Granda, L.A.; Espinach, F.X.; Mendez, J.A.; Julian, F.; Mutjé, P. Tensile properties and micromechanical analysis of stone groundwood from softwood reinforced bio-based polyamide11 composites. Compos. Sci. Technol. 2016, 132, 123–130. [Google Scholar] [CrossRef]
- Tarrés, Q.; Oliver-Ortega, H.; Alcalà, M.; Espinach, F.X.; Mutjé, P.; Delgado-Aguilar, M. Research on the Strengthening Advantages on Using Cellulose Nanofibers as Polyvinyl Alcohol Reinforcement. Polymers 2020, 12, 974. [Google Scholar] [CrossRef]
- Canché-Escamilla, G.; Rodríguez-Trujillo, G.; Herrera-Franco, P.J.; Mendizábal, E.; Puig, J.E. Preparation and characterization of henequen cellulose grafted with methyl methacrylate and its application in composites. J. Appl. Polym. Sci. 1997, 66, 339–346. [Google Scholar] [CrossRef]
- Sain, S.; Bose, M.; Ray, D.; Mukhopadhyay, A.; Sengupta, S.; Kar, T.; Ennis, C.J.; Rahman, P.K.S.M.; Misra, M. A comparative study of polymethylmethacrylate/cellulose nanocomposites prepared by in situ polymerization and ex situ dispersion techniques. J. Reinf. Plast. Compos. 2013, 32, 147–159. [Google Scholar] [CrossRef]
- Mangipudi, K.R.; Onck, P.R. Tensile failure of two-dimensional quasi-brittle foams. Int. J. Solids Struct. 2012, 49, 2823–2829. [Google Scholar] [CrossRef][Green Version]
- Dong, H.; Strawhecker, K.E.; Snyder, J.F.; Orlicki, J.A.; Reiner, R.S.; Rudie, A.W. Cellulose nanocrystals as a reinforcing material for electrospun poly(methyl methacrylate) fibers: Formation, properties and nanomechanical characterization. Carbohydr. Polym. 2012, 87, 2488–2495. [Google Scholar] [CrossRef]
- Anžlovar, A.; Huskić, M.; Žagar, E. Modification of nanocrystalline cellulose for application as a reinforcing nanofiller in PMMA composites. Cellulose 2016, 23, 505–518. [Google Scholar] [CrossRef]
- Jones, R.N.; Ramsay, D.A.; Keir, D.S.; Dobriner, K. The Intensities of Carbonyl Bands in the Infrared Spectra of Steroids 1. J. Am. Chem. Soc. 1952, 74, 80–88. [Google Scholar] [CrossRef]
- Wang, W.; Liang, T.; Zhang, B.; Bai, H.; Ma, P.; Dong, W. Green functionalization of cellulose nanocrystals for application in reinforced poly(methyl methacrylate) nanocomposites. Carbohydr. Polym. 2018, 202, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The effect of polymerisation conditions on the kinetics and mechanisms of thermal degradation of PMMA. Polym. Degrad. Stab. 2002, 77, 435–439. [Google Scholar] [CrossRef]
- Ferriol, M.; Gentilhomme, A.; Cochez, M.; Oget, N.; Mieloszynski, J.L. Thermal degradation of poly(methyl methacrylate) (PMMA): Modelling of DTG and TG curves. Polym. Degrad. Stab. 2003, 79, 271–281. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Brown, J.E.; Inaba, A.; Hatada, K.; Kitayama, T.; Masuda, E. Effects of Weak Linkages on the Thermal and Oxidative Degradation of Poly(methyl methacrylates). Macromolecules 1986, 19, 2160–2168. [Google Scholar] [CrossRef]
- Oliver-Ortega, H.; Méndez, J.A.; Mutjé, P.; Tarrés, Q.; Espinach, F.X.; Ardanuy, M. Evaluation of Thermal and Thermomechanical Behaviour of Bio-Based Polyamide 11 Based Composites Reinforced with Lignocellulosic Fibres. Polymers (Basel) 2017, 9, 522. [Google Scholar] [CrossRef] [PubMed]
- Ardanuy, M.; Antunes, M.; Velasco, J.I. Vegetable fibres from agricultural residues as thermo-mechanical reinforcement in recycled polypropylene-based green foams. Waste Manag. 2012, 32, 256–263. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Nicolae, C. Micro- and nano-mechanical characterization of polyamide 11 and its composites containing cellulose nanofibers. Eur. Polym. J. 2013, 49, 3857–3866. [Google Scholar] [CrossRef]



| Property | KBC Aerogel | KBC Nanofiber Network |
|---|---|---|
| Density (g·cm−3) | 0.014 ± 0.002 | 0.807 ± 0.146 |
| Porosity (%) | 99.0 ± 0.2 | 44.5 ± 8.5 |
| Strength | * 227 ± 63 kPa | ** 172 ± 24 MPa |
| Stiffness | * 153 ± 22 * kPa | ** 8.0 ± 1.9 GPa |
| Sample | Young’s Modulus (GPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| PMMA | 2.1 ± 0.1 | 63.4 ± 1.2 | 6.4 ± 0.7 |
| PMMA-KBC | 4.0 ± 0.2 | 55.1 ± 0.7 | 1.4 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliver-Ortega, H.; Geng, S.; Espinach, F.X.; Oksman, K.; Vilaseca, F. Bacterial Cellulose Network from Kombucha Fermentation Impregnated with Emulsion-Polymerized Poly(methyl methacrylate) to Form Nanocomposite. Polymers 2021, 13, 664. https://doi.org/10.3390/polym13040664
Oliver-Ortega H, Geng S, Espinach FX, Oksman K, Vilaseca F. Bacterial Cellulose Network from Kombucha Fermentation Impregnated with Emulsion-Polymerized Poly(methyl methacrylate) to Form Nanocomposite. Polymers. 2021; 13(4):664. https://doi.org/10.3390/polym13040664
Chicago/Turabian StyleOliver-Ortega, Helena, Shiyu Geng, Francesc Xavier Espinach, Kristiina Oksman, and Fabiola Vilaseca. 2021. "Bacterial Cellulose Network from Kombucha Fermentation Impregnated with Emulsion-Polymerized Poly(methyl methacrylate) to Form Nanocomposite" Polymers 13, no. 4: 664. https://doi.org/10.3390/polym13040664
APA StyleOliver-Ortega, H., Geng, S., Espinach, F. X., Oksman, K., & Vilaseca, F. (2021). Bacterial Cellulose Network from Kombucha Fermentation Impregnated with Emulsion-Polymerized Poly(methyl methacrylate) to Form Nanocomposite. Polymers, 13(4), 664. https://doi.org/10.3390/polym13040664











