Thermal Stability and Flammability of Epoxy Composites Filled with Multi-Walled Carbon Nanotubes, Boric Acid, and Sodium Bicarbonate
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. TG Results
3.2. Hardness
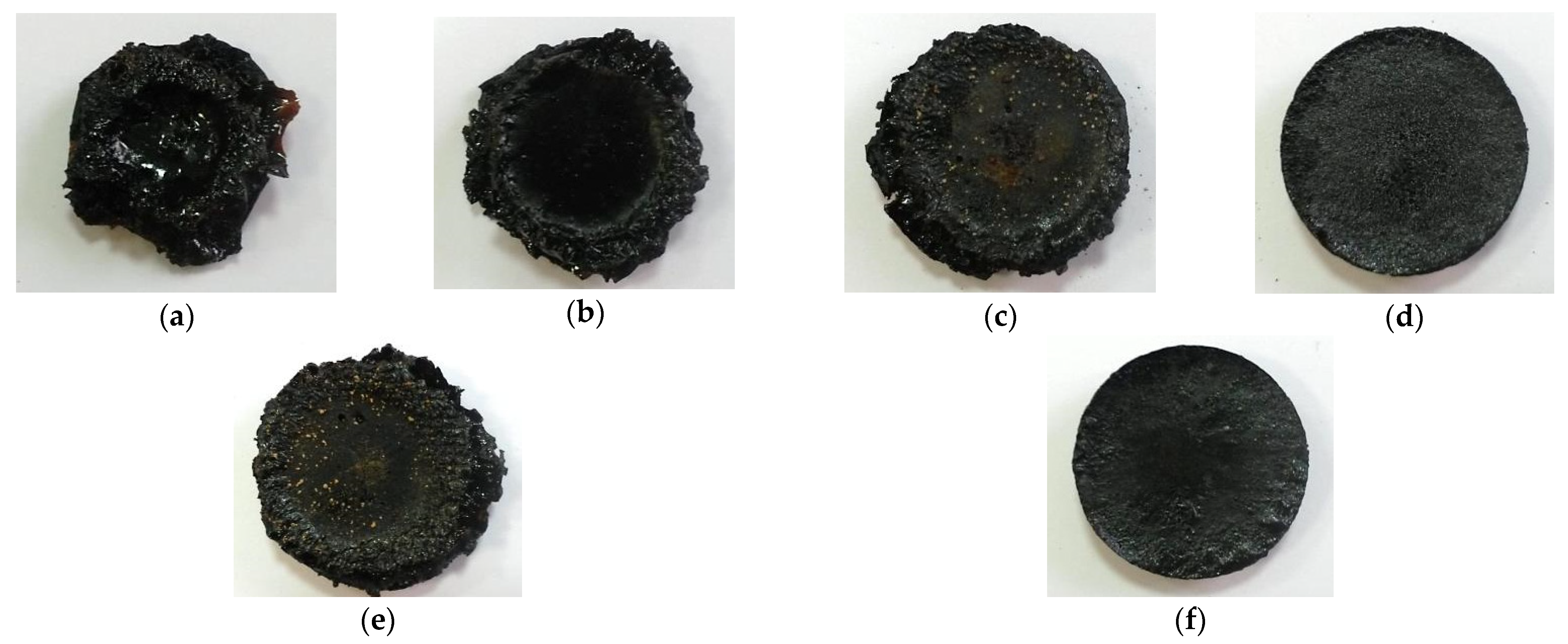
3.3. Flammability Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.; Neville, K. Handbook of Epoxy Resins; McGraw-Hill: New York, NY, USA, 1967. [Google Scholar]
- Mohan, P. A Critical Review: The Modification, Properties, and Applications of Epoxy Resins. Polym.-Plast. Technol. Eng. 2013, 52, 107–125. [Google Scholar] [CrossRef]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Chairat, A.; Joulia, X.; Floquet, P.; Vergnes, H.; Ablitzer, C.; Fiquet, O.; Brothier, M. Thermal degradation kinetics of a commercial epoxy resin—Comparative analysis of parameter estimation methods. J. Appl. Polym. Sci. 2015, 132, 6–9. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Flame retardancy of thermoplastic polyester—A review of the recent literature. Polym. Int. 2005, 54, 11–35. [Google Scholar] [CrossRef]
- Schmidt, C.; Ciesielski, M.; Greiner, L.; Döring, M. Novel organophosphorus flame retardants and their synergistic application in novolac epoxy resin. Polym. Degrad. Stab. 2018, 158, 190–201. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Kausar, A.; Rafique, I.; Muhammad, B. Review of Applications of Polymer/Carbon Nanotubes and Epoxy/CNT Composites. Polym.-Plast. Technol. Eng. 2016, 55, 1167–1191. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Wang, X. Synergistic effects of organically modified montmorillonite on the flame-retardant and smoke suppression properties of transparent intumescent fire-retardant coatings. Prog. Org. Coat. 2018, 122, 107–118. [Google Scholar] [CrossRef]
- Bannov, A.G.; Nazarenko, O.B.; Maksimovskii, E.A.; Popov, M.V.; Berdyugina, I. Thermal behavior and flammability of epoxy composites based on multi-walled carbon nanotubes and expanded graphite: A comparative study. Appl. Sci. 2020, 10, 6928. [Google Scholar] [CrossRef]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R. Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Arao, Y. Flame retardancy of polymer nanocomposite. In Flame Retardants. Engineering Materials; Visakh, P., Arao, Y., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Kononova, S.V.; Gubanova, G.N.; Korytkova, E.N.; Sapegin, D.A.; Setnickova, K.; Petrychkovych, R.; Uchytil, P. Polymer Nanocomposite Membranes. Appl. Sci. 2018, 8, 1181. [Google Scholar] [CrossRef]
- Toldy, A.; Szebényi, G.; Molnár, K.; Tóth, L.F.; Magyar, B.; Hliva, V.; Czigány, T.; Szolnoki, B. The effect of multilevel carbon reinforcements on the fire performance, conductivity, and mechanical properties of epoxy composites. Polymers (Basel) 2019, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, M.; Dodson, E.A.; Datta, S. Effect of graphene on the properties of flax fabric reinforced epoxy composites. Adv. Compos. Mater. 2020, 29, 443–458. [Google Scholar] [CrossRef]
- Feng, Y.; He, C.; Wen, Y.; Ye, Y.; Zhou, X.; Xie, X.; Mai, Y.W. Improving thermal and flame retardant properties of epoxy resin by functionalized graphene containing phosphorous, nitrogen and silicon elements. Compos. Part A Appl. Sci. Manuf. 2017, 103, 74–83. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, H.; Li, M.; Chen, T.; Xu, Y.; Yuan, C.; Zeng, B.; Dai, L. Effect of functionalized graphene oxide with phosphaphenanthrene and isocyanurate on flammability, mechanical properties, and thermal stability of epoxy composites. J. Appl. Polym. Sci. 2020, 137, 1–11. [Google Scholar] [CrossRef]
- Ganguli, S.; Aglan, H.; Dennig, P.; Irvin, G. Effect of loading and surface modification of MWCNTs on the fracture behavior of epoxy nanocomposites. J. Reinf. Plast. Compos. 2006, 25, 175–188. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, J.T.; Kim, H.C.; Rhee, K.Y.; Kathi, J. Thermal and mechanical properties of epoxy/carbon fiber composites reinforced with multi-walled carbon nanotubes. J. Macromol. Sci. Part B Phys. 2012, 51, 358–367. [Google Scholar] [CrossRef]
- Jin, F.-L.; Park, S.-J. Recent Advances in Carbon-Nanotube-Based Epoxy Composites. Carbon Lett. 2013, 14, 1–13. [Google Scholar] [CrossRef]
- Beyer, G. Short communication: Carbon nanotubes as flame retardants for polymers. Fire Mater. 2002, 26, 291–293. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Du, F.; Douglas, J.F.; Winey, K.I.; Harris, R.H.; Shields, J.R. Nanoparticle networks reduce the flammability of polymer nanocomposites. Nat. Mater. 2005, 4, 928–933. [Google Scholar] [CrossRef]
- Cipiriano, B.H.; Kashiwagi, T.; Raghavan, S.R.; Yang, Y.; Grulke, E.A.; Yamamoto, K.; Shields, J.R.; Douglas, J.F. Effects of aspect ratio of MWNT on the flammability properties of polymer nanocomposites. Polymer (Guildf) 2007, 48, 6086–6096. [Google Scholar] [CrossRef]
- Schartel, B.; Braun, U.; Knoll, U.; Bartholmai, M.; Goering, H.; Neubert, D.; Pötschke, P. Mechanical, thermal, and fire behavior of bisphenol a polycarbonate/multiwall carbon nanotube nanocomposites. Polym. Eng. Sci. 2007, 48, 149–158. [Google Scholar] [CrossRef]
- Berdyugina, I.S.; Steksova, Y.P.; Shibaev, A.A.; Maksimovskii, E.A.; Bannov, A.G. Thermal degradation of epoxy composites based on thermally expanded graphite and multiwalled carbon nanotubes. Russ. J. Appl. Chem. 2016, 89, 1447–1453. [Google Scholar] [CrossRef]
- Sonnier, R.; Bokobza, L.; Concha-Lozano, N. Influence of multiwall carbon nanotube (MWCNT) dispersion on ignition of poly(dimethylsiloxane)-MWCNT composites. Polym. Adv. Technol. 2015, 26, 277–286. [Google Scholar] [CrossRef]
- Jen, Y.M.; Huang, J.C. Synergistic effect on the thermomechanical and electrical properties of epoxy composites with the enhancement of carbon nanotubes and graphene nano platelets. Materials (Basel) 2019, 12, 255. [Google Scholar] [CrossRef]
- Singh, A.K.; Parhi, A.; Panda, B.P.; Mohanty, S.; Nayak, S.K.; Gupta, M.K. Aligned multi-walled carbon nanotubes (MWCNT) and vapor grown carbon fibers (VGCF) reinforced epoxy adhesive for thermal conductivity applications. J. Mater. Sci. Mater. Electron. 2017, 28, 17655–17674. [Google Scholar] [CrossRef]
- Ma, P.C.; Kim, J.K.; Tang, B.Z. Effects of silane functionalization on the properties of carbon nanotube/epoxy nanocomposites. Compos. Sci. Technol. 2007, 67, 2965–2972. [Google Scholar] [CrossRef]
- Wladyka-Przybylak, M.; Wesolek, D.; Gieparda, W.; Boczkowska, A.; Ciecierska, E. Functionalization effect on physico-mechanical properties of multi-walled carbon nanotubes/epoxy composites. Polym. Adv. Technol. 2011, 22, 48–59. [Google Scholar] [CrossRef]
- Wang, X.; Kalali, E.N.; Wan, J.T.; Wang, D.Y. Carbon-family materials for flame retardant polymeric materials. Prog. Polym. Sci. 2017, 69, 22–46. [Google Scholar] [CrossRef]
- Kuan, C.F.; Chen, W.J.; Li, Y.L.; Chen, C.H.; Kuan, H.C.; Chiang, C.L. Flame retardance and thermal stability of carbon nanotube epoxy composite prepared from sol-gel method. J. Phys. Chem. Solids 2010, 71, 539–543. [Google Scholar] [CrossRef]
- Yang, K.; Gu, M. The effects of triethylenetetramine grafting of multi-walled carbon nanotubes on its dispersion, filler-matrix interfacial interaction and the thermal properties of epoxy nanocomposites. Polym. Eng. Sci. 2009, 49, 2158–2167. [Google Scholar] [CrossRef]
- Ruiz, J.A.R.; Vincent, M.; Agassant, J.-F.; Sadik, T.; Pillon, C.; Carrot, C. Polymer foaming with chemical blowing agents: Experiment and modeling. Polym. Eng. Sci. 2015, 55, 2018–2029. [Google Scholar] [CrossRef]
- Mazzon, E.; Habas-Ulloa, A.; Habas, J.P. Lightweight rigid foams from highly reactive epoxy resins derived from vegetable oil for automotive applications. Eur. Polym. J. 2015, 68, 546–557. [Google Scholar] [CrossRef]
- Girish, S.; Devendra, K.; Bharath, K.N. Effect of Sodium bicarbonate on Fire behaviour of tilled E- Glass Reinforced Epoxy Composites. IOP Conf. Ser. Mater. Sci. Eng. 2016, 149, 012120. [Google Scholar] [CrossRef]
- Shen, K.K.; Kochesfahani, S.; Jouffret, F. Boron based flame retardants and flame retardancy. In Fire Retardancy of Polymeric Materials; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Awoyemi, L.; Westermark, U. Effects of borate impregnation on the response of wood strength to heat treatment. Wood Sci. Technol. 2005, 39, 484–491. [Google Scholar] [CrossRef]
- Huber, C.; Setoodeh Jahromy, S.; Jordan, C.; Schreiner, M.; Harasek, M.; Werner, A.; Winter, F. Boric Acid: A High Potential Candidate for Thermochemical Energy Storage. Energies 2019, 12, 1086. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, S.; Xu, J.; Chen, Z. Kinetic Study of Boron Oxide Prepared by Dehydration of Boric Acid. Asian J. Chem. 2015, 27, 1001–1004. [Google Scholar] [CrossRef]
- Nazarenko, O.B.; Melnikova, T.V.; Visakh, P.M. Combined effect of zeolite and boric acid on thermal behavior of epoxy composites. J. Therm. Anal. Calorim. 2017, 128, 169–175. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Zhao, L.; Li, W.; Zhang, H.; Yu, X.; Zhang, Z. High performance shape memory epoxy/carbon nanotube nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 311–320. [Google Scholar] [CrossRef]
- Visakh, P.M.; Nazarenko, O.B.; Amelkovich, Y.A.; Melnikova, T.V. Effect of zeolite and boric acid on epoxy-based composites. Polym. Adv. Technol. 2016, 27, 1098–1101. [Google Scholar] [CrossRef]
- Arshad, M.A.; Maaroufi, A.; Benavente, R.; Pinto, G. Thermal Degradation Mechanisms of Epoxy Composites Filled With Tin Particles. Polym. Compos. 2015, 38, 1529–1540. [Google Scholar] [CrossRef]
- Zhou, Y.; Pervin, F.; Lewis, L.; Jeelani, S. Experimental study on the thermal and mechanical properties of multi-walled carbon nanotube-reinforced epoxy. Mater. Sci. Eng. A 2007, 452–453, 657–664. [Google Scholar] [CrossRef]
- Rahman, M.M.; Zainuddin, S.; Hosur, M.V.; Robertson, C.J.; Kumar, A.; Trovillion, J.; Jeelani, S. Effect of NH2-MWCNTs on crosslink density of epoxy matrix and ILSS properties of e-glass/epoxy composites. Compos. Struct. 2013, 95, 213–221. [Google Scholar] [CrossRef]
- Rubab, Z.; Afzal, A.; Siddiqi, H.M.; Saeed, S. Preparation, characterization, and enhanced thermal and mechanical properties of epoxy-titania composites. Sci. World J. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Burganov, R.R.; Mochalova, E.N.; Galikhanov, M.F.; Bannov, A.G.; Shibaev, A.A. Electret materials based on an epoxy oligomer and multi-walled carbon nanotubes (MWNT-1020). Mendeleev Commun. 2017, 27, 38–40. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Sugimoto, K.-I.; Song, S.-M.; Gotoh, Y.; Ohkoshi, Y.; Endo, M. Mechanical and physical properties of epoxy composites reinforced by vapor grown carbon nanofibers. Carbon 2005, 43, 2199–2208. [Google Scholar] [CrossRef]
- Gojny, F.H.; Schulte, K. Functionalisation effect on the thermo-mechanical behaviour of multi-wall carbon nanotube/epoxy-composites. Compos. Sci. Technol. 2004, 64, 2303–2308. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Bian, C.; Zhong, Y.; Jing, X. The thermal stability and pyrolysis mechanism of boron-containing phenolic resins: The effect of phenyl borates on the char formation. Appl. Surf. Sci. 2015, 331, 519–529. [Google Scholar] [CrossRef]
- Balci, S.; Sezgi, N.A.; Eren, E. Boron oxide production kinetics using boric acid as raw material. Ind. Eng. Chem. Res. 2012, 51, 11091–11096. [Google Scholar] [CrossRef]




| No. | Sample | Epoxy Resin | H3BO3 | Na2CO3 | MWCNTs |
|---|---|---|---|---|---|
| 1 | E0 | 100 | 0 | 0 | 0 |
| 2 | E/0.5MWCNT | 100 | 0 | 0 | 0.5 |
| 3 | E/10B | 100 | 10 | 0 | 0 |
| 4 | E/15B | 100 | 15 | 0 | 0 |
| 5 | E/10S | 100 | 0 | 10 | 0 |
| 6 | E/15S | 100 | 0 | 15 | 0 |
| 7 | E/10B/0.5MWCNT | 100 | 10 | 0 | 0.5 |
| 8 | E/15B/0.5MWCNT | 100 | 15 | 0 | 0.5 |
| 9 | E/10S/0.5MWCNT | 100 | 0 | 10 | 0.5 |
| 10 | E/15S/0.5MWCNT | 100 | 0 | 15 | 0.5 |
| Sample | T5 (°C) | T50 (°C) | T90 (°C) | Residue at 690 °C (%) | Tmax (°C) | Tg (C) |
|---|---|---|---|---|---|---|
| E0 | 314.9 | 397.1 | 627.8 | 0.2 | 361.2 | 126.1 |
| E/0.5MWCNT | 291.1 | 398.5 | 608.7 | 0.0 | 358.3 | 139.8 |
| E/10B | 229.3 | 412.6 | – | 18.5 | 355.0 | 174.6 |
| E/15B | 266.1 | 418.8 | – | 23.1 | 354.7 | 160.2 |
| E/10S | 279.2 | 381.8 | 640.3 | 5.6 | 366.9 | 174.5 |
| E/15S | 181.9 | 378.2 | 636.1 | 7.5 | 360.1 | 170.4 |
| E/10B/0.5MWCNT | 222.4 | 416.1 | – | 13.4 | 357.1 | 169.5 |
| E/15B/0.5MWCNT | 272.7 | 432.8 | – | 17.4 | 359.1 | 163.8 |
| E/10S/0.5MWCNT | 260.7 | 385.9 | 640.4 | 6.2 | 363.2 | 175.9 |
| E/15S/0.5MWCNT | 265.4 | 381.4 | 647.1 | 6.9 | 362.2 | 172.7 |
| Sample | Shore D |
|---|---|
| E0 | 81.2 ± 2.7 |
| E/0.5MWCNT | 85.3 ± 2.5 |
| E/10B | 79.6 ± 4.3 |
| E/15B | 74.7 ± 2.9 |
| E/10S | 79.6 ± 4.3 |
| E/15S | 75.2 ± 3.4 |
| E/10B/0.5MWCNT | 75.0 ± 2.6 |
| E/15B/0.5MWCNT | 73.9 ± 3.4 |
| E/10S/0.5MWCNT | 75.9 ± 1.8 |
| E/15S/0.5MWCNT | 78.4 ± 1.9 |
| Sample | Ignition Temperature, °C | Time-to-Ignition |
|---|---|---|
| E0 | 308 | 7 min 17 s |
| E/0.5MWCNT | 315 | 7 min 26 s |
| E/10B | 318 | 7 min 1 s |
| E/15B | 318 | 7 min 12 s |
| E/10S | 320 | 6 min 36 s |
| E/15S | 318 | 6 min 32 s |
| E/10B/0.5MWCNT | 312 | 7 min 1 s |
| E/15B/0.5MWCNT | 318 | 7 min 13 s |
| E/10S/0.5MWCNT | 306 | 6 min 53 s |
| E/15S/0.5MWCNT | 312 | 6 min 16 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarenko, O.B.; Amelkovich, Y.A.; Bannov, A.G.; Berdyugina, I.S.; Maniyan, V.P. Thermal Stability and Flammability of Epoxy Composites Filled with Multi-Walled Carbon Nanotubes, Boric Acid, and Sodium Bicarbonate. Polymers 2021, 13, 638. https://doi.org/10.3390/polym13040638
Nazarenko OB, Amelkovich YA, Bannov AG, Berdyugina IS, Maniyan VP. Thermal Stability and Flammability of Epoxy Composites Filled with Multi-Walled Carbon Nanotubes, Boric Acid, and Sodium Bicarbonate. Polymers. 2021; 13(4):638. https://doi.org/10.3390/polym13040638
Chicago/Turabian StyleNazarenko, Olga B., Yulia A. Amelkovich, Alexander G. Bannov, Irina S. Berdyugina, and Visakh P. Maniyan. 2021. "Thermal Stability and Flammability of Epoxy Composites Filled with Multi-Walled Carbon Nanotubes, Boric Acid, and Sodium Bicarbonate" Polymers 13, no. 4: 638. https://doi.org/10.3390/polym13040638
APA StyleNazarenko, O. B., Amelkovich, Y. A., Bannov, A. G., Berdyugina, I. S., & Maniyan, V. P. (2021). Thermal Stability and Flammability of Epoxy Composites Filled with Multi-Walled Carbon Nanotubes, Boric Acid, and Sodium Bicarbonate. Polymers, 13(4), 638. https://doi.org/10.3390/polym13040638






