Tuning Photophysical Properties of Donor/Acceptor Hybrid Thin- Film via Addition of SiO2/TiO2 Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
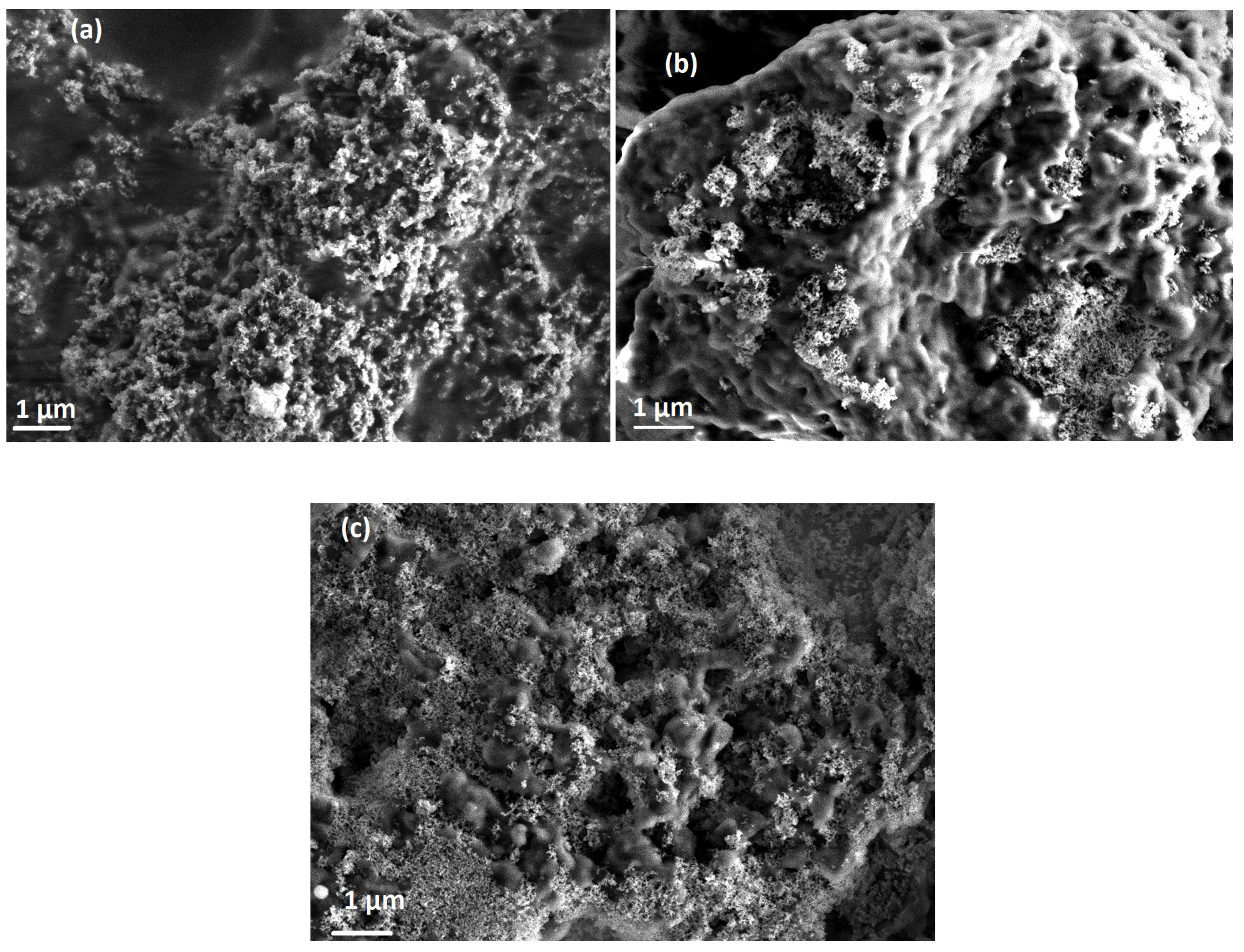
3. Results
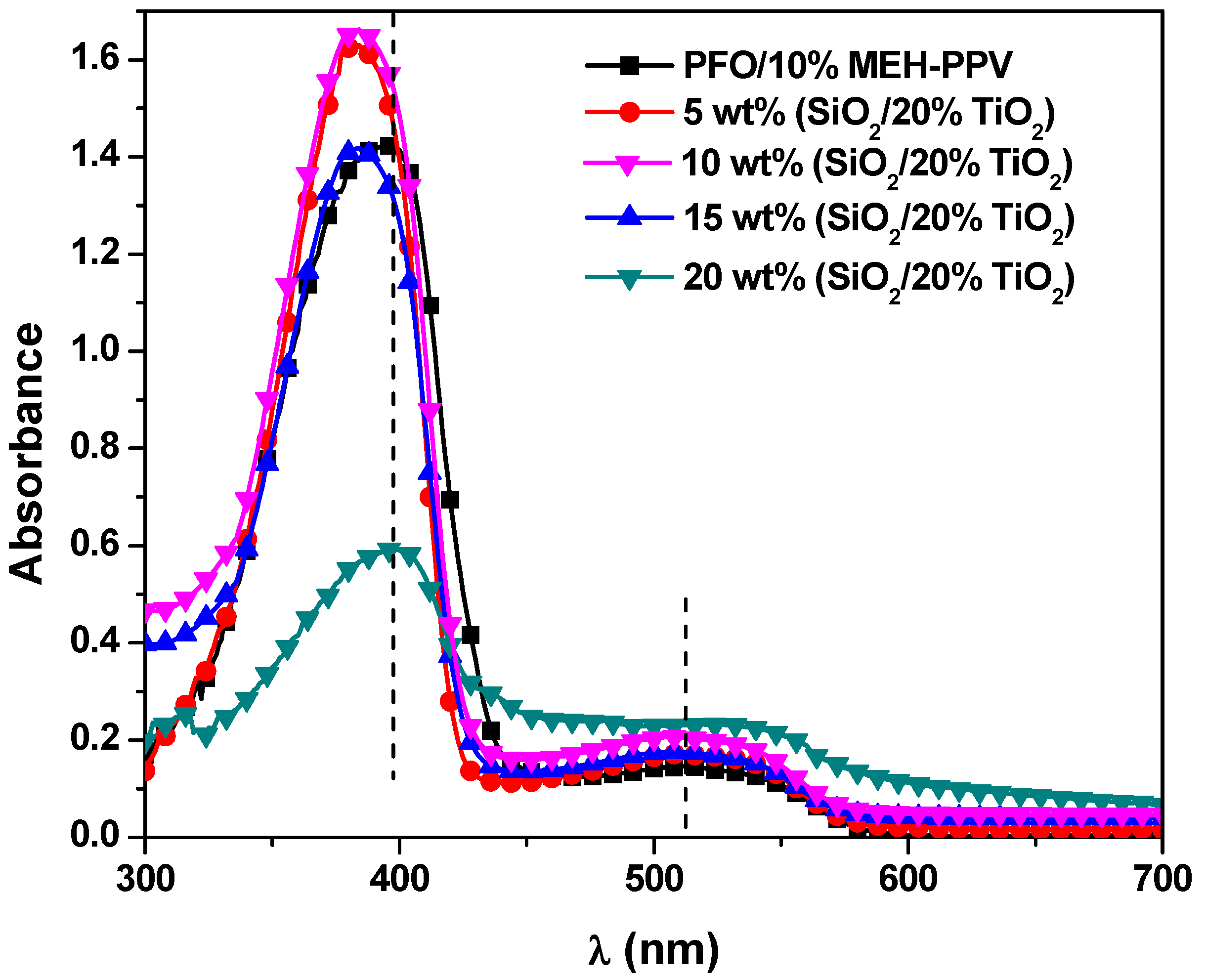
3.1. Optical Properties
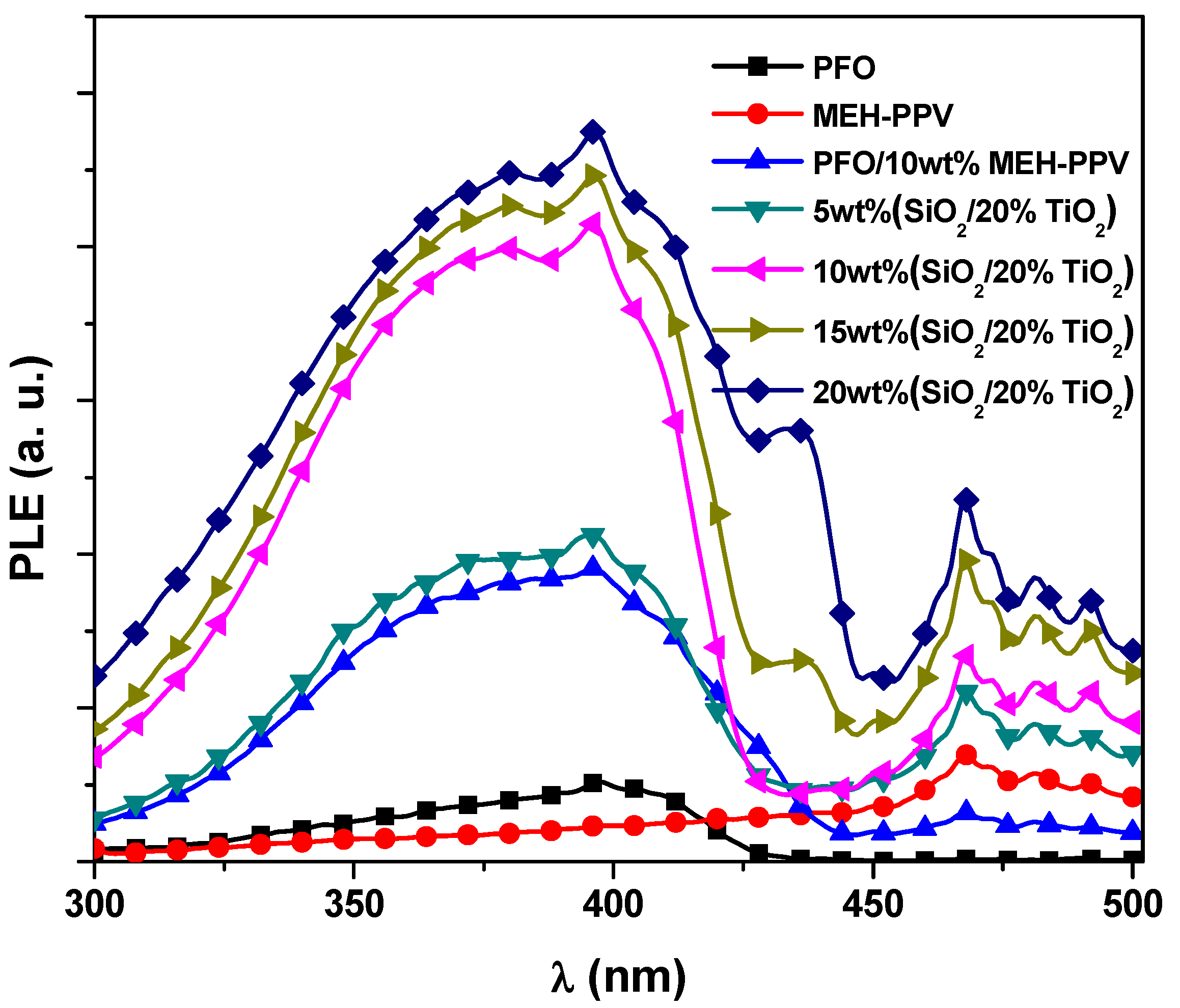
3.2. Energy Transfer Parameters
3.2.1. Quantum Yield and Lifetime of the Donor in the Hybrids
3.2.2. Stern–Volmer (kSV) and Quenching Rate (kq) Constants
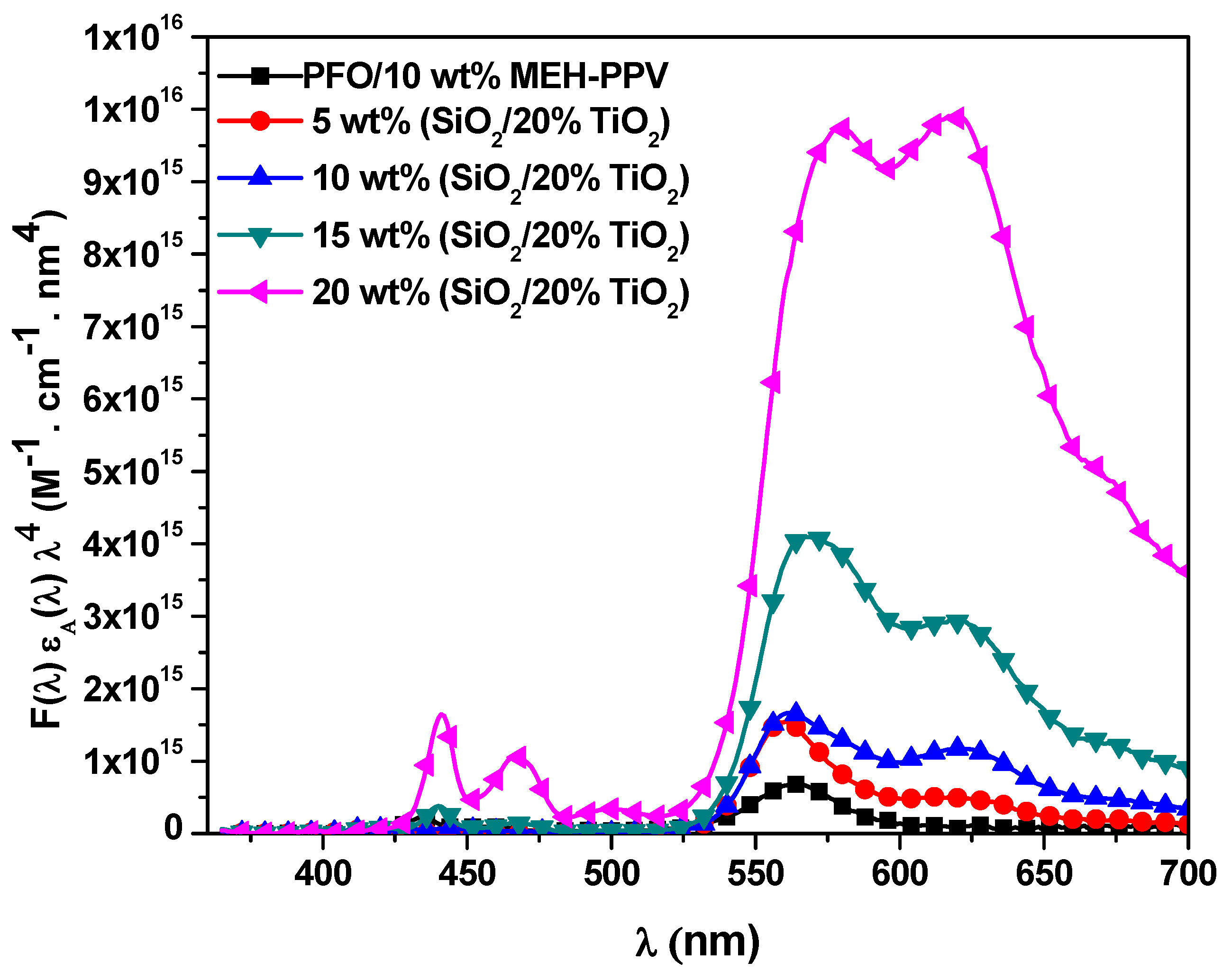
3.2.3. Förster Radius, Energy Transfer Rate, and Energy Transfer Lifetime
3.2.4. Critical Concentration of Acceptor (Ao) and Conjugated Length (Aπ)
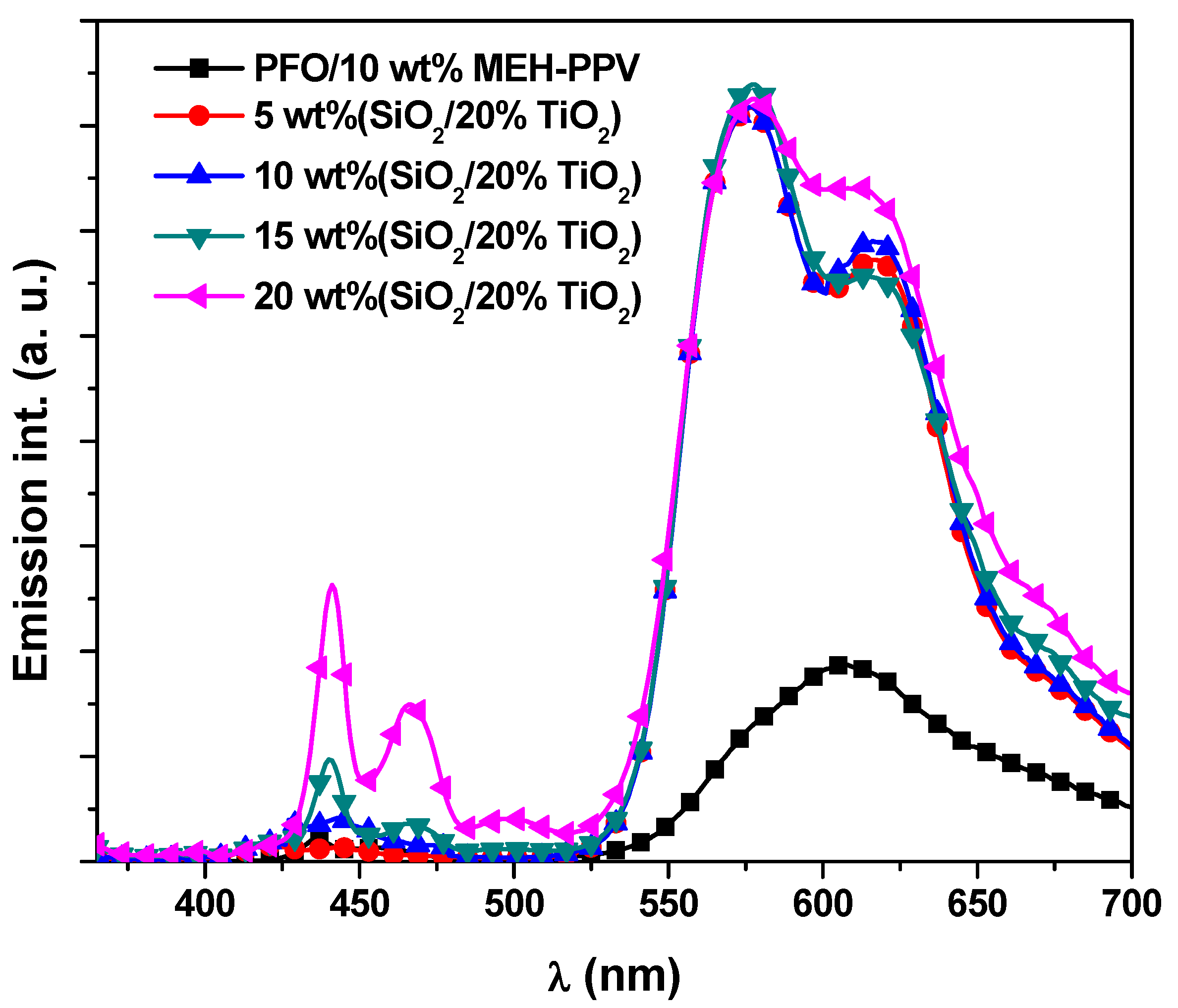
3.3. Lifetime Decay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Asbahi, B.A.; Jumali, M.H.H.; Yap, C.C.; Flaifel, M.H.; Salleh, M.M. Photophysical properties and energy transfer mechanism of PFO/Fluorol 7GA hybrid thin films. J. Lumin. 2013, 142, 57–65. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Jumali, M.H.H.; AlSalhi, M.S. Enhanced Optoelectronic Properties of PFO/Fluorol 7GA Hybrid Light Emitting Diodes via Additions of TiO2 Nanoparticles. Polymers 2016, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-T. Evolution of Red Organic Light-Emitting Diodes: Materials and Devices. Chem. Mater. 2004, 16, 4389–4400. [Google Scholar] [CrossRef]
- Buckley, A.; Rahn, M.; Hill, J.; Cabanillas-Gonzalez, J.; Fox, A.; Bradley, D. Energy transfer dynamics in polyfluorene-based polymer blends. Chem. Phys. Lett. 2001, 339, 331–336. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Alsalhi, M.S.; Al-Dwayyan, A.S.; Jumali, M.H. Förster-type energy transfer mechanism in PF2/6 to MEH-PPV conjugated polymers. J. Lumin. 2012, 132, 386–390. [Google Scholar] [CrossRef]
- Wang, R.; Shi, Z.; Zhang, C.; Zhang, A.; Chen, J.; Guo, W.; Sun, Z. Facile synthesis and controllable bromination of asymmetrical intermediates of perylene monoanhy-dride/monoimide diester. Dyes Pigment. 2013, 98, 450–458. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Qaid, S.M.; Jumali, M.H.; AlSalhi, M.S.; Aldwayyan, A.S. Long-range dipole–dipole energy transfer enhancement via addition of SiO2/TiO2 nanocomposite in PFO/MEH-PPV hybrid thin films. J. Appl. Polym. Sci. 2019, 136, 47845. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Sen, T.; Patra, A. Host–guest energy transfer: Semiconducting polymer nanoparticles and au nanoparticles. J. Phys. Chem. C 2010, 114, 11787–11795. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A. Influence of SiO2/TiO2 nanocomposite on the optoelectronic properties of PFO/MEH-PPV-based OLED devices. Polymers 2018, 10, 800. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Qaid, S.M.; Ghaithan, H.M.; Farooq, W. Enhancing the Optical and Optoelectronic Properties of MEH-PPV-Based Light-Emitting Diodes by Adding SiO2/TiO2 Nanocomposites. J. Noncryst. Solids 2020, 552, 120429. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Piccirillo, G.; Bollino, F. PEG-based organic–inorganic hybrid coatings prepared by the sol–gel dip-coating process for biomed-ical applications. Polym. Eng. Sci. 2017, 57, 478–484. [Google Scholar] [CrossRef]
- Adnan, M.M.; Dalod, A.R.M.; Balci, M.H.; Glaum, J.; Einarsrud, M.-A. In Situ Synthesis of Hybrid Inorganic–Polymer Nanocomposites. Polymers 2018, 10, 1129. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; He, Y.; Yoon, Y.J.; Jung, J.; Zhang, G.; Lin, Z. Light-enabled reversible self-assembly and tunable optical properties of stable hairy nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E1391–E1400. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A. Influence of anatase titania nanoparticles content on optical and structural properties of amorphous silica. Mater. Res. Bull. 2017, 89, 286–291. [Google Scholar] [CrossRef]
- Jumali, M.H.H.; Al-Asbahi, B.A.; Yap, C.C.; Salleh, M.M.; Alsalhi, M.S. Optical properties of poly (9,9′-di-n-octylfluorenyl-2.7-diyl)/amorphous SiO2 nanocomposite thin films. Sains Malays. 2013, 42, 1151–1157. [Google Scholar]
- Ginger, D.; Greenham, N. Charge separation in conjugated-polymer/nanocrystal blends. Synth. Met. 1999, 101, 425–428. [Google Scholar] [CrossRef]
- Yang, S.; Nguyen, T.; Le Rendu, P.; Hsu, C. Optical and electrical properties of PPV/SiO2 and PPV/TiO2 composite materials. Compos. Part A Appl. Sci. Manuf. 2005, 36, 509–513. [Google Scholar] [CrossRef]
- Zou, J.; Le Rendu, P.; Musa, I.; Yang, S.; Dan, Y.; Ton-That, C.; Nguyen, T. Investigation of the optical properties of polyfluorene/ZnO nanocomposites. Thin Solid Films 2011, 519, 3997–4003. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, M.S.; Joo, J. Hybrid nanostructures using π-conjugated polymers and nanoscale metals: Synthesis, characteristics, and optoelectronic applications. Chem. Soc. Rev. 2010, 39, 2439–2452. [Google Scholar] [CrossRef] [PubMed]
- Jumali, M.H.H.; Al-Asbahi, B.A.; Yap, C.C.; Salleh, M.M.; Alsalhi, M.S. Optoelectronic property enhancement of conjugated polymer in poly (9,9′-di-n-octylfluorenyl-2.7-diyl)/titania nanocomposites. Thin Solid Films 2012, 524, 257–262. [Google Scholar] [CrossRef]
- Geng, H.; Wang, M.; Han, S.; Peng, R. Enhanced performance of hybrid photovoltaic devices via surface-modifying metal oxides with conjugated polymer. Sol. Energy Mater. Sol. Cells 2010, 94, 547–553. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Turro, N.J. Modern Molecular Photochemistry; University Science Books: Sausalito, CA, USA, 1991. [Google Scholar]
- Albani, J. Structure and Dynamics of Macromolecules: Absorption and Fluorescence Studies; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Schwartz, B.J. Conjugated polymers as molecular materials: How chain conformation and film morphology influence energy transfer and interchain interactions. Annu. Rev. Phys. Chem. 2003, 54, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Brand, L. Resonance Energy Transfer: Methods and Applications. Anal. Biochem. 1994, 218, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Silfvast, W.T. Laser Fundamentals; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Seth, D.; Chakrabarty, D.; Chakraborty, A.; Sarkar, N. Study of energy transfer from 7-amino coumarin donors to rhodamine 6G acceptor in non-aqueous reverse micelles. Chem. Phys. Lett. 2005, 401, 546–552. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Matsubara, Y.; Ochi, T.; Wakamiya, T.; Yoshida, Z.-I. How the π Conjugation Length Affects the Fluorescence Emission Efficiency. J. Am. Chem. Soc. 2008, 130, 13867–13869. [Google Scholar] [CrossRef]






| STNCs (wt%) | φDA | τDA (ps) | knr (ns)−1 | ksv (µM)−1 | kq × 1015 (M.S)−1 | Aπ (Å) | A0 (mM) | A1/2 (µM) |
|---|---|---|---|---|---|---|---|---|
| 0 | 0.0137 | 6.57 | 150.1 | 1.24 | 3.57 | −4.278 | 5.4 | 0.81 |
| 5 | 0.0389 | 18.7 | 51.4 | 0.420 | 1.21 | −3.207 | 3.7 | 2.38 |
| 10 | 0.0814 | 39.1 | 23.5 | 0.188 | 0.543 | −2.424 | 2.9 | 5.32 |
| 15 | 0.0907 | 43.6 | 20.9 | 0.166 | 0.480 | −2.305 | 1.8 | 6.02 |
| 20 | 0.2650 | 127 | 5.8 | 0.041 | 0.119 | −1.020 | 1.0 | 24.3 |
| STNCs (wt%) | J(λ) × 1015 (M−1.cm−1.nm4) | R0 (Å) | RDA (Å) | kET (ns)−1 | τET (ps) | TDR (ns)−1 |
|---|---|---|---|---|---|---|
| 0 | 0.82 | 43.7 | 22.6 | 149 | 6.70 | 152 |
| 5 | 1.68 | 49.3 | 30.6 | 50.6 | 19.7 | 53.5 |
| 10 | 2.82 | 53.7 | 38.1 | 22.6 | 44.1 | 25.6 |
| 15 | 7.18 | 62.8 | 45.5 | 20.1 | 49.9 | 22.9 |
| 20 | 21.8 | 75.6 | 69.0 | 4.96 | 201 | 7.85 |
| At λem. 440 nm | At λem. 580 nm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| STNCs (wt%) | Relative Amplitude | Donor Lifetime | Relative Amplitude | Acceptor Lifetime (ps) | |||||
| B | τD (ps) | χ2 | B1 | B2 | τ1 | τ2 | τA (ps) | χ2 | |
| 0 | 0.316 | 60 | 1.784 | 0.049 | 0.004 | 461 | 1766 | 772 | 1.138 |
| 5 | 0.160 | 110 | 1.198 | 0.160 | - | 275 | - | 275 | 1.069 |
| 10 | 0.432 | 116 | 1.095 | 0.432 | - | 234 | - | 234 | 1.069 |
| 15 | 0.574 | 114 | 0.912 | 0.298 | - | 264 | - | 264 | 0.848 |
| 20 | 0.660 | 178 | 0.982 | 0.555 | - | 253 | - | 253 | 0.908 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Asbahi, B.A.; Hj. Jumali, M.H.; AlSalhi, M.S.; Qaid, S.M.H.; Fatehmulla, A.; Mujamammi, W.M.; Ghaithan, H.M. Tuning Photophysical Properties of Donor/Acceptor Hybrid Thin- Film via Addition of SiO2/TiO2 Nanocomposites. Polymers 2021, 13, 611. https://doi.org/10.3390/polym13040611
Al-Asbahi BA, Hj. Jumali MH, AlSalhi MS, Qaid SMH, Fatehmulla A, Mujamammi WM, Ghaithan HM. Tuning Photophysical Properties of Donor/Acceptor Hybrid Thin- Film via Addition of SiO2/TiO2 Nanocomposites. Polymers. 2021; 13(4):611. https://doi.org/10.3390/polym13040611
Chicago/Turabian StyleAl-Asbahi, Bandar Ali, Mohammad Hafizuddin Hj. Jumali, M. S. AlSalhi, Saif M. H. Qaid, Amanullah Fatehmulla, Wafa Musa Mujamammi, and Hamid M. Ghaithan. 2021. "Tuning Photophysical Properties of Donor/Acceptor Hybrid Thin- Film via Addition of SiO2/TiO2 Nanocomposites" Polymers 13, no. 4: 611. https://doi.org/10.3390/polym13040611
APA StyleAl-Asbahi, B. A., Hj. Jumali, M. H., AlSalhi, M. S., Qaid, S. M. H., Fatehmulla, A., Mujamammi, W. M., & Ghaithan, H. M. (2021). Tuning Photophysical Properties of Donor/Acceptor Hybrid Thin- Film via Addition of SiO2/TiO2 Nanocomposites. Polymers, 13(4), 611. https://doi.org/10.3390/polym13040611










