Application of Zwitterions in Forward Osmosis: A Short Review
Abstract
1. Introduction
2. Classification of Fouling Types in FO
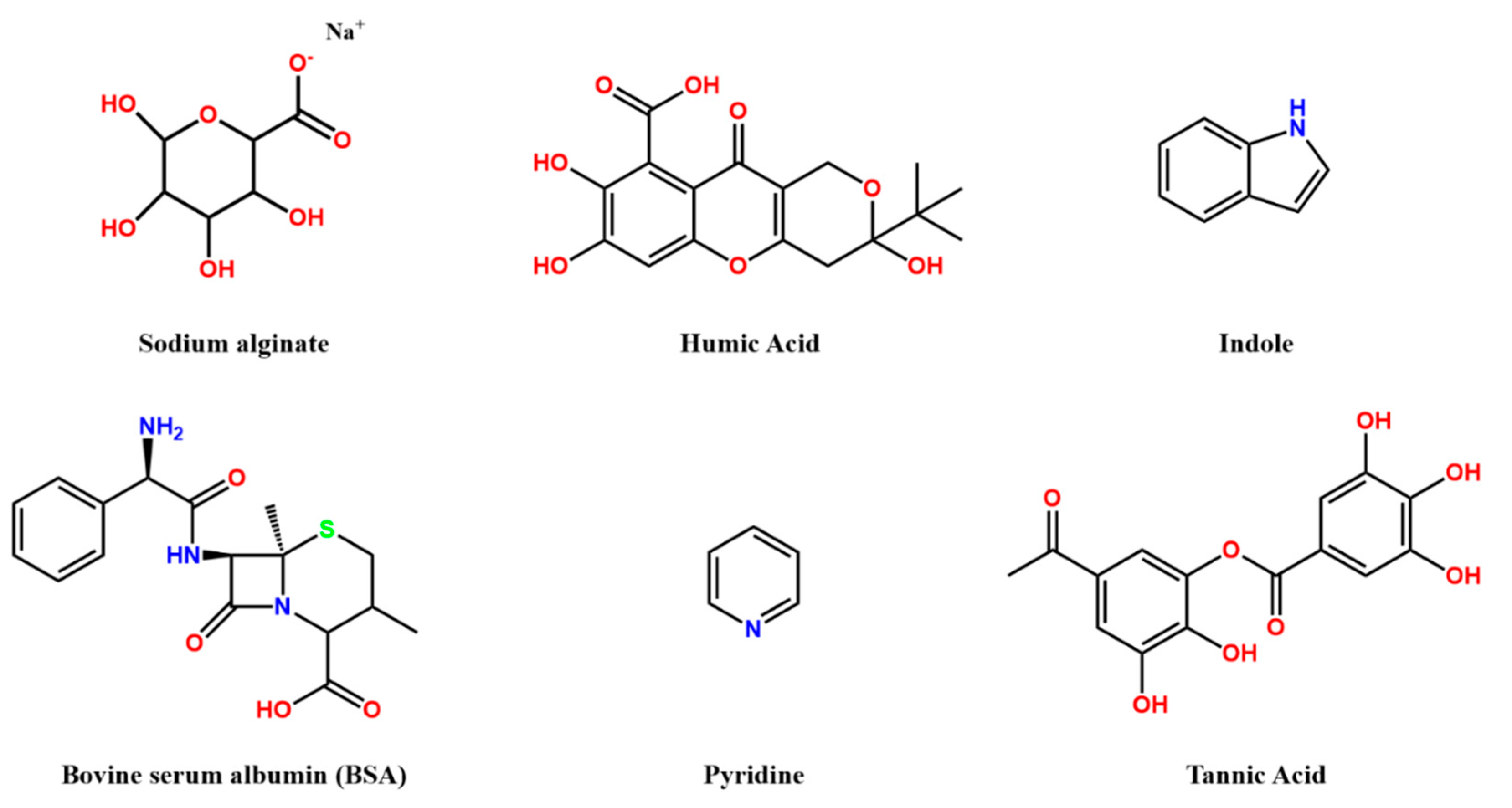
2.1. Organic Fouling
- (1)
- Autochthonous: They are obtained from extracellular macromolecules from microorganism and carbon fixation by algae and aquatic plants.
- (2)
- Allochthones: They are obtained from the decayed parts of plants and animals.
2.2. Inorganic Fouling
2.3. Biofouling
- (1)
- The transportation of bacterial cells near the membrane-feed solution interface and attachment of bacterial cells on the membrane surface. This step is reversible in nature and is mainly governed by van der Waal’s forces.
- (2)
- In the subsequent step, there is a formation of a bacterial colony leading to a stronger interaction with membrane materials, formation of biofilms, and eventually modification of the membrane surface properties. This step is irreversible in nature.
2.4. Colloidal Fouling
- (1)
- Cake layer hydraulic resistance
- (2)
- Cake enhanced osmotic pressure (CEOP)
3. Zwitterionic Membrane
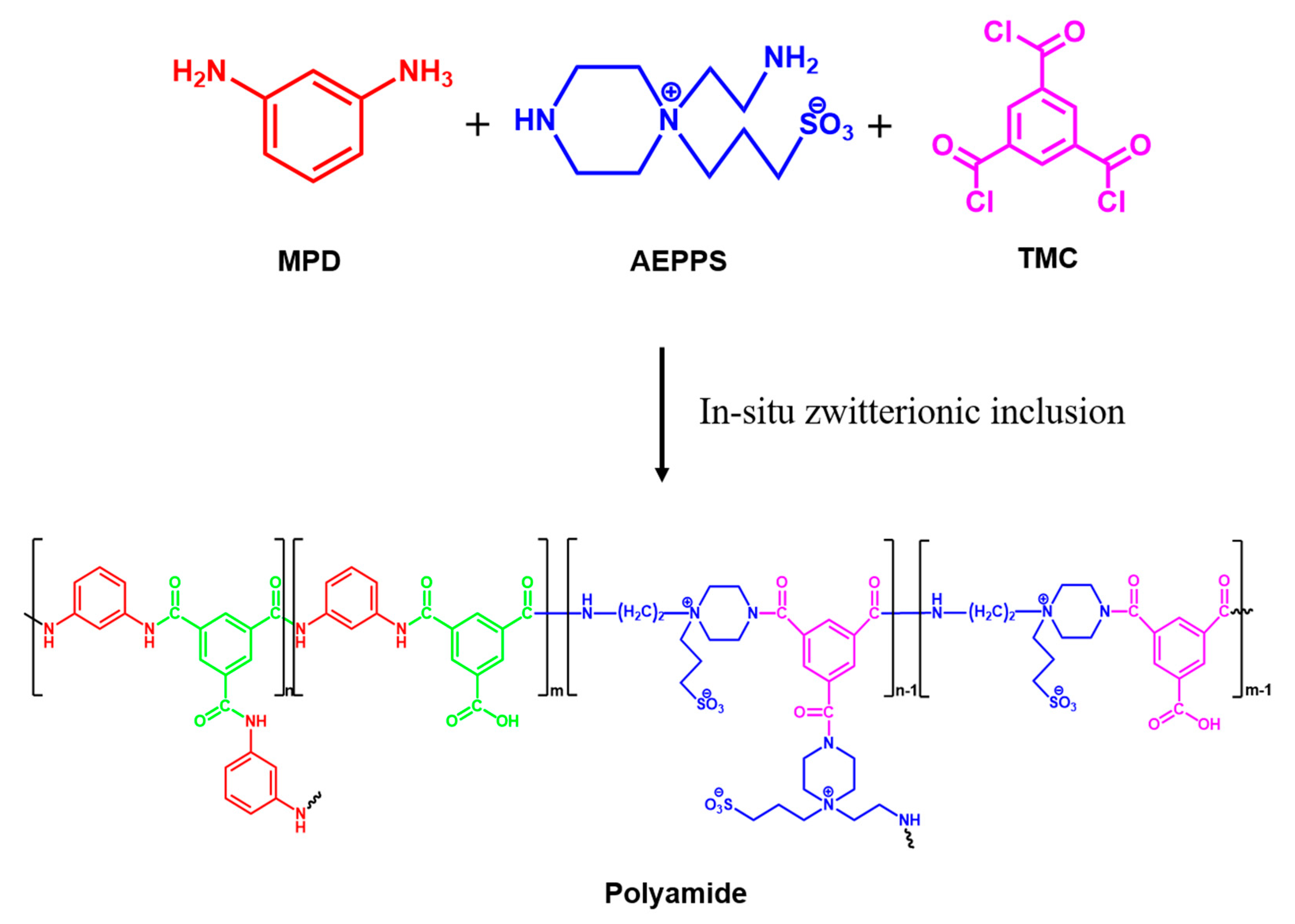
3.1. In-Situ
3.2. Second Interfacial Polymerization (SIP)
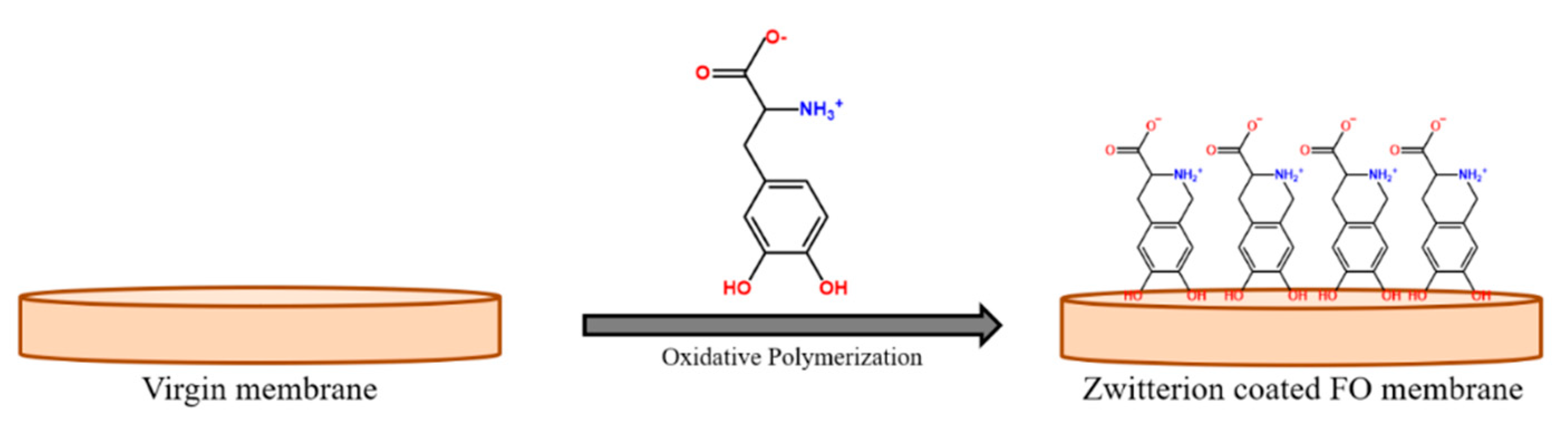
3.3. Coating
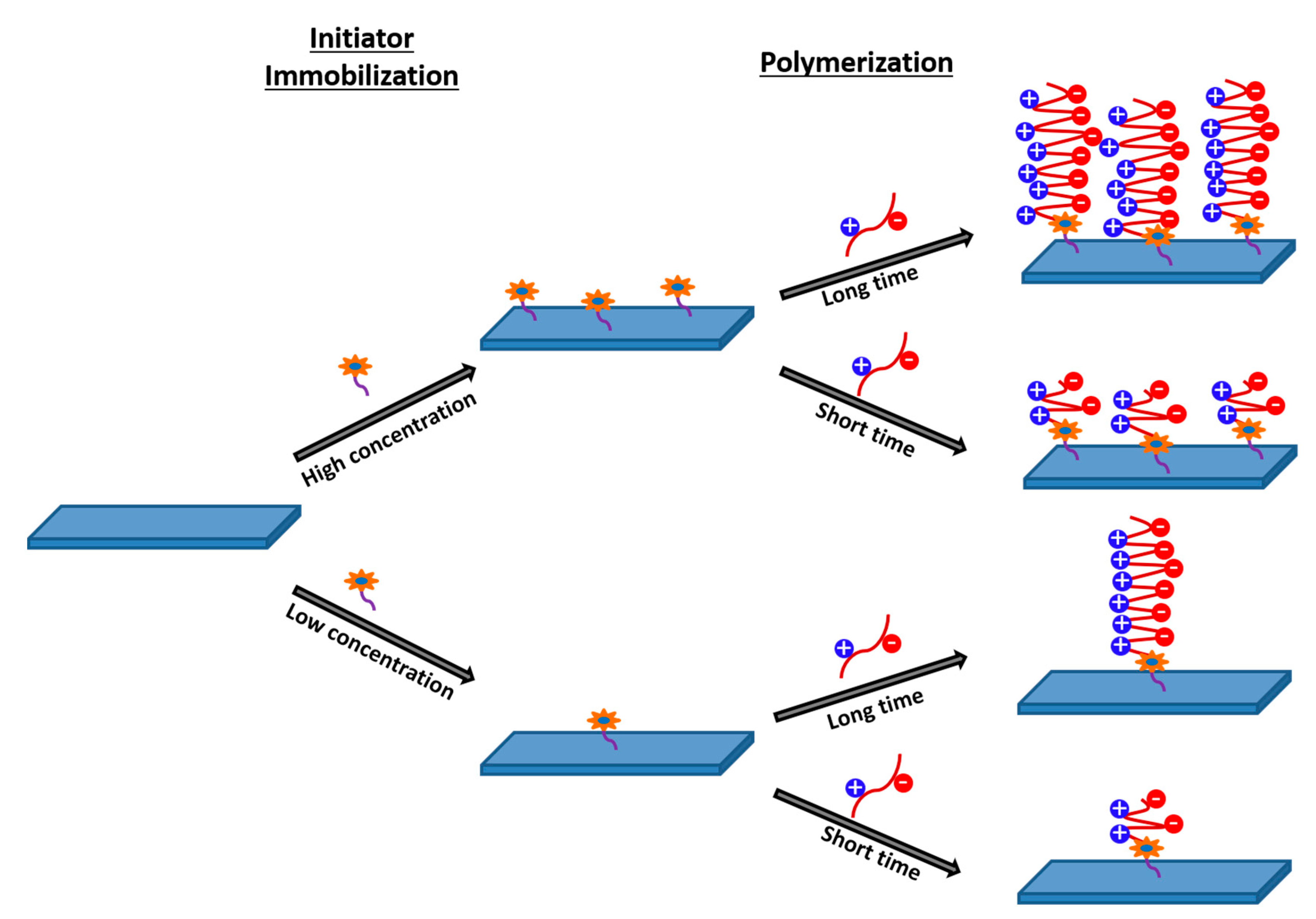
3.4. Atom Transfer Radical Polymerization (ATRP)
- (1)
- Simple hydraulic washing: Feed: DI water; Draw solution: DI water; pH 7
- (2)
- Osmotic backwashing: Feed: 2 M NaCl, Draw solution: DI water; pH 7
- (3)
- Acid cleaning: Feed: 0.1% Citric acid; Draw solution: DI water; pH 3
- (4)
- Alkaline cleaning: Feed: 0.1% NaOH, Draw solution: DI water; pH 12
- (5)
- Surfactant cleaning: Feed: 0.1% SDBS solution; Draw solution: DI water; pH 9
4. Draw Solution
- (1)
- Osmotic pressure
- (2)
- Charge
- (3)
- Polarity
- (4)
- Molecular weight
Osmotic Pressure:
Charge:
Polarity:
Molecular Weight:
5. Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Water permeability |
| AEP | 1-(2-Aminoethyl) piperazine |
| AEPPS | N-aminoethyl piperazinepropane sulfonate |
| Ag NPs | Silver nanoparticles |
| AL-DS | Active layer facing the draw solution |
| APIS | (1-(3-Aminopropyl) imidazole) propane sulfonate |
| ATRP | Atom transfer radical polymerization |
| BSA | Bovine serum albumin |
| CA | Cellulose acetate |
| Cb | Bulk salt concentration |
| CBMA | Carboxybetaine methacrylate |
| CEOP | Cake enhanced osmotic pressure |
| CFU | Colony forming unit |
| CP | Concentration polarization |
| CP* | Cake-enhanced concentration polarization |
| CQDs | Carbon quantum dots |
| CTA | Cellulose triacetate |
| DS | Draw solution |
| ECP | External concentration polarization |
| FO | Forward osmosis |
| FS | Feed solution |
| GO | Graphene oxide |
| HEMA | 2-Hydroxyethyl methacrylate |
| ICP | Internal concentration polarization |
| J | Water flux |
| J0 | Initial flux |
| k* | Cake-hindered mass transfer coefficient |
| ki | Rate constant of the ith component |
| KL | Langmuir adsorption coefficient |
| L-DOPA | Polyamino acid 3-(3,4-dihydroxyphenyl)-L-alanine |
| MD | Membrane distillation |
| NF | Nanofiltration |
| OEG | Oligo(ethylene glycol) |
| PBMA | Phosphobetaine methacrylate |
| PES | Polyethersulfone |
| PMAPS | Poly[3-(N-2-methacryloylxyethyl-N,N-dimethyl)-ammonatopropanesulfonate] |
| PSBMA | Poly [2-(methacryloyloxy)-ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide |
| Ra | Resistance due to organic foulant adsorption |
| RC | Resistance due to cake formation |
| RCP | Resistance due to concentration polarization |
| Ri | Intrinsic resistance of the membrane in presence of pure water |
| ri | Initial rate of interaction between organic matter and the membrane surface |
| RO | Reverse osmosis |
| Ro | Salt rejection |
| SIP | Second interfacial polymerization |
| T | Temperature (K) |
| TFC | Thin film composite |
| VOPSs | Vertically oriented porous substrates |
| Z-CNTs | Zwitterion functionalized carbon nanotubes |
| Φ | Molar osmotic coefficient |
| Osmotic pressure difference | |
| Viscosity coefficient |
References
- Alihemati, Z.; Hashemifard, S.A.; Matsuura, T.; Ismail, A.F.; Hilal, N. Current status and challenges of fabricating thin film composite forward osmosis membrane: A comprehensive roadmap. Desalination 2020, 491, 114557. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Glater, J. The early history of reverse osmosis membrane development. Desalination 1998, 117, 297–309. [Google Scholar] [CrossRef]
- Belfort, G. Membrane Filtration with Liquids: A Global Approach with Prior Successes, New Developments and Unresolved Challenges. Angew. Chem. Int. Ed. 2018, 58, 1892–1902. [Google Scholar]
- Mulder, J.X. Basic Principles of Membrane Technology; Springer Science & Business Media: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Amy, G.; Ghaffour, N.; Li, Z.; Francis, L.; Linares, R.V.; Missimer, T.; Lattemann, S. Membrane-based seawater desalination: Present and future prospects. Desalination 2017, 401, 16–21. [Google Scholar] [CrossRef]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Chung, T.-S.; Zhang, S.; Wang, K.Y.; Su, J.; Ling, M.M. Forward osmosis processes: Yesterday, today and tomorrow. Desalination 2012, 287, 78–81. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Qiu, C.; Wang, Y.-N.; Wang, R.; Tang, C.Y. Comparison of NF-like and RO-like thin film composite osmotically-driven membranes—Implications for membrane selection and process optimization. J. Membr. Sci. 2013, 427, 460–471. [Google Scholar] [CrossRef]
- Yao, Z.; Peng, L.E.; Guo, H.; Qing, W.; Mei, Y.; Tang, C.Y. Seawater pretreatment with an NF-like forward osmotic membrane: Membrane preparation, characterization and performance comparison with RO-like membranes. Desalination 2019, 470. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Sengupta, A.; Chen, S.-T.; Hung, W.-S.; Lai, J.-Y.; Upadhyaya, L.; Qian, X.; Wickramasinghe, S.R. Novel thin-film composite forward osmosis membrane using polyethylenimine and its impact on membrane performance. Sep. Sci. Technol. 2020, 55, 590–600. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Sengupta, A.; Chen, S.-T.; Huang, S.-H.; Hu, C.-C.; Hung, W.-S.; Chang, Y.; Qian, X.; Wickramasinghe, S.R.; Lee, K.-R.; et al. Zwitterion augmented polyamide membrane for improved forward osmosis performance with significant antifouling characteristics. Sep. Purif. Technol. 2019, 212, 316–325. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Chen, S.-T.; Patra, T.; Hsu, C.-H.; Sengupta, A.; Hung, W.-S.; Huang, S.-H.; Qian, X.; Wickramasinghe, R.; Chang, Y. Zwitterionic forward osmosis membrane modified by fast second interfacial polymerization with enhanced antifouling and antimicrobial properties for produced water pretreatment. Desalination 2019, 469, 114090. [Google Scholar] [CrossRef]
- Chen, G.Q.; Artemi, A.; Lee, J.; Gras, S.L.; Kentish, S.E. A pilot scale study on the concentration of milk and whey by forward osmosis. Sep. Purif. Technol. 2019, 215, 652–659. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Wang, R.; Li, W.; Tang, C.Y. Whey recovery using forward osmosis—Evaluating the factors limiting the flux performance. J. Membr. Sci. 2017, 533, 179–189. [Google Scholar] [CrossRef]
- Li, B.; Japip, S.; Chung, T.-S. Molecularly tunable thin-film nanocomposite membranes with enhanced molecular sieving for organic solvent forward osmosis. Nat. Commun. 2020, 11, 1198. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Li, X.; Chung, T.-S. The forward osmosis-pressure retarded osmosis (FO-PRO) hybrid system: A new process to mitigate membrane fouling for sustainable osmotic power generation. J. Membr. Sci. 2018, 559, 63–74. [Google Scholar] [CrossRef]
- Rastogi, N.K. Opportunities and challenges in application of forward osmosis in food processing. Crit. Rev. Food Sci. Nutr. 2016, 56, 266–291. [Google Scholar] [CrossRef]
- Chun, Y.; Mulcahy, D.; Zou, L.; Kim, I.S. A Short Review of Membrane Fouling in Forward Osmosis Processes. Membranes 2017, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chung, T.-S. Pharmaceutical concentration using organic solvent forward osmosis for solvent recovery. Nat. Commun. 2018, 9, 1426. [Google Scholar] [CrossRef]
- Dong, X.; Ge, Q. Metal Ion-Bridged Forward Osmosis Membranes for Efficient Pharmaceutical Wastewater Reclamation. Acs Appl. Mater. Interfaces 2019, 11, 37163–37171. [Google Scholar] [CrossRef]
- Xiong, S.; Xu, S.; Zhang, S.; Phommachanh, A.; Wang, Y. Highly permeable and antifouling TFC FO membrane prepared with CD-EDA monomer for protein enrichment. J. Membr. Sci. 2019, 572, 281–290. [Google Scholar] [CrossRef]
- Wu, W.; Shi, Y.; Liu, G.; Fan, X.; Yu, Y. Recent development of graphene oxide based forward osmosis membrane for water treatment: A critical review. Desalination 2020, 491, 114452. [Google Scholar] [CrossRef]
- Siddiqui, F.A.; She, Q.; Fane, A.G.; Field, R.W. Exploring the differences between forward osmosis and reverse osmosis fouling. J. Membr. Sci. 2018, 565, 241–253. [Google Scholar] [CrossRef]
- Yadav, S.; Saleem, H.; Ibrar, I.; Naji, O.; Hawari, A.A.; Alanezi, A.A.; Zaidi, S.J.; Altaee, A.; Zhou, J. Recent developments in forward osmosis membranes using carbon-based nanomaterials. Desalination 2020, 482, 114375. [Google Scholar] [CrossRef]
- McGinnis, R.L.; Elimelech, M. Energy requirements of ammonia–carbon dioxide forward osmosis desalination. Desalination 2007, 207, 370–382. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Marchand, E.A.; Childress, A.E. The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes. Desalination 2009, 239, 10–21. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Organic fouling of forward osmosis membranes: Fouling reversibility and cleaning without chemical reagents. J. Membr. Sci. 2010, 348, 337–345. [Google Scholar] [CrossRef]
- Arjmandi, M.; Peyravi, M.; Altaee, A.; Arjmandi, A.; Pourafshari Chenar, M.; Jahanshahi, M.; Binaeian, E. A state-of-the-art protocol to minimize the internal concentration polarization in forward osmosis membranes. Desalination 2020, 480, 114355. [Google Scholar] [CrossRef]
- Kahrizi, M.; Lin, J.; Ji, G.; Kong, L.; Song, C.; Dumée, L.F.; Sahebi, S.; Zhao, S. Relating forward water and reverse salt fluxes to membrane porosity and tortuosity in forward osmosis: CFD modelling. Sep. Purif. Technol. 2020, 241, 116727. [Google Scholar] [CrossRef]
- Cath, T.Y.; Elimelech, M.; McCutcheon, J.R.; McGinnis, R.L.; Achilli, A.; Anastasio, D.; Brady, A.R.; Childress, A.E.; Farr, I.V.; Hancock, N.T.; et al. Standard Methodology for Evaluating Membrane Performance in Osmotically Driven Membrane Processes. Desalination 2013, 312, 31–38. [Google Scholar] [CrossRef]
- Bui, N.-N.; Lind, M.L.; Hoek, E.M.V.; McCutcheon, J.R. Electrospun nanofiber supported thin film composite membranes for engineered osmosis. J. Membr. Sci. 2011, 385–386, 10–19. [Google Scholar] [CrossRef]
- Bui, N.-N.; McCutcheon, J.R. Nanoparticle-embedded nanofibers in highly permselective thin-film nanocomposite membranes for forward osmosis. J. Membr. Sci. 2016, 518, 338–346. [Google Scholar] [CrossRef]
- Liang, H.-Q.; Hung, W.-S.; Yu, H.-H.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y.; Xu, Z.-K. Forward osmosis membranes with unprecedented water flux. J. Membr. Sci. 2017, 529, 47–54. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; McGinnis, R.L.; Elimelech, M. A novel ammonia—carbon dioxide forward (direct) osmosis desalination process. Desalination 2005, 174, 1–11. [Google Scholar] [CrossRef]
- Chang, H.; Li, T.; Liu, B.; Vidic, R.D.; Elimelech, M.; Crittenden, J.C. Potential and implemented membrane-based technologies for the treatment and reuse of flowback and produced water from shale gas and oil plays: A review. Desalination 2019, 455, 34–57. [Google Scholar] [CrossRef]
- Lee, W.J.; Ng, Z.C.; Hubadillah, S.K.; Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F.; Hilal, N. Fouling mitigation in forward osmosis and membrane distillation for desalination. Desalination 2020, 480, 114338. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Bakly, S.; Khanafer, D.; Altaee, A.; Padmanaban, V.C.; Samal, A.K.; Hawari, A.H. Organic Fouling in Forward Osmosis: A Comprehensive Review. Water 2020, 12, 1505. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Chen, S.-T.; Ang, M.B.M.Y.; Patra, T.; Castilla-Casadiego, D.A.; Fan, R.; Almodovar, J.; Hung, W.-S.; Wickramasinghe, S.R. High-Performance Polyacrylic Acid-Grafted PVDF Nanofiltration Membrane with Good Antifouling Property for the Textile Industry. Polymers 2020, 12, 2443. [Google Scholar] [CrossRef]
- Zhang, G.; Zhan, Y.; He, S.; Zhang, L.; Zeng, G.; Chiao, Y.H. Construction of superhydrophilic/underwater superoleophobic polydopamine-modified h-BN/poly (arylene ether nitrile) composite membrane for stable oil-water emulsions separation. Polym. Adv. Technol. 2020, 31, 1007–1018. [Google Scholar] [CrossRef]
- Zeng, G.; Wei, K.; Yang, D.; Yan, J.; Zhou, K.; Patra, T.; Sengupta, A.; Chiao, Y.-H. Improvement in performance of PVDF ultrafiltration membranes by co-incorporation of dopamine and halloysite nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 124142. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Qi, L.; Hu, Y.; Liu, Z.; An, X.; Bar-Zeev, E. Improved Anti-Biofouling Performance of Thin -Film Composite Forward-Osmosis Membranes Containing Passive and Active Moieties. Environ. Sci. Technol. 2018, 52, 9684–9693. [Google Scholar] [CrossRef]
- Sin, M.-C.; Chen, S.-H.; Chang, Y. Hemocompatibility of zwitterionic interfaces and membranes. Polym. J. 2014, 46, 436–443. [Google Scholar] [CrossRef]
- Luk, Y.-Y.; Kato, M.; Mrksich, M. Self-assembled monolayers of alkanethiolates presenting mannitol groups are inert to protein adsorption and cell attachment. Langmuir 2000, 16, 9604–9608. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Patra, T.; Ang, M.B.M.Y.; Chen, S.-T.; Almodovar, J.; Qian, X.; Wickramasinghe, R.; Hung, W.-S.; Huang, S.-H.; Chang, Y. Zwitterion Co-Polymer PEI-SBMA Nanofiltration Membrane Modified by Fast Second Interfacial Polymerization. Polymers 2020, 12, 269. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Control of surface forces through hydrated boundary layers. Curr. Opin. Colloid Interface Sci. 2019, 44, 94–106. [Google Scholar] [CrossRef]
- Erfani, A.; Seaberg, J.; Aichele, C.P.; Ramsey, J.D. Interactions between Biomolecules and Zwitterionic Moieties: A Review. Biomacromolecules 2020, 21, 2557–2573. [Google Scholar] [CrossRef] [PubMed]
- Chiao, Y.-H.; Chen, S.-T.; Sivakumar, M.; Ang, M.B.M.Y.; Patra, T.; Almodovar, J.; Wickramasinghe, S.R.; Hung, W.-S.; Lai, J.-Y. Zwitterionic Polymer Brush Grafted on Polyvinylidene Difluoride Membrane Promoting Enhanced Ultrafiltration Performance with Augmented Antifouling Property. Polymers 2020, 12, 1303. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Ang, M.B.M.Y.; Huang, Y.-X.; DePaz, S.S.; Chang, Y.; Almodovar, J.; Wickramasinghe, S.R. A “Graft to” Electrospun Zwitterionic Bilayer Membrane for the Separation of Hydraulic Fracturing-Produced Water via Membrane Distillation. Membranes 2020, 10, 402. [Google Scholar] [CrossRef]
- Han, L.; Tan, Y.Z.; Xu, C.; Xiao, T.; Trinh, T.A.; Chew, J.W. Zwitterionic grafting of sulfobetaine methacrylate (SBMA) on hydrophobic PVDF membranes for enhanced anti-fouling and anti-wetting in the membrane distillation of oil emulsions. J. Membr. Sci. 2019, 588, 117196. [Google Scholar] [CrossRef]
- Zhou, Q.; Lei, X.-P.; Li, J.-H.; Yan, B.-F.; Zhang, Q.-Q. Antifouling, adsorption and reversible flux properties of zwitterionic grafted PVDF membrane prepared via physisorbed free radical polymerization. Desalination 2014, 337, 6–15. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Shon, H.K.; Vigneswaran, S.; Kim, I.S.; Cho, J.; Ngo, H.H. Fouling of ultrafiltration membrane by effluent organic matter: A detailed characterization using different organic fractions in wastewater. J. Membr. Sci. 2006, 278, 232–238. [Google Scholar] [CrossRef]
- Yuan, W.; Zydney, A.L. Humic Acid Fouling during Ultrafiltration. Environ. Sci. Technol. 2000, 34, 5043–5050. [Google Scholar] [CrossRef]
- Parida, V.; Ng, H.Y. Forward osmosis organic fouling: Effects of organic loading, calcium and membrane orientation. Desalination 2013, 312, 88–98. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Silica scaling and scaling reversibility in forward osmosis. Desalination 2013, 312, 75–81. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Gypsum Scaling and Cleaning in Forward Osmosis: Measurements and Mechanisms. Environ. Sci. Technol. 2010, 44, 2022–2028. [Google Scholar] [CrossRef]
- Chun, Y.; Zaviska, F.; Kim, S.-J.; Mulcahy, D.; Yang, E.; Kim, I.S.; Zou, L. Fouling characteristics and their implications on cleaning of a FO-RO pilot process for treating brackish surface water. Desalination 2016, 394, 91–100. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Yangali-Quintanilla, V.; Valladares-Linares, R.; Li, Q.; Zhan, T.; Amy, G. Flux patterns and membrane fouling propensity during desalination of seawater by forward osmosis. Water Res. 2012, 46, 195–204. [Google Scholar] [CrossRef]
- Flemming, H.C.; Schaule, G.; Griebe, T.; Schmitt, J.; Tamachkiarowa, A. Biofouling—the Achilles heel of membrane processes. Desalination 1997, 113, 215–225. [Google Scholar] [CrossRef]
- Veza, J.M.; Ortiz, M.; Sadhwani, J.J.; Gonzalez, J.E.; Santana, F.J. Measurement of biofouling in seawater: Some practical tests. Desalination 2008, 220, 326–334. [Google Scholar] [CrossRef]
- Saeed, M.O.; Jamaluddin, A.T.; Tisan, I.A.; Lawrence, D.A.; Al-Amri, M.M.; Chida, K. Biofouling in a seawater reverse osmosis plant on the Red Sea coast, Saudi Arabia. Desalination 2000, 128, 177–190. [Google Scholar] [CrossRef]
- Goulter, R.M.; Gentle, I.R.; Dykes, G.A. Issues in determining factors influencing bacterial attachment: A review using the attachment of Escherichia coli to abiotic surfaces as an example. Lett. Appl. Microbiol. 2009, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ibrar, I.; Naji, O.; Sharif, A.; Malekizadeh, A.; Alhawari, A.; Alanezi, A.A.; Altaee, A. A Review of Fouling Mechanisms, Control Strategies and Real-Time Fouling Monitoring Techniques in Forward Osmosis. Water 2019, 11, 695. [Google Scholar] [CrossRef]
- Singh, G.; Song, L. Experimental correlations of pH and ionic strength effects on the colloidal fouling potential of silica nanoparticles in crossflow ultrafiltration. J. Membr. Sci. 2007, 303, 112–118. [Google Scholar] [CrossRef]
- Cohen, R.; Probstein, R. Colloidal fouling of reverse osmosis membranes. J. Colloid Interface Sci. 1986, 114, 194–207. [Google Scholar] [CrossRef]
- Boo, C.; Lee, S.; Elimelech, M.; Meng, Z.; Hong, S. Colloidal fouling in forward osmosis: Role of reverse salt diffusion. J. Membr. Sci. 2012, 390–391, 277–284. [Google Scholar] [CrossRef]
- Dizon, G.V.; Venault, A. Direct in-situ modification of PVDF membranes with a zwitterionic copolymer to form bi-continuous and fouling resistant membranes. J. Membr. Sci. 2018, 550, 45–58. [Google Scholar] [CrossRef]
- Lee, W.J.; Goh, P.S.; Lau, W.J.; Ong, C.S.; Ismail, A.F. Antifouling zwitterion embedded forward osmosis thin film composite membrane for highly concentrated oily wastewater treatment. Sep. Purif. Technol. 2019, 214, 40–50. [Google Scholar] [CrossRef]
- Romero-Vargas Castrillón, S.; Lu, X.; Shaffer, D.L.; Elimelech, M. Amine enrichment and poly(ethylene glycol) (PEG) surface modification of thin-film composite forward osmosis membranes for organic fouling control. J. Membr. Sci. 2014, 450, 331–339. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, J.; Gao, S.; Zhang, Z.; Cui, F.; Tang, C.Y. In situ surface modification of thin film composite forward osmosis membranes with sulfonated poly (arylene ether sulfone) for anti-fouling in emulsified oil/water separation. J. Membr. Sci. 2017, 527, 26–34. [Google Scholar] [CrossRef]
- Venault, A.; Chang, Y. Designs of Zwitterionic Interfaces and Membranes. Langmuir 2019, 35, 1714–1726. [Google Scholar] [CrossRef]
- Chen, Y.; Ge, Q. A Bifunctional Zwitterion That Serves as Both a Membrane Modifier and a Draw Solute for Forward Osmosis Wastewater Treatment. Acs Appl. Mater. Interfaces 2019, 11, 36118–36129. [Google Scholar] [CrossRef]
- Zou, S.; Smith, E.D.; Lin, S.; Martin, S.M.; He, Z. Mitigation of bidirectional solute flux in forward osmosis via membrane surface coating of zwitterion functionalized carbon nanotubes. Environ. Int. 2019, 131, 104970. [Google Scholar] [CrossRef]
- Nguyen, A.; Azari, S.; Zou, L. Coating zwitterionic amino acid l-DOPA to increase fouling resistance of forward osmosis membrane. Desalination 2013, 312, 82–87. [Google Scholar] [CrossRef]
- Carter, B.M.; Sengupta, A.; Qian, X.; Ulbricht, M.; Wickramasinghe, S.R. Controlling external versus internal pore modification of ultrafiltration membranes using surface-initiated AGET-ATRP. J. Membr. Sci. 2018, 554, 109–116. [Google Scholar] [CrossRef]
- Tripathi, T.; Kamaz, M.; Wickramasinghe, S.R.; Sengupta, A. Designing Electric Field Responsive Ultrafiltration Membranes by Controlled Grafting of Poly (Ionic Liquid) Brush. Int. J. Environ. Res. Public Health 2020, 17, 271. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Wickramasinghe, R. Activator Generated Electron Transfer Combined Atom Transfer Radical Polymerization (AGET-ATRP) for Controlled Grafting Location of Glycidyl Methacrylate on Regenerated Cellulose Ultrafiltration Membranes. J. Membr. Sci. Res. 2020, 6, 90–98. [Google Scholar]
- Liu, C.; Lee, J.; Ma, J.; Elimelech, M. Antifouling Thin-Film Composite Membranes by Controlled Architecture of Zwitterionic Polymer Brush Layer. Environ. Sci. Technol. 2017, 51, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lee, J.; Small, C.; Ma, J.; Elimelech, M. Comparison of organic fouling resistance of thin-film composite membranes modified by hydrophilic silica nanoparticles and zwitterionic polymer brushes. J. Membr. Sci. 2017, 544, 135–142. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, S.; Tian, J.; Shan, S.; Takagi, R.; Cui, F.; Bai, L.; Matsuyama, H. Investigation of Cleaning Strategies for an Antifouling Thin-Film Composite Forward Osmosis Membrane for Treatment of Polymer-Flooding Produced Water. Ind. Eng. Chem. Res. 2019, 58, 994–1003. [Google Scholar] [CrossRef]
- Ju, C.; Kang, H. Zwitterionic polymers showing upper critical solution temperature behavior as draw solutes for forward osmosis. Rsc Adv. 2017, 7, 56426–56432. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Lauber, L.; Roest, K.; Harmsen, D.J.H.; Post, J.W.; Rietveld, L.C.; van Lier, J.B.; Cornelissen, E.R. Zwitterions as alternative draw solutions in forward osmosis for application in wastewater reclamation. J. Membr. Sci. 2014, 460, 82–90. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiao, Y.-H.; Sengupta, A.; Ang, M.B.M.Y.; Chen, S.-T.; Haan, T.Y.; Almodovar, J.; Hung, W.-S.; Wickramasinghe, S.R. Application of Zwitterions in Forward Osmosis: A Short Review. Polymers 2021, 13, 583. https://doi.org/10.3390/polym13040583
Chiao Y-H, Sengupta A, Ang MBMY, Chen S-T, Haan TY, Almodovar J, Hung W-S, Wickramasinghe SR. Application of Zwitterions in Forward Osmosis: A Short Review. Polymers. 2021; 13(4):583. https://doi.org/10.3390/polym13040583
Chicago/Turabian StyleChiao, Yu-Hsuan, Arijit Sengupta, Micah Belle Marie Yap Ang, Shu-Ting Chen, Teow Yeit Haan, Jorge Almodovar, Wei-Song Hung, and S. Ranil Wickramasinghe. 2021. "Application of Zwitterions in Forward Osmosis: A Short Review" Polymers 13, no. 4: 583. https://doi.org/10.3390/polym13040583
APA StyleChiao, Y.-H., Sengupta, A., Ang, M. B. M. Y., Chen, S.-T., Haan, T. Y., Almodovar, J., Hung, W.-S., & Wickramasinghe, S. R. (2021). Application of Zwitterions in Forward Osmosis: A Short Review. Polymers, 13(4), 583. https://doi.org/10.3390/polym13040583









