Development of Antimicrobial PLA Composites for Fused Filament Fabrication
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Feedstock and Filament Fabrication
2.3. Characterization Techniques
2.3.1. Chemical Structural Characterization
2.3.2. Thermal Characterization
2.3.3. Mechanical Characterization
2.4. Antimicrobial Efficacy
3. Results and Discussion
3.1. Chemical Structural Characterization
3.1.1. FTIR
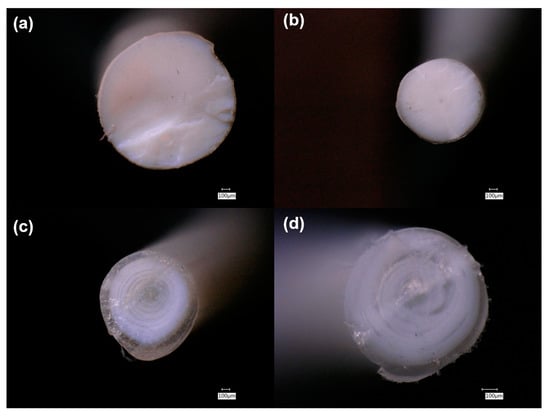
3.1.2. MXRF
3.2. Thermal Characterization
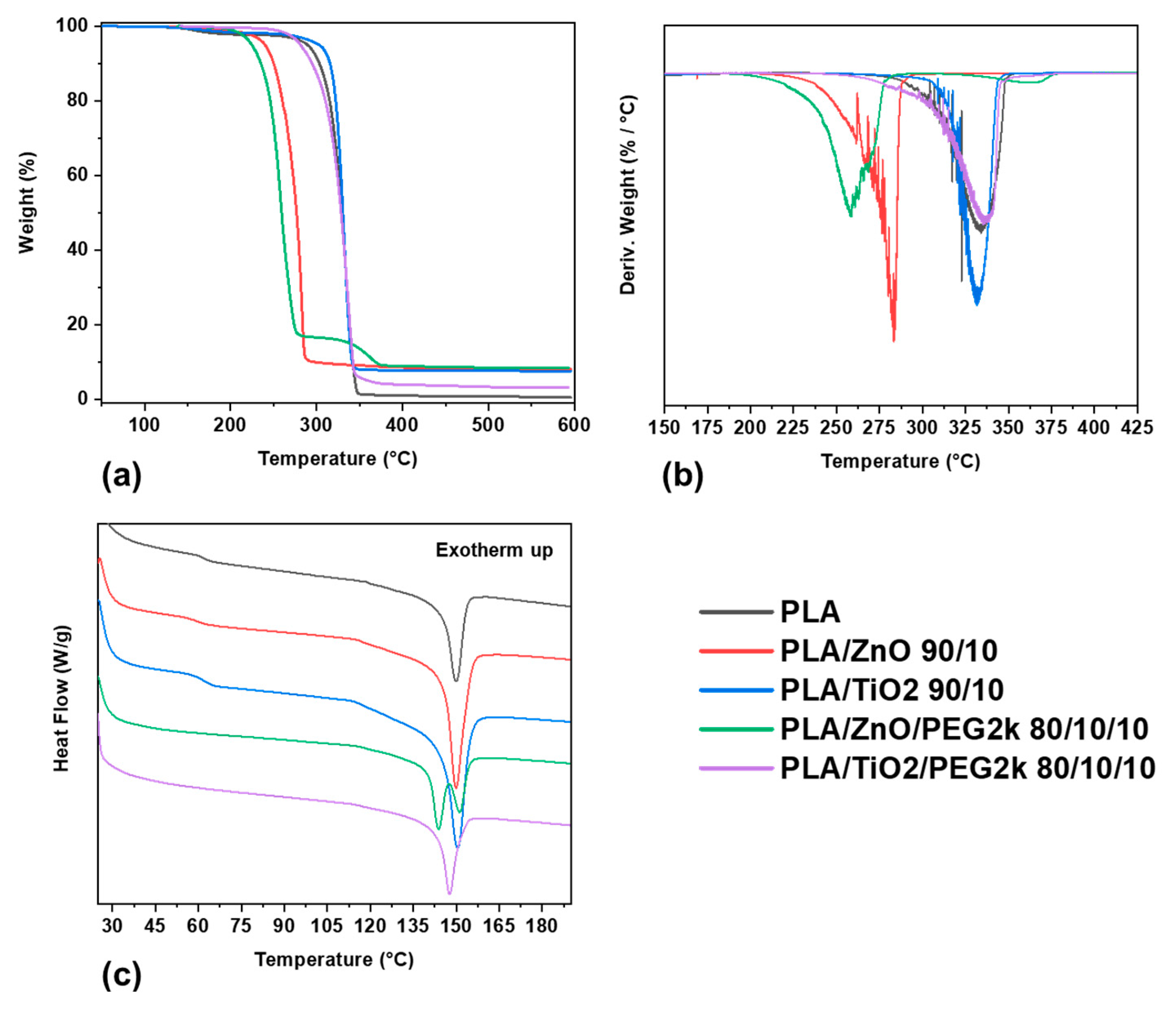
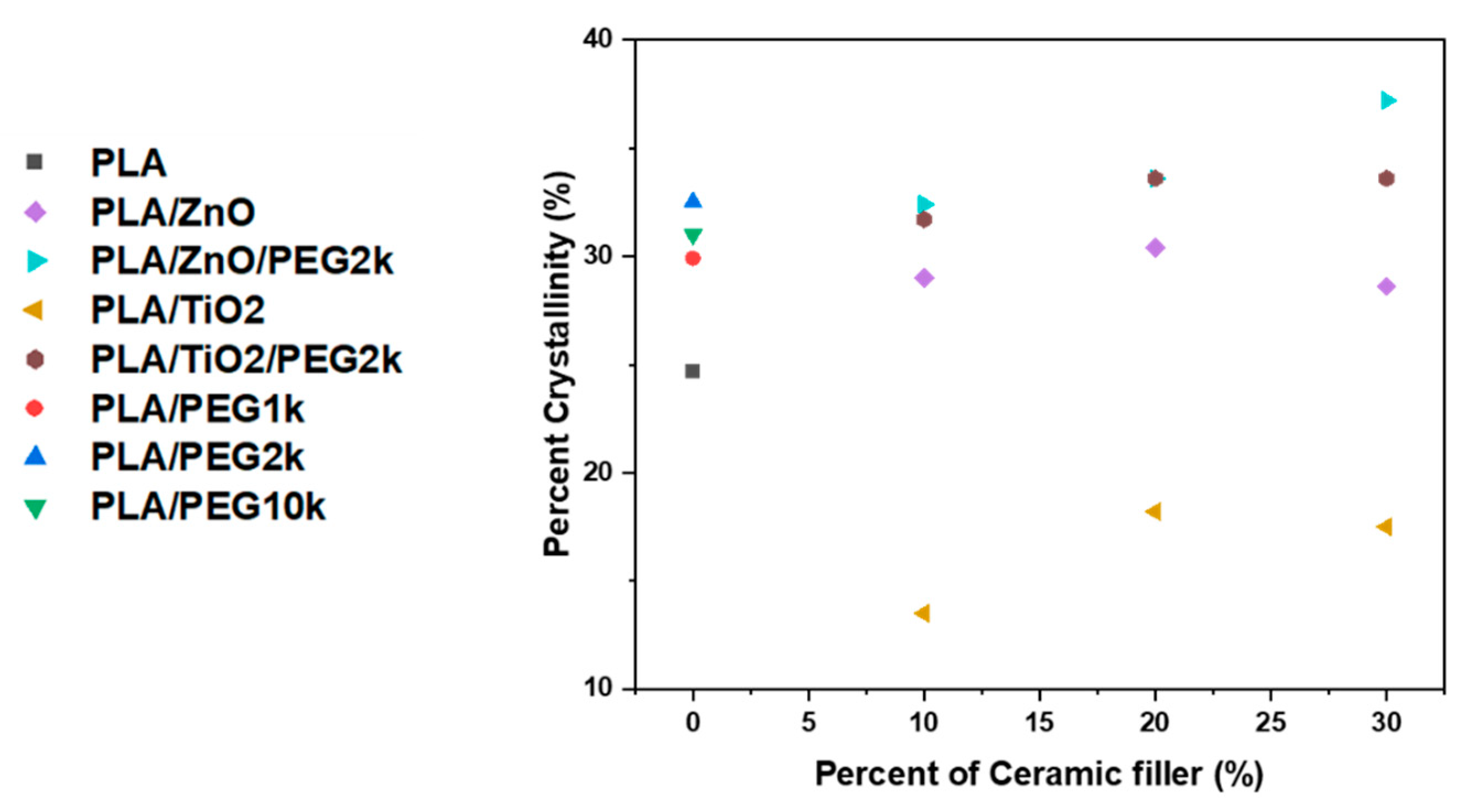
3.2.1. Thermal Phase Behavior
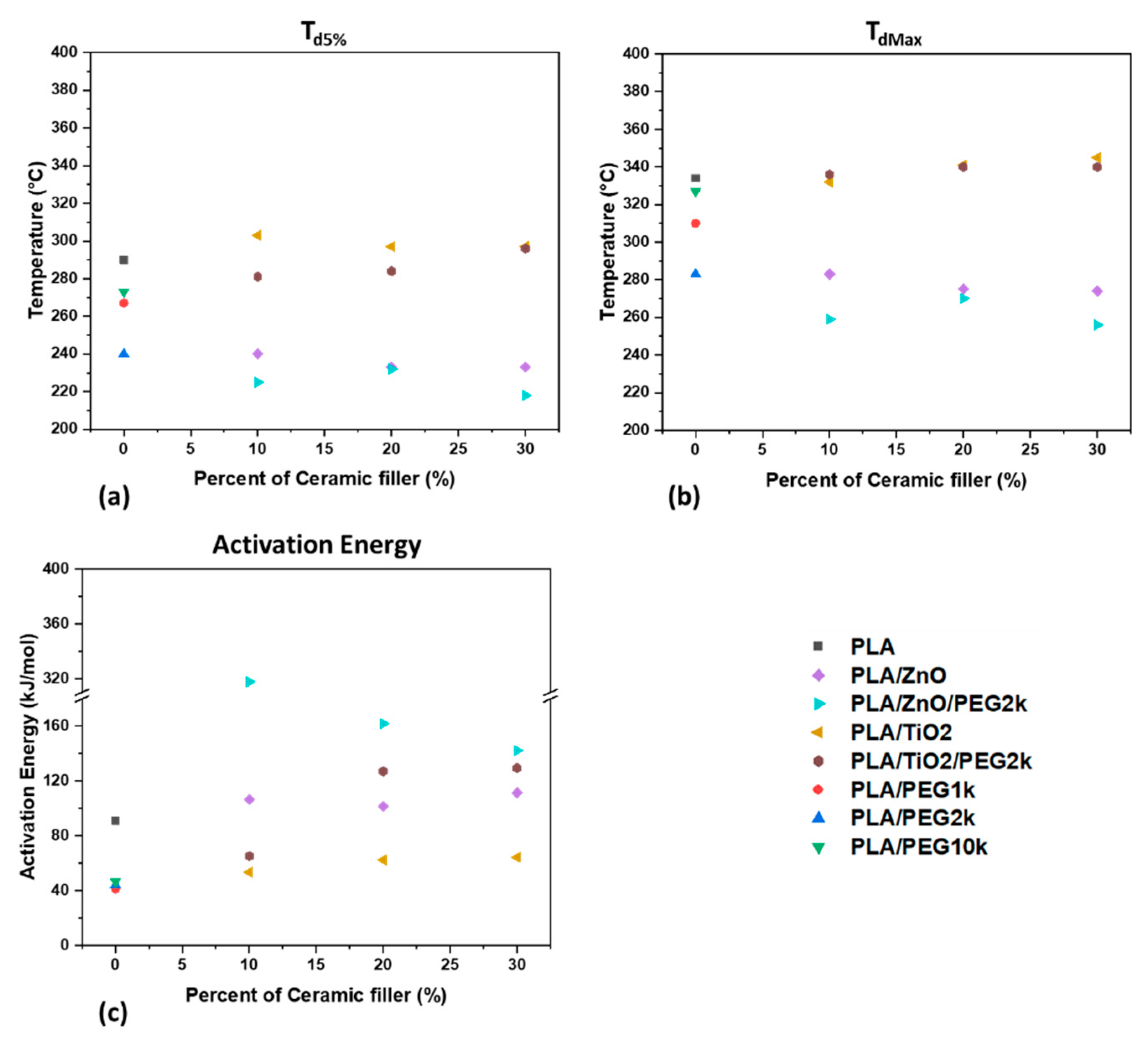
3.2.2. Thermal Stability
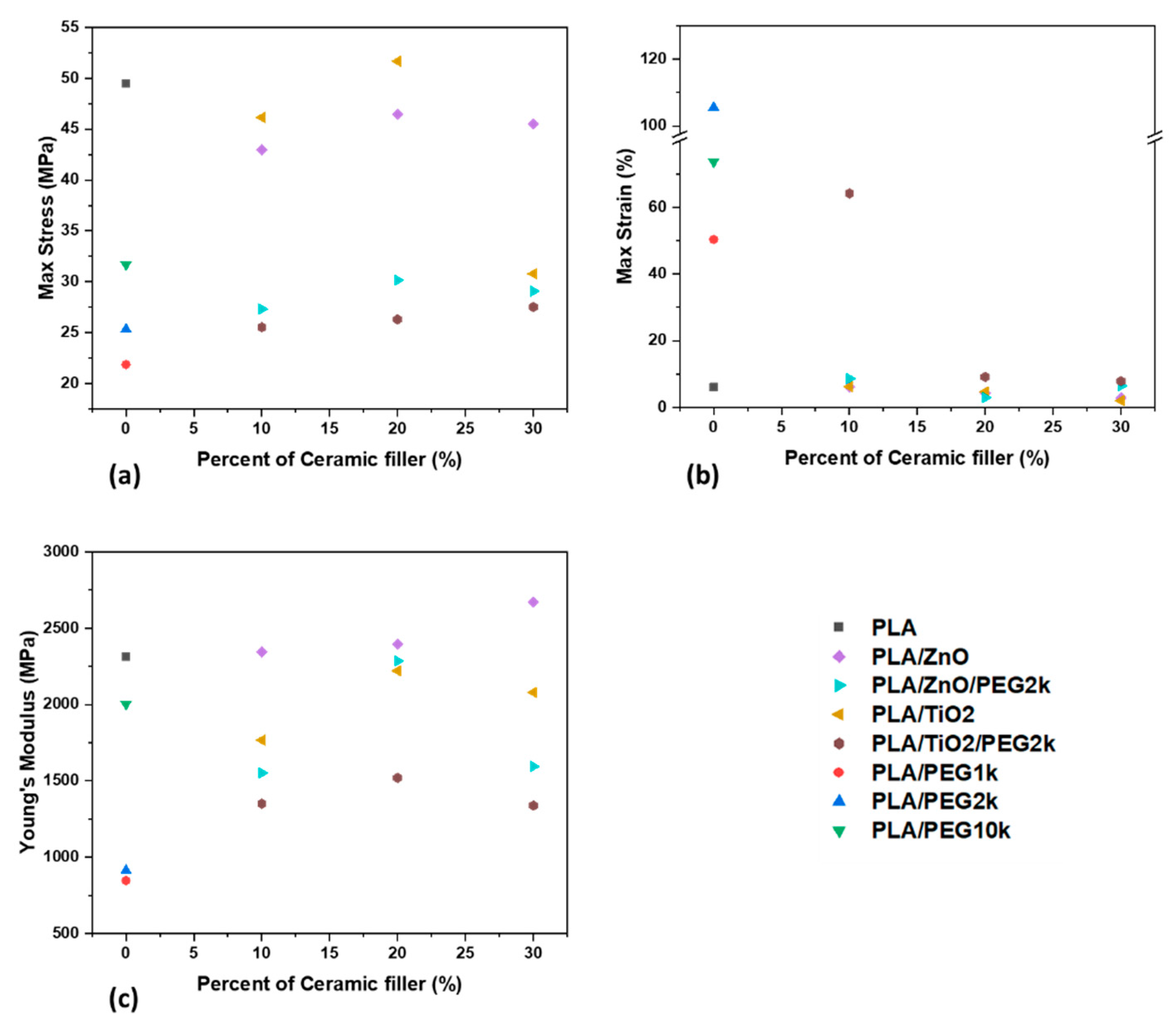
3.3. Mechaniacal Characterization
3.4. Antimicrobial Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Royte, E. Corn plastic to the rescue. Smithson. Mag. 2006, 37, 84–88. [Google Scholar]
- Avinc, O.; Khoddami, A. Overview of Poly(lactic acid) (PLA) Fibre. Fibre Chem. 2009, 41, 391–401. [Google Scholar] [CrossRef]
- Sawyer, D.J. Bioprocessing—No longer a field of dreams. In Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 2003. [Google Scholar]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Marino, A.; Nostro, A. Antimicrobial additives for poly(lactic acid) materials and their applications: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 7739–7756. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gramlich, W.M.; Gardner, D.J. Improving the impact strength of Poly(lactic acid) (PLA) in fused layer modeling (FLM). Polymer 2017, 114, 242–248. [Google Scholar] [CrossRef]
- Thanki, P.N.; Dellacherie, E.; Six, J. Surface characteristic of PLA and PLGA films. Appl. Surf. Sci. 2006, 253, 2758–2764. [Google Scholar]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current advances and future perspectives of 3D printing natural-derived biopolymers. Carbohydr. Polym. 2019, 207, 297–316. [Google Scholar] [CrossRef]
- Thompson, M.K.; Moroni, G.; Vaneker, T.; Fadel, G.; Campbell, R.I.; Gibson, I.; Bernard, A.; Schulz, J.; Graf, P.; Ahuja, B.; et al. Design for Additive Manufacturing: Trends, opportunities, considerations, and constraints. CIRP Ann. 2016, 65, 737–760. [Google Scholar] [CrossRef]
- Levy, G.N.; Schindel, R.; Kruth, J. Rapid manufacturing and rapid tooling with layer manufacturing (LM) technologies, state of the art and future perspectives. CIRP Ann. 2003, 52, 589–609. [Google Scholar] [CrossRef]
- Bayer, I.S. Thermomechanical Properties of Polylactic Acid-Graphene Composites: A State-of-the-Art Review for Biomedical Applications. Materials 2017, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiu, Z. Crystallization kinetics and morphology of biodegradable poly (l-lactic acid)/graphene oxide nano-composites: Influences of graphene oxide loading and crystallization temperature. Thermochim. Acta 2012, 527, 40–46. [Google Scholar]
- Kim, I.-H.; Jeong, Y.G. Polylactide/exfoliated graphite nanocomposites with enhanced thermal stability, mechanical modulus, and electrical conductivity. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 850–858. [Google Scholar] [CrossRef]
- Hassouna, F.; Raquez, J.-M.; Addiego, F.; Dubois, P.; Toniazzo, V.; Ruch, D. New approach on the development of plasticized polylactide (PLA): Grafting of poly (ethylene gly-col)(PEG) via reactive extrusion. Eur. Polym. J. 2011, 47, 2134–2144. [Google Scholar] [CrossRef]
- You, Y.; Lee, S.W.; Youk, J.H.; Min, B.-M.; Lee, S.J.; Park, W.H. In vitro degradation behaviour of non-porous ultra-fine poly(glycolic acid)/poly(l-lactic acid) fibres and porous ultra-fine poly(glycolic acid) fibres. Polym. Degrad. Stab. 2005, 90, 441–448. [Google Scholar] [CrossRef]
- Li, H.-Z.; Chen, S.-C.; Wang, Y.-Z. Thermoplastic PVA/PLA Blends with Improved Processability and Hydrophobicity. Ind. Eng. Chem. Res. 2014, 53, 17355–17361. [Google Scholar] [CrossRef]
- Grande, R.; Pessan, L.A.; Carvalho, A.J. Ternary melt blends of poly(lactic acid)/poly(vinyl alcohol)-chitosan. Ind. Crops Prod. 2015, 72, 159–165. [Google Scholar] [CrossRef]
- Bartczak, Z.; Galeski, A.; Kowalczuk, M.; Sobota, M.; Malinowski, R. Tough blends of poly (lactide) and amorphous poly ([R, S]-3-hydroxy butyrate)–morphology and prop-erties. Eur. Polym. J. 2013, 49, 3630–3641. [Google Scholar] [CrossRef]
- Dong, W.; Ma, P.; Wang, S.; Chen, M.; Cai, X.; Zhang, Y. Effect of partial crosslinking on morphology and properties of the poly(β-hydroxybutyrate)/poly(d,l-lactic acid) blends. Polym. Degrad. Stab. 2013, 98, 1549–1555. [Google Scholar] [CrossRef]
- Sedlarik, V.; Saha, N.; Sedlarikova, J.; Sáha, P. Biodegradation of Blown Films Based on Poly(lactic acid) under Natural Conditions. Macromol. Symp. 2008, 272, 100–103. [Google Scholar] [CrossRef]
- Mallick, S.; Ahmad, Z.; Touati, F.; Bhadra, J.; Shakoor, R.A.; Al-Thani, N.J. PLA-TiO2 nanocomposites: Thermal, morphological, structural, and humidity sensing properties. Ceram. Int. 2018, 44, 16507–16513. [Google Scholar] [CrossRef]
- Man, C.; Zhang, C.; Liu, Y.; Wang, W.; Ren, W.; Jiang, L.; Reisdorffer, F.; Nguyen, T.P.; Dan, Y. Poly (lactic acid)/titanium dioxide composites: Preparation and performance under ultraviolet irradiation. Polym. Degrad. Stab. 2012, 97, 856–862. [Google Scholar] [CrossRef]
- Segura González, E.A.; Olmos, D.; Lorente, M.Á.; Vélaz, I.; González-Benito, J. Preparation and characterization of polymer composite materials based on PLA/TiO2 for an-tibacterial packaging. Polymers 2018, 10, 1365. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef]
- Doumbia, A.S.; Vezin, H.; Ferreira, M.; Campagne, C.; Devaux, E. Studies of polylactide/zinc oxide nanocomposites: Influence of surface treatment on zinc oxide anti-bacterial activities in textile nanocomposites. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Ponnamma, D.; Cabibihan, J.-J.; Rajan, M.; Pethaiah, S.S.; Deshmukh, K.; Gogoi, J.P.; Pasha, S.K.; Ahamed, M.B.; Krishnegowda, J.; Chandrashekar, B.; et al. Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater. Sci. Eng. C 2019, 98, 1210–1240. [Google Scholar] [CrossRef]
- Marra, A.; Cimmino, S.; Silvestre, C. Effect of TiO2 and ZnO on PLA degradation in various media. Adv. Mater. Sci. 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Buerki-Thurnherr, T.; Xiao, L.; Diener, L.; Arslan, O.; Hirsch, C.; Maeder-Althaus, X.; Grieder, K.; Wampfler, B.; Mathur, S.; Wick, P.; et al. In vitro mechanistic study towards a better understanding of ZnO nanoparticle toxicity. Nanotoxicology 2013, 7, 402–416. [Google Scholar] [CrossRef]
- Pandurangan, M.; Kim, D.H. In vitro toxicity of zinc oxide nanoparticles: A review. J. Nanopart. Res. 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Zhu, X.; Chang, Y.; Chen, Y. Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere 2010, 78, 209–215. [Google Scholar] [CrossRef]
- Menard, A.; Drobne, D.; Jemec, A. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ. Pollut. 2011, 159, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Buzarovska, A. PLA Nanocomposites with Functionalized TiO2 Nanoparticles. Polym. Technol. Eng. 2013, 52, 280–286. [Google Scholar] [CrossRef]
- Wang, X.-J.; Huang, Z.; Wei, M.-Y.; Lu, T.; Nong, D.-D.; Zhao, J.-X.; Gao, X.-Y.; Teng, L.-J. Catalytic effect of nanosized ZnO and TiO2 on thermal degradation of poly(lactic acid) and isoconversional kinetic analysis. Thermochim. Acta 2019, 672, 14–24. [Google Scholar] [CrossRef]
- Temenoff, J.S.; Mikos, A.G. Biomaterials: The Intersection of Biology and Materials Science; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2008; Volume 1. [Google Scholar]
- Bijarimi, M.; Ahmad, S.; Rasid, R.; Khushairi, M.A.; Zakir, M. Poly (lactic acid)/Poly (ethylene glycol) blends: Mechanical, thermal and morphological properties. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016. [Google Scholar]
- Phan, Q.T.; Le, M.H.; Le, T.T.H.; Tran, T.H.H.; Xuan, P.N.; Ha, P.T. Characteristics and cytotoxicity of folate-modified curcumin-loaded PLA-PEG micellar nano systems with various PLA:PEG ratios. Int. J. Pharm. 2016, 507, 32–40. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, T.V.; Chowdhury, S.R.; Reddy, S.R. Compatibility confirmation and refinement of thermal and mechanical properties of poly (lactic acid)/poly (ethylene- co -glycidyl methacrylate) blend reinforced by hexagonal boron nitride. React. Funct. Polym. 2017, 117, 1–9. [Google Scholar] [CrossRef]
- Jia, S.; Yu, D.; Zhu, Y.; Wang, Z.; Chen, L.; Fu, L. Morphology, Crystallization and Thermal Behaviors of PLA-Based Composites: Wonderful Effects of Hybrid GO/PEG via Dynamic Impregnating. Polymers 2017, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Toncheva, A.; Mincheva, R.; Kancheva, M.; Manolova, N.; Rashkov, I.; Dubois, P.; Markova, N. Antibacterial PLA/PEG electrospun fibers: Comparative study between grafting and blending PEG. Eur. Polym. J. 2016, 75, 223–233. [Google Scholar] [CrossRef]
- Greenwald, R. PEG drugs: An overview. J. Control. Release 2001, 74, 159–171. [Google Scholar] [CrossRef]
- Brounstein, Z.; Talley, S.; Dumont, J.H.; Zhao, J.; Lee, K.-S.; Labouriau, A. Fused filament fabrication of polymer composites for extreme environments. J. Mater. Res. 2020, 35, 1493–1503. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nat. Cell Biol. 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Ebrahimi-Kahrizsangi, R.; Abbasi, M. Evaluation of reliability of Coats-Redfern method for kinetic analysis of non-isothermal TgA. Trans. Nonferrous Met. Soc. China 2008, 18, 217–221. [Google Scholar] [CrossRef]
- Brems, A.; Baeyens, J.; Beerlandt, J.; Dewil, R. Thermogravimetric pyrolysis of waste polyethylene-terephthalate and polystyrene: A critical assessment of kinetics modelling. Resour. Conserv. Recycl. 2011, 55, 772–781. [Google Scholar] [CrossRef]
- Gao, W.; Chen, K.; Xiang, Z.; Yang, F.; Zeng, J.; Li, J.; Yang, R.; Rao, G.; Tao, H. Kinetic study on pyrolysis of tobacco residues from the cigarette industry. Ind. Crops Prod. 2013, 44, 152–157. [Google Scholar] [CrossRef]
- Butbunchu, N.; Pathom-Aree, W. Actinobacteria as Promising Candidate for Polylactic Acid Type Bioplastic Degradation. Front. Microbiol. 2019, 10, 2834. [Google Scholar] [CrossRef] [PubMed]
- Popelka, Š.; Machová, L.; Rypáček, F. Adsorption of poly(ethylene oxide)–block–polylactide copolymers on polylactide as studied by ATR-FTIR spectroscopy. J. Colloid Interface Sci. 2007, 308, 291–299. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.B.; Yunus, W.M.Z.W.; Hussein, M.Z. Poly(lactic acid)/Poly(ethylene glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers 2013, 6, 93–104. [Google Scholar] [CrossRef]
- Jayaramudu, J.; Das, K.; Sonakshi, M.; Reddy, G.S.M.; Aderibigbe, B.; Sadiku, R.; Ray, S.S. Structure and properties of highly toughened biodegradable polylactide/ZnO biocomposite films. Int. J. Biol. Macromol. 2014, 64, 428–434. [Google Scholar] [CrossRef]
- Yuniarto, K.; Purwanto, Y.A.; Purwanto, S.; Welt, B.A.; Purwadaria, H.K.; Sunarti, T.C. Infrared and Raman studies on polylactide acid and polyethylene glycol-400 blend. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016. [Google Scholar]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly (lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Murariu, M.; Paint, Y.; Murariu, O.; Raquez, J.-M.; Bonnaud, L.; Dubois, P. Current progress in the production of PLA-ZnO nanocomposites: Beneficial effects of chain extender addition on key properties. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Lizundia, E.; Pérez-Álvarez, L.; Sáenz-Pérez, M.; Patrocinio, D.; Vilas, J.L.; León, L.M. Physical aging and mechanical performance of poly(l-lactide)/ZnO nanocomposites. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Nonato, R.; Mei, L.; Bonse, B.; Chinaglia, E.; Morales, A. Nanocomposites of PLA containing ZnO nanofibers made by solvent cast 3D printing: Production and characterization. Eur. Polym. J. 2019, 114, 271–278. [Google Scholar] [CrossRef]
- Carrion, F.; Sanes, J.; Bermúdez, M.-D. Influence of ZnO nanoparticle filler on the properties and wear resistance of pol-ycarbonate. Wear 2007, 262, 1504–1510. [Google Scholar] [CrossRef]
- Pantani, R.; Gorrasi, G.; Vigliotta, G.; Murariu, M.; Dubois, P. PLA-ZnO nanocomposite films: Water vapor barrier properties and specific end-use characteristics. Eur. Polym. J. 2013, 49, 3471–3482. [Google Scholar] [CrossRef]
- Brems, A.; Baeyens, J.; Vandecasteele, C.; Dewil, R. Polymeric Cracking of Waste Polyethylene Terephthalate to Chemicals and Energy. J. Air Waste Manag. Assoc. 2011, 61, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, A.K.; Mohanty, S.P.; Nayak, S.K. Effect of PEG on PLA/PEG blend and its nanocomposites: A study of thermo-mechanical and morphological characterization. Polym. Compos. 2013, 35, 283–293. [Google Scholar] [CrossRef]
- Abe, H.; Takahashi, N.; Kim, K.J.; Mochizuki, M.; Doi, Y. Thermal Degradation Processes of End-Capped Poly(l-lactide)s in the Presence and Absence of Residual Zinc Catalyst. Biomacromolecules 2004, 5, 1606–1614. [Google Scholar] [CrossRef]


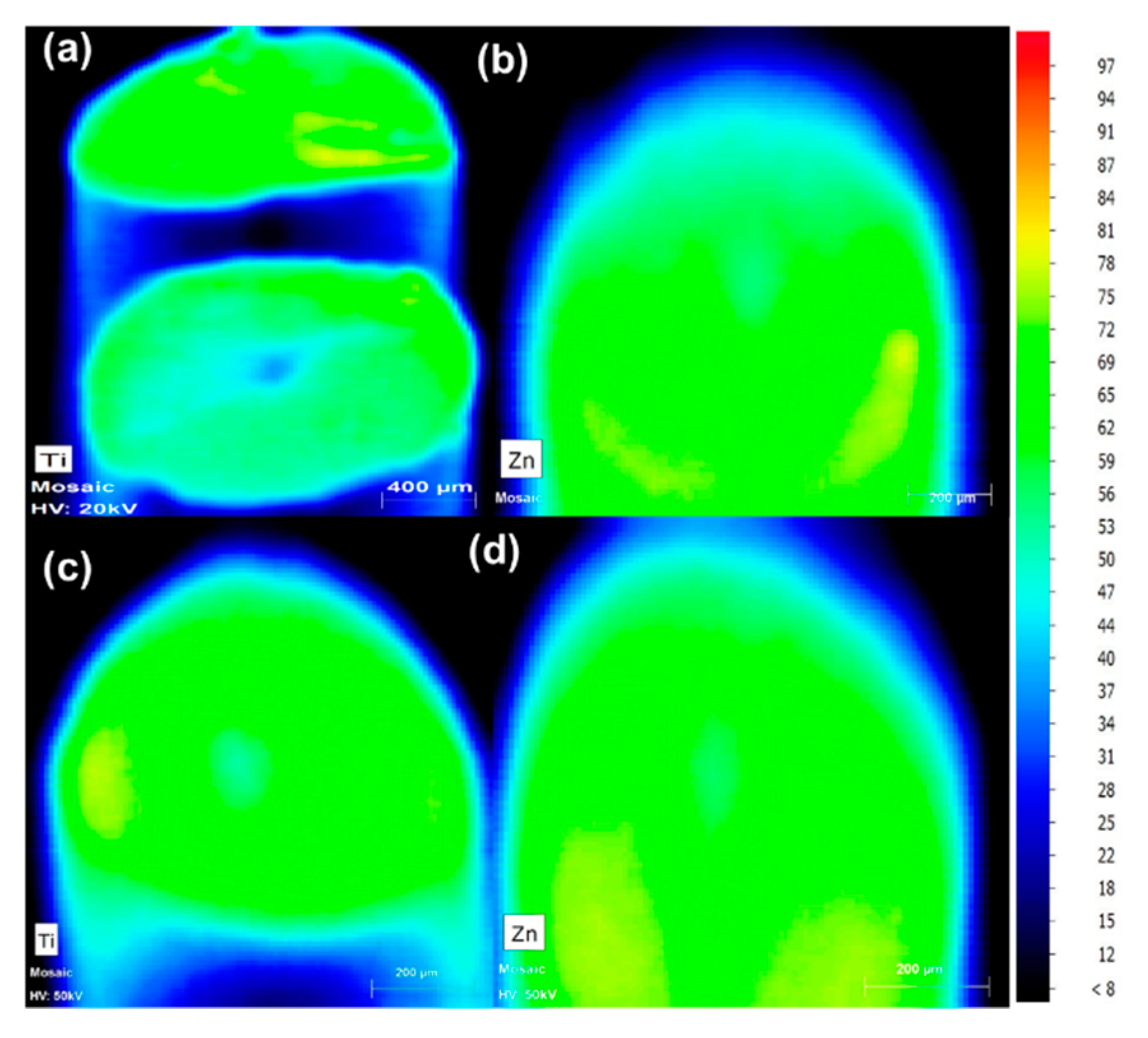

| Sample | Material |
|---|---|
| PLA | 100 wt% PLA |
| PLA/PEG1k 90/10 | 90 wt% PLA, 10 wt% PEG (MW, 1 kDa) |
| PLA/PEG2k 90/10 | 90 wt% PLA, 10 wt% PEG (MW, 2 kDa) |
| PLA/PEG10k 90/10 | 90 wt% PLA, 10 wt% PEG (MW, 10 kDa) |
| PLA/ZnO 90/10 | 90 wt% PLA, 10 wt% ZnO |
| PLA/ZnO 80/20 | 80 wt% PLA, 20 wt% ZnO |
| PLA/ZnO 70/30 | 70 wt% PLA, 30 wt% ZnO |
| PLA/ZnO/PEG2k 80/10/10 | 80 wt% PLA, 10 wt% ZnO, 10 wt% PEG (MW, 2 kDa) |
| PLA/ZnO/PEG2k 70/20/10 | 70 wt% PLA, 20 wt% ZnO, 10 wt% PEG (MW, 2 kDa) |
| PLA/ZnO/PEG2k 60/30/10 | 60 wt% PLA, 30 wt% ZnO, 10 wt% PEG (MW, 2 kDa) |
| PLA/TiO2 90/10 | 90 wt% PLA, 10 wt% TiO2 |
| PLA/TiO2 80/20 | 80 wt% PLA, 20 wt% TiO2 |
| PLA/TiO2 70/30 | 70 wt% PLA, 30 wt% TiO2 |
| PLA/TiO2/PEG2k 80/10/10 | 80 wt% PLA, 10 wt% TiO2, 10 wt% PEG (MW, 2 kDa) |
| PLA/TiO2/PEG2k 70/20/10 | 70 wt% PLA, 20 wt% TiO2, 10 wt% PEG (MW, 2 kDa) |
| PLA/TiO2/PEG2k 60/30/10 | 60 wt% PLA, 30 wt% TiO2, 10 wt% PEG (MW, 2 kDa) |
| Peak Number | Wavenumber (cm−1) | Vibrational Mode |
|---|---|---|
| 1 | 1080, 1187 | C-O stretching |
| 2 | 1361 | Symmetric -CH3 bending |
| 3 | 1452 | Asymmetric -CH3 bending |
| 4 | 1746 | C = O stretching |
| 5 | 2946 | Asymmetric -CH3 stretching |
| 6 | 2995 | Symmetric -CH3 stretching |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brounstein, Z.; Yeager, C.M.; Labouriau, A. Development of Antimicrobial PLA Composites for Fused Filament Fabrication. Polymers 2021, 13, 580. https://doi.org/10.3390/polym13040580
Brounstein Z, Yeager CM, Labouriau A. Development of Antimicrobial PLA Composites for Fused Filament Fabrication. Polymers. 2021; 13(4):580. https://doi.org/10.3390/polym13040580
Chicago/Turabian StyleBrounstein, Zachary, Chris M. Yeager, and Andrea Labouriau. 2021. "Development of Antimicrobial PLA Composites for Fused Filament Fabrication" Polymers 13, no. 4: 580. https://doi.org/10.3390/polym13040580
APA StyleBrounstein, Z., Yeager, C. M., & Labouriau, A. (2021). Development of Antimicrobial PLA Composites for Fused Filament Fabrication. Polymers, 13(4), 580. https://doi.org/10.3390/polym13040580