Effects of Two Melt Extrusion Based Additive Manufacturing Technologies and Common Sterilization Methods on the Properties of a Medical Grade PLGA Copolymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Filament and Scaffold Fabrication
2.3. Scaffold Sterilization
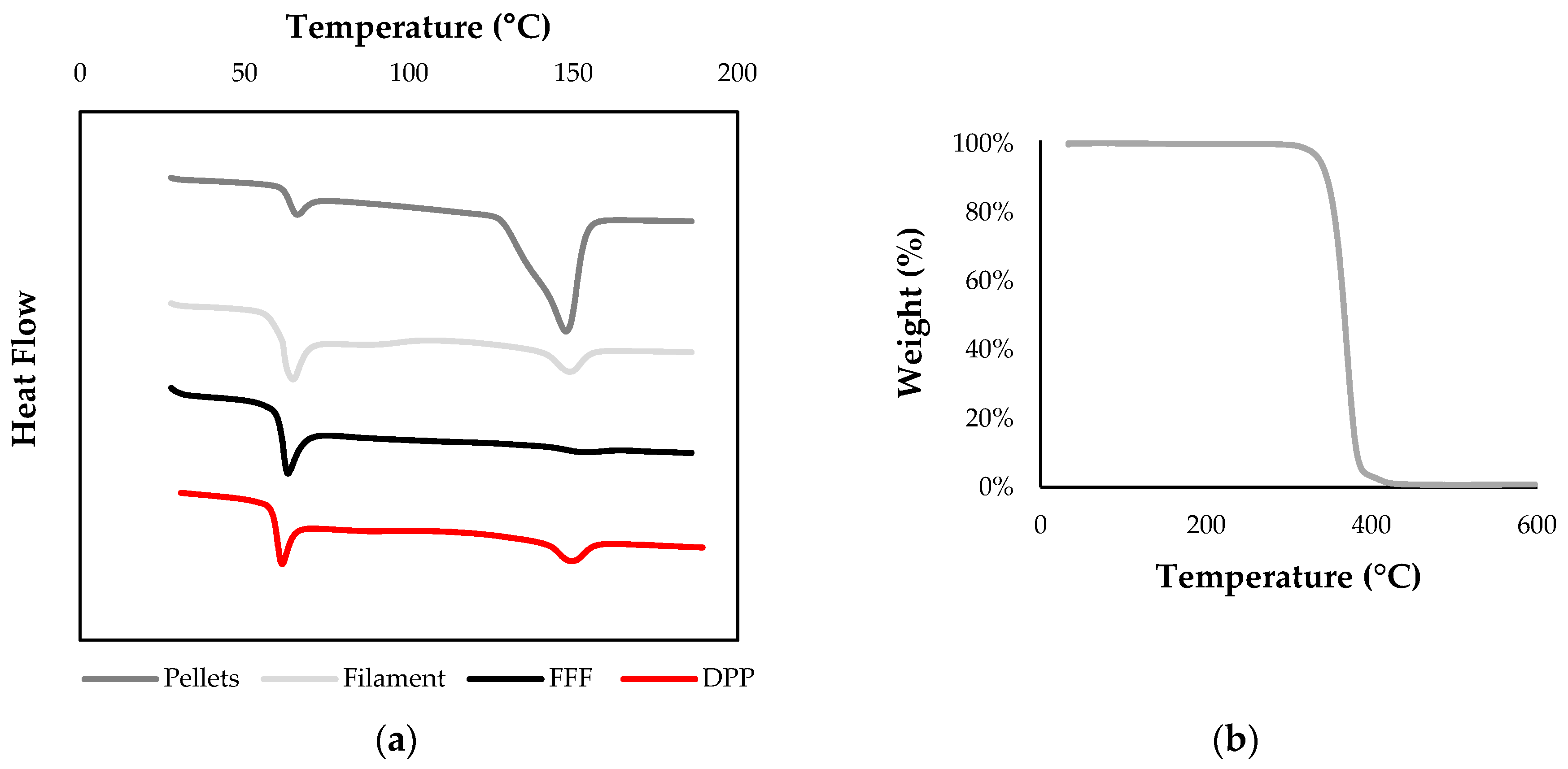
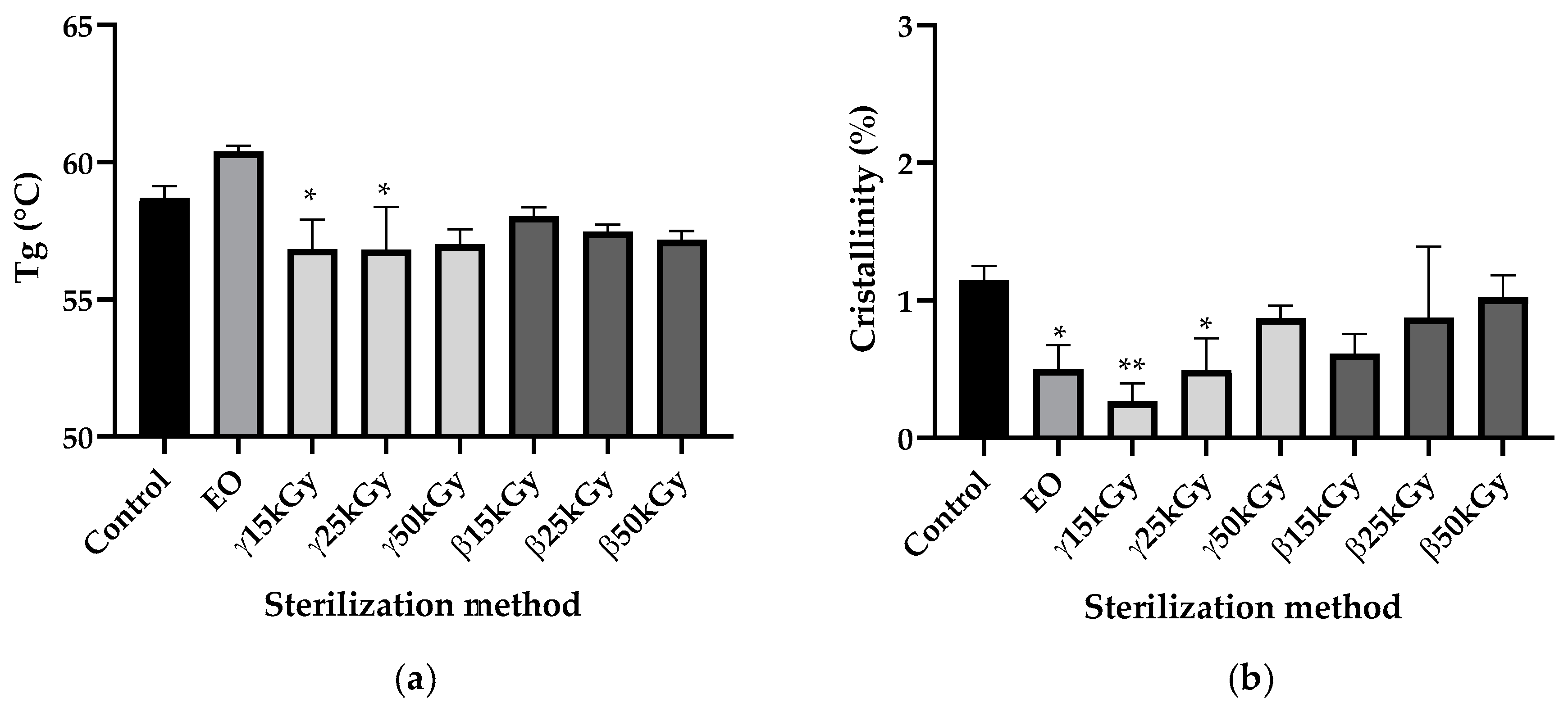
2.4. DSC
2.5. TGA
2.6. Gel Permeation Chromatography (GPC)
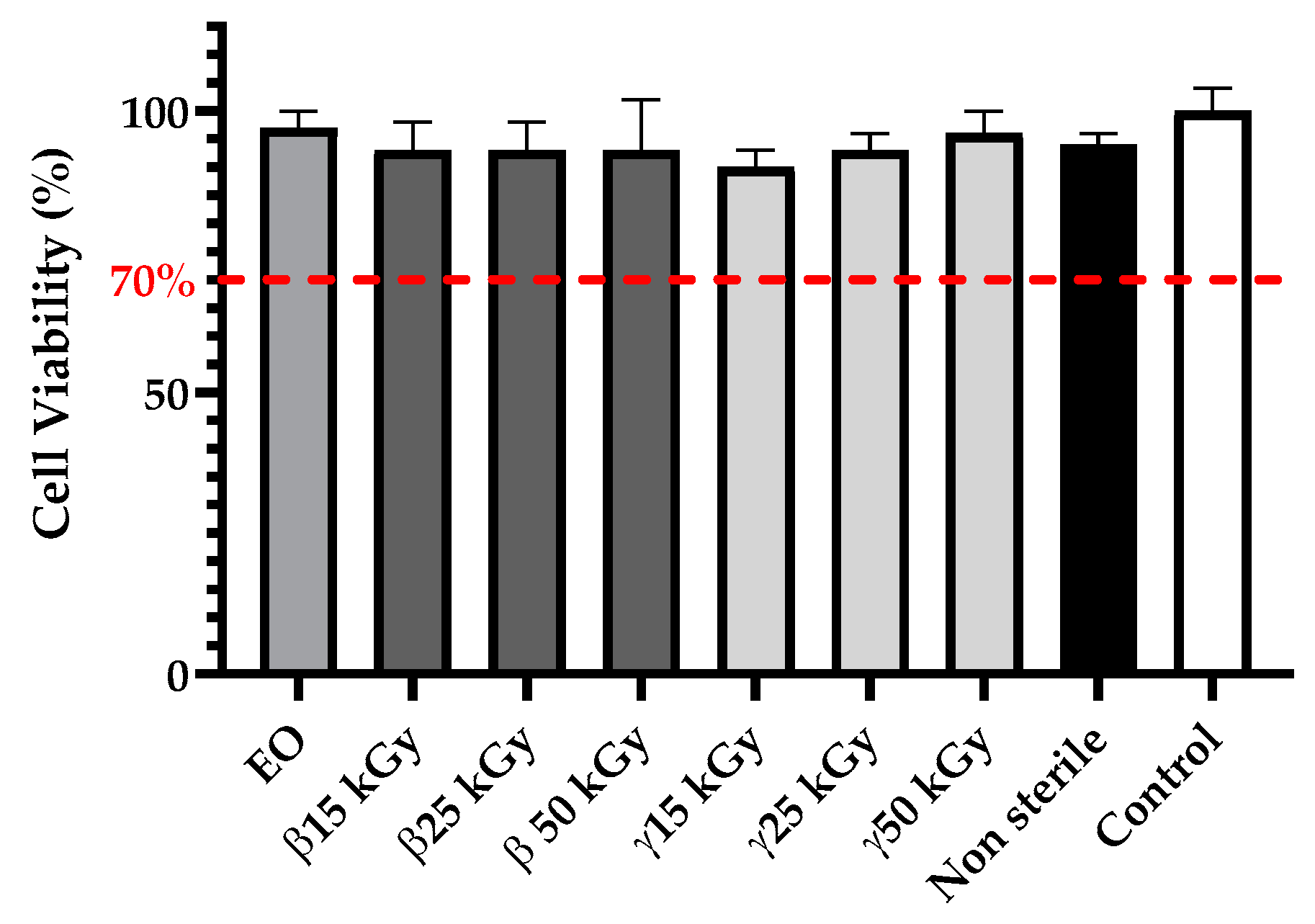
2.7. Cytotoxicity Assay
3. Results
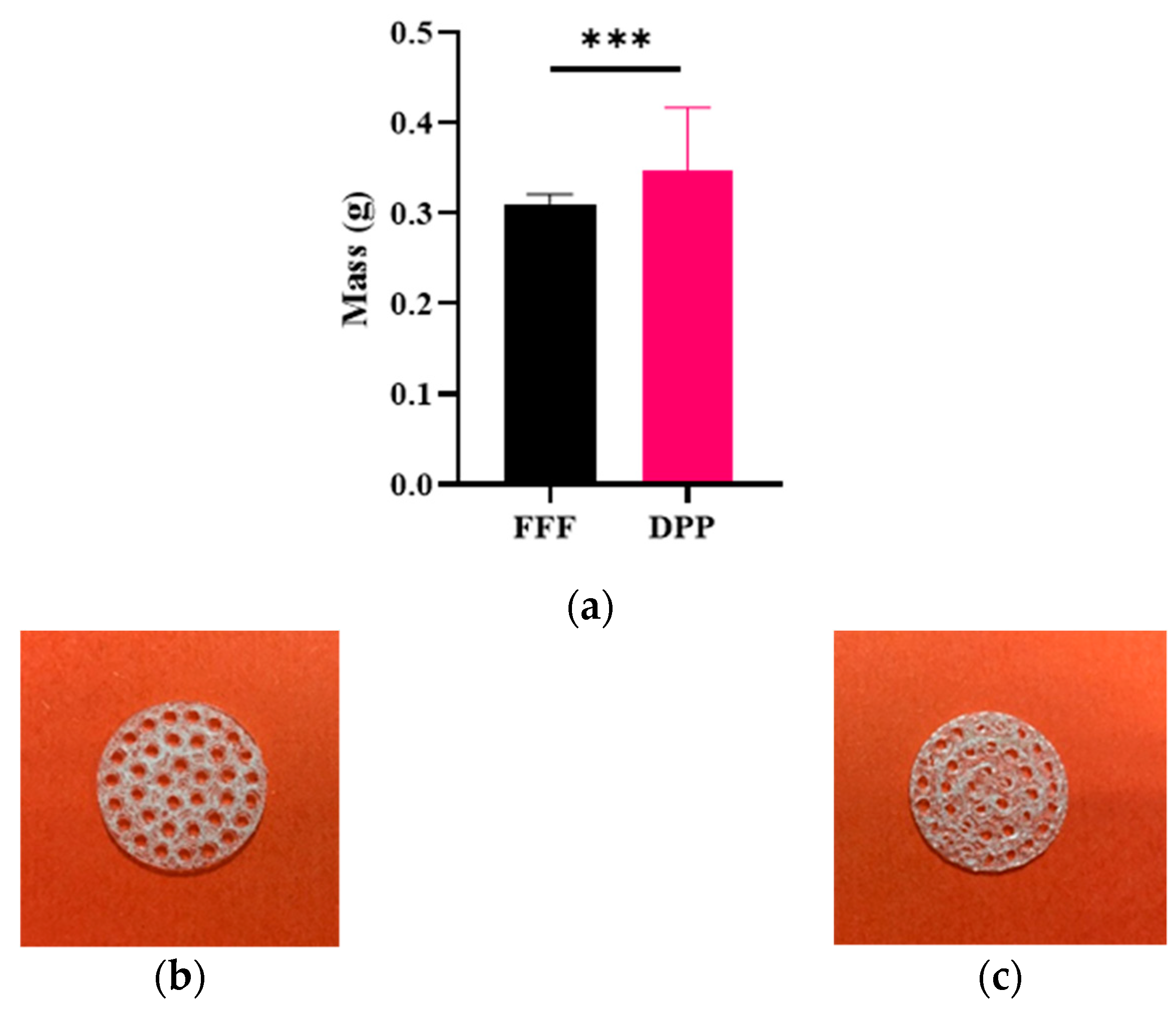
3.1. Repetability and Printing Quality
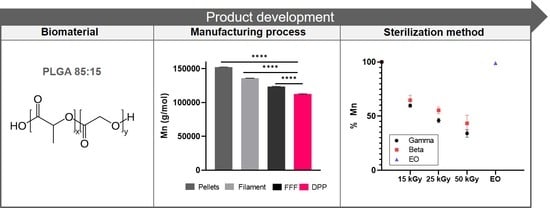
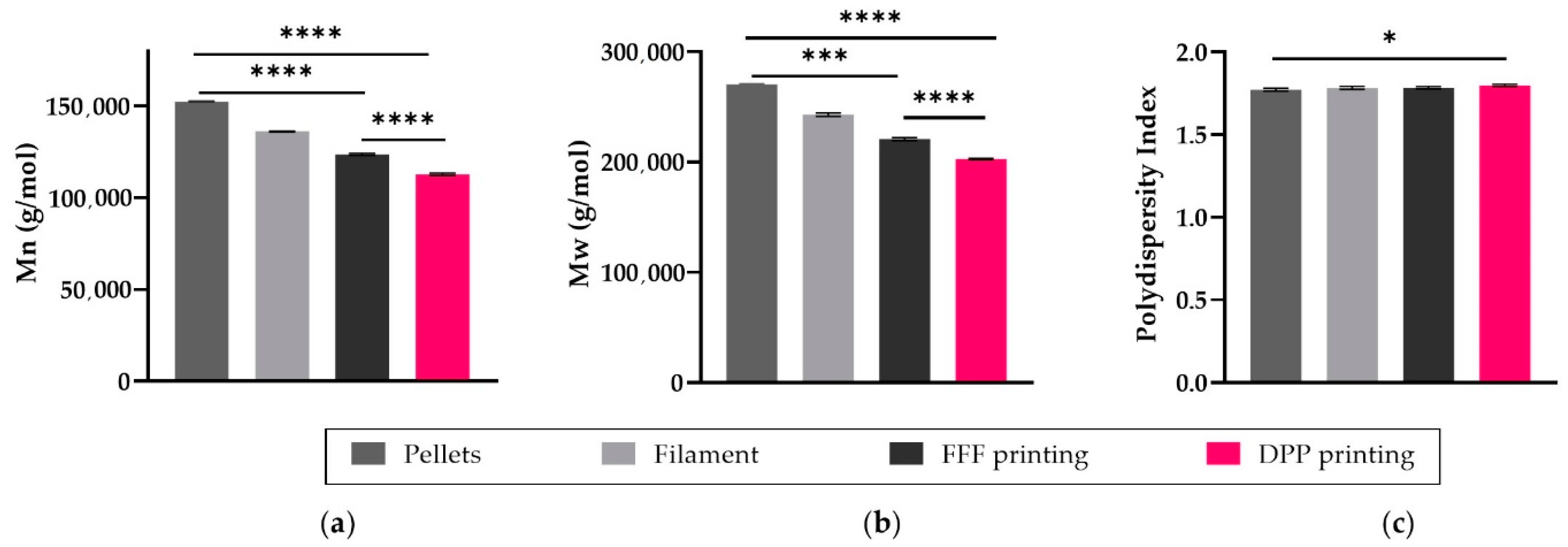
3.2. Impact of the Manufacturing Process
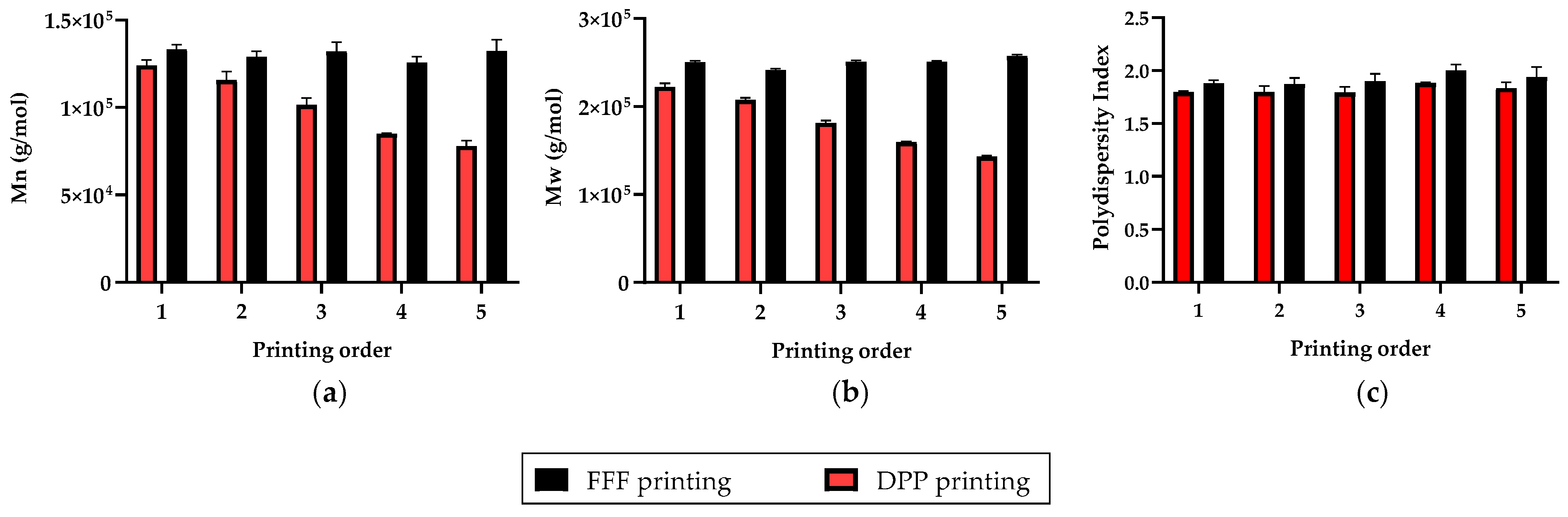
3.2.1. Molecular Weight
3.2.2. Thermal Analysis
3.3. Impact of Sterilization
3.4. Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franchetti, M.; Kress, C. An Economic Analysis Comparing the Cost Feasibility of Replacing Injection Molding Processes with Emerging Additive Manufacturing Techniques. Int. J. Adv. Manuf. Technol. 2017, 88, 2573–2579. [Google Scholar] [CrossRef]
- Nikoubashman, O.; Heringer, S.; Feher, K.; Brockmann, M.-A.; Sellhaus, B.; Dreser, A.; Kurtenbach, K.; Pjontek, R.; Jockenhövel, S.; Weis, J.; et al. Development of a Polymer-Based Biodegradable Neurovascular Stent Prototype: A Preliminary In Vitro and In Vivo Study. Macromol. Biosci. 2018, 18, 1700292. [Google Scholar] [CrossRef]
- Liao, L.; Peng, C.; Li, S.; Lu, Z.; Fan, Z. Evaluation of Bioresorbable Polymers as Potential Stent Material—In Vivo Degradation Behavior and Histocompatibility. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- de Melo, L.P.; Salmoria, G.V.; Fancello, E.A.; de Mello Roesler, C.R. Effect of Injection Molding Melt Temperatures on PLGA Craniofacial Plate Properties during In Vitro Degradation. Int. J. Biomater. 2017, 2017, 1256537. [Google Scholar] [CrossRef]
- Maurus, P.B.; Kaeding, C.C. Bioabsorbable Implant Material Review. Oper. Tech. Sports Med. 2004, 12, 158–160. [Google Scholar] [CrossRef]
- Konan, S.; Haddad, F.S. A Clinical Review of Bioabsorbable Interference Screws and Their Adverse Effects in Anterior Cruciate Ligament Reconstruction Surgery. Knee 2009, 16, 6–13. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and Safety of PLA and Its Copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Mohseni, M.; Hutmacher, D.W.; Castro, N.J. Independent Evaluation of Medical-Grade Bioresorbable Filaments for Fused Deposition Modelling/Fused Filament Fabrication of Tissue Engineered Constructs. Polymers 2018, 10, 40. [Google Scholar] [CrossRef]
- Prabhu, B.; Karau, A.; Wood, A.; Dadsetan, M.; Liedtke, H.; DeWitt, T. Bioresorbable Materials for Orthopedic Applications (Lactide and Glycolide Based). In Orthopedic Biomaterials: Progress in Biology, Manufacturing, and Industry Perspectives; Li, B., Webster, T., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 287–344. ISBN 978-3-319-89542-0. [Google Scholar]
- Gunatillake, P.A.; Adhikari, R. Biodegradable Synthetic Polymers for Tissue Engineering. Eur. Cell Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Chye Joachim Loo, S.; Ping Ooi, C.; Chiang Freddy Boey, Y. Influence of Electron-Beam Radiation on the Hydrolytic Degradation Behaviour of Poly(Lactide-Co-Glycolide) (PLGA). Biomaterials 2005, 26, 3809–3817. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic Degradation and Erosion of Polyester Biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef]
- Ahlinder, A.; Fuoco, T.; Finne-Wistrand, A. Medical Grade Polylactide, Copolyesters and Polydioxanone: Rheological Properties and Melt Stability. Polym. Test. 2018, 72, 214–222. [Google Scholar] [CrossRef]
- Ahlinder, A.; Fuoco, T.; Morales-López, Á.; Yassin, M.A.; Mustafa, K.; Finne-Wistrand, A. Nondegradative Additive Manufacturing of Medical Grade Copolyesters of High Molecular Weight and with Varied Elastic Response. J. Appl. Polym. Sci. 2020, 137, 48550. [Google Scholar] [CrossRef]
- Jain, S.; Fuoco, T.; Yassin, M.A.; Mustafa, K.; Wistrand, A.F. Printability and Critical Insight into Polymer Properties during Direct- Extrusion Based 3D Printing of Medical Grade Polylactide and Copolyesters. Biomacromolecules 2019, 21, 388–396. [Google Scholar] [CrossRef]
- Sterilization of Implantable Polymer-Based Medical Devices: A Review. ScienceDirect. Available online: https://www-sciencedirect-com.ressources-electroniques.univ-lille.fr/science/article/pii/S0378517317311304?via%3Dihub (accessed on 30 July 2019).
- Sterilization Techniques for Biodegradable Scaffolds in Tissue Engineering Applications. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4874054/ (accessed on 30 July 2019).
- Holy, C.E.; Cheng, C.; Davies, J.E.; Shoichet, M.S. Optimizing the Sterilization of PLGA Scaffolds for Use in Tissue Engineering. Biomaterials 2000, 22, 25–31. [Google Scholar] [CrossRef]
- Haim Zada, M.; Kumar, A.; Elmalak, O.; Mechrez, G.; Domb, A.J. Effect of Ethylene Oxide and Gamma (γ-) Sterilization on the Properties of a PLCL Polymer Material in Balloon Implants. ACS Omega 2019, 4, 21319–21326. [Google Scholar] [CrossRef]
- Pietrzak, W.S. Effects of Ethylene Oxide Sterilization on 82: 18 PLLA/PGA Copolymer Craniofacial Fixation Plates. J. Craniofac. Surg. 2010, 21, 177–181. [Google Scholar] [CrossRef]
- Phillip, E.; Murthy, N.S.; Bolikal, D.; Narayanan, P.; Kohn, J.; Lavelle, L.; Bodnar, S.; Pricer, K. Ethylene Oxide’s Role as a Reactive Agent during Sterilization: Effects of Polymer Composition and Device Architecture. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101B, 532–540. [Google Scholar] [CrossRef]
- Ethylene Oxide Sterilization of Medical Devices: A Review. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0196655307000521 (accessed on 18 February 2020).
- Ethylene Oxide Potential Toxicity: Expert Review of Medical Devices: Vol 5, No 3. Available online: https://www.tandfonline.com/doi/abs/10.1586/17434440.5.3.323 (accessed on 18 February 2020).
- Lucas, A.D.; Merritt, K.; Hitchins, V.M.; Woods, T.O.; McNamee, S.G.; Lyle, D.B.; Brown, S.A. Residual Ethylene Oxide in Medical Devices and Device Material. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 66, 548–552. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/jbm.b.10036 (accessed on 18 February 2020).
- Kinetics of the Aeration of Ethylene-Oxide Sterilized Plastics. Available online: https://www.sciencedirect.com/science/article/pii/0142961280900381 (accessed on 18 February 2020).
- Aeration of Ethylene Oxide-Sterilized Polymers. Available online: https://www.sciencedirect.com/science/article/pii/0142961286901080 (accessed on 18 February 2020).
- FDA. Ethylene Oxide Sterilization for Medical Devices. Available online: https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/ethylene-oxide-sterilization-medical-devices (accessed on 18 February 2020).
- Loo, S.C.J.; Ooi, C.P.; Boey, Y.C.F. Radiation Effects on Poly (Lactide-Co-Glycolide) (PLGA) and Poly(l-Lactide) (PLLA). Polym. Degrad. Stab. 2004, 83, 259–265. [Google Scholar] [CrossRef]
- Davison, L.; Themistou, E.; Buchanan, F.; Cunningham, E. Low Temperature Gamma Sterilization of a Bioresorbable Polymer, PLGA. Radiat. Phys. Chem. 2018, 143, 27–32. [Google Scholar] [CrossRef]
- Montanari, L.; Cilurzo, F.; Selmin, F.; Conti, B.; Genta, I.; Poletti, G.; Orsini, F.; Valvo, L. Poly (Lactide-Co-Glycolide) Microspheres Containing Bupivacaine: Comparison between Gamma and Beta Irradiation Effects. J. Control. Release 2003, 90, 281–290. [Google Scholar] [CrossRef]
- Cingolani, A.; Casalini, T.; Caimi, S.; Klaue, A.; Sponchioni, M.; Rossi, F.; Perale, G. A Methodologic Approach for the Selection of Bio-Resorbable Polymers in the Development of Medical Devices: The Case of Poly(l-Lactide-Co-ε-Caprolactone). Polymers 2018, 10, 851. [Google Scholar] [CrossRef]
- Tsuji, H.; Mizuno, A.; Ikada, Y. Properties and Morphology of Poly(L-lactide). III. Effects of Initial Crystallinity on Long-term in Vitro Hydrolysis of High Molecular Weight Poly(L-lactide) Film in Phosphate-buffered Solution. J. Appl. Polym. Sci. 2000, 77, 1452–1464. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/1097-4628%2820000815%2977%3A7%3C1452%3A%3AAID-APP7%3E3.0.CO%3B2-S (accessed on 12 May 2020).
- Widmer, M.S.; Gupta, P.K.; Lu, L.; Meszlenyi, R.K.; Evans, G.R.D.; Brandt, K.; Savel, T.; Gurlek, A.; Patrick, C.W.; Mikos, A.G. Manufacture of Porous Biodegradable Polymer Conduits by an Extrusion Process for Guided Tissue Regeneration. Biomaterials 1998, 19, 1945–1955. [Google Scholar] [CrossRef]
- Shim, J.-H.; Kim, J.Y.; Park, J.K.; Hahn, S.K.; Rhie, J.-W.; Kang, S.-W.; Lee, S.-H.; Cho, D.-W. Effect of Thermal Degradation of SFF-Based PLGA Scaffolds Fabricated Using a Multi-Head Deposition System Followed by Change of Cell Growth Rate. J. Biomater. Sci. Polym. Ed. 2010, 21, 1069–1080. [Google Scholar] [CrossRef]
- Ragaert, K.; Cardon, L.; Dekeyser, A.; Degrieck, J. Machine Design and Processing Considerations for the 3D Plotting of Thermoplastic Scaffolds. Biofabrication 2010, 2, 014107. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Soufivand, A.A.; Abolfathi, N.; Hashemi, A.; Lee, S.J. The Effect of 3D Printing on the Morphological and Mechanical Properties of Polycaprolactone Filament and Scaffold. Polym. Adv. Technol. 2020, 31, 1038–1046. [Google Scholar] [CrossRef]
- Peltola, S.M.; Melchels, F.P.W.; Grijpma, D.W.; Kellomäki, M. A Review of Rapid Prototyping Techniques for Tissue Engineering Purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef]
- Faisant, N.; Siepmann, J.; Richard, J.; Benoit, J.P. Mathematical Modeling of Drug Release from Bioerodible Microparticles: Effect of Gamma-Irradiation. Eur. J. Pharm. Biopharm. 2003, 56, 271–279. [Google Scholar] [CrossRef]
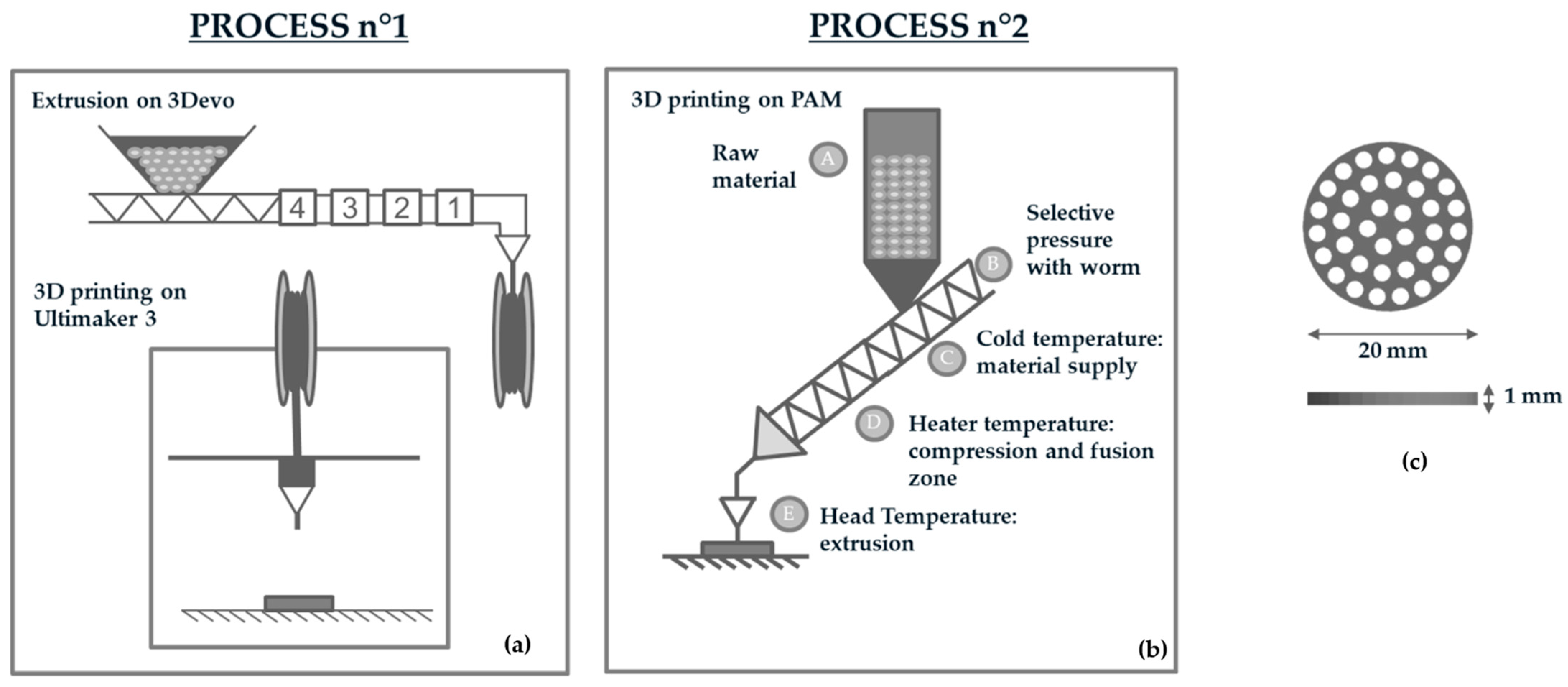

| PROCESS n°1 | |||||||
| Filament Extrusion Parameters | Zone 1 Temperature (°C) | Zone 2 Temperature (°C) | Zone 3 Temperature (°C) | Zone 4 Temperature (°C) | Extrusion speed (rpm) | ||
| 205 °C | 210 °C | 210 °C | 200 °C | 5 rpm | |||
| Printing Profile on FFF Printer | Printing Temperature | Bed Temperature | Printing speed | Flow (%) | |||
| 200 °C | 65 °C | 60 mm/s | 100% | ||||
| PROCESS n°2 | |||||||
| Printing Profile on DPP Printer | Cold Temperature (°C) | Extruder Temperature (D) | Head Temperature (E) | Bed Temperature | Printing speed | Flow | |
| 65 °C | 170 °C | 210 °C | 65 °C | 10 mm/s | 100% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradwohl, M.; Chai, F.; Payen, J.; Guerreschi, P.; Marchetti, P.; Blanchemain, N. Effects of Two Melt Extrusion Based Additive Manufacturing Technologies and Common Sterilization Methods on the Properties of a Medical Grade PLGA Copolymer. Polymers 2021, 13, 572. https://doi.org/10.3390/polym13040572
Gradwohl M, Chai F, Payen J, Guerreschi P, Marchetti P, Blanchemain N. Effects of Two Melt Extrusion Based Additive Manufacturing Technologies and Common Sterilization Methods on the Properties of a Medical Grade PLGA Copolymer. Polymers. 2021; 13(4):572. https://doi.org/10.3390/polym13040572
Chicago/Turabian StyleGradwohl, Marion, Feng Chai, Julien Payen, Pierre Guerreschi, Philippe Marchetti, and Nicolas Blanchemain. 2021. "Effects of Two Melt Extrusion Based Additive Manufacturing Technologies and Common Sterilization Methods on the Properties of a Medical Grade PLGA Copolymer" Polymers 13, no. 4: 572. https://doi.org/10.3390/polym13040572
APA StyleGradwohl, M., Chai, F., Payen, J., Guerreschi, P., Marchetti, P., & Blanchemain, N. (2021). Effects of Two Melt Extrusion Based Additive Manufacturing Technologies and Common Sterilization Methods on the Properties of a Medical Grade PLGA Copolymer. Polymers, 13(4), 572. https://doi.org/10.3390/polym13040572