Solution Blowing Spinning Technology towards Green Development of Urea Sensor Nanofibers Immobilized with Hydrazone Probe
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Cellulose Nanofibers (CNF)
2.3. Preparation of Colorimetric Cellulose Nanofibers (CNF-TCFH) Sensor
2.4. Methods
2.5. Urea Sensing
3. Results and Discussion
3.1. Preparation of Cellulose Nanofibers Biosensor for Urea
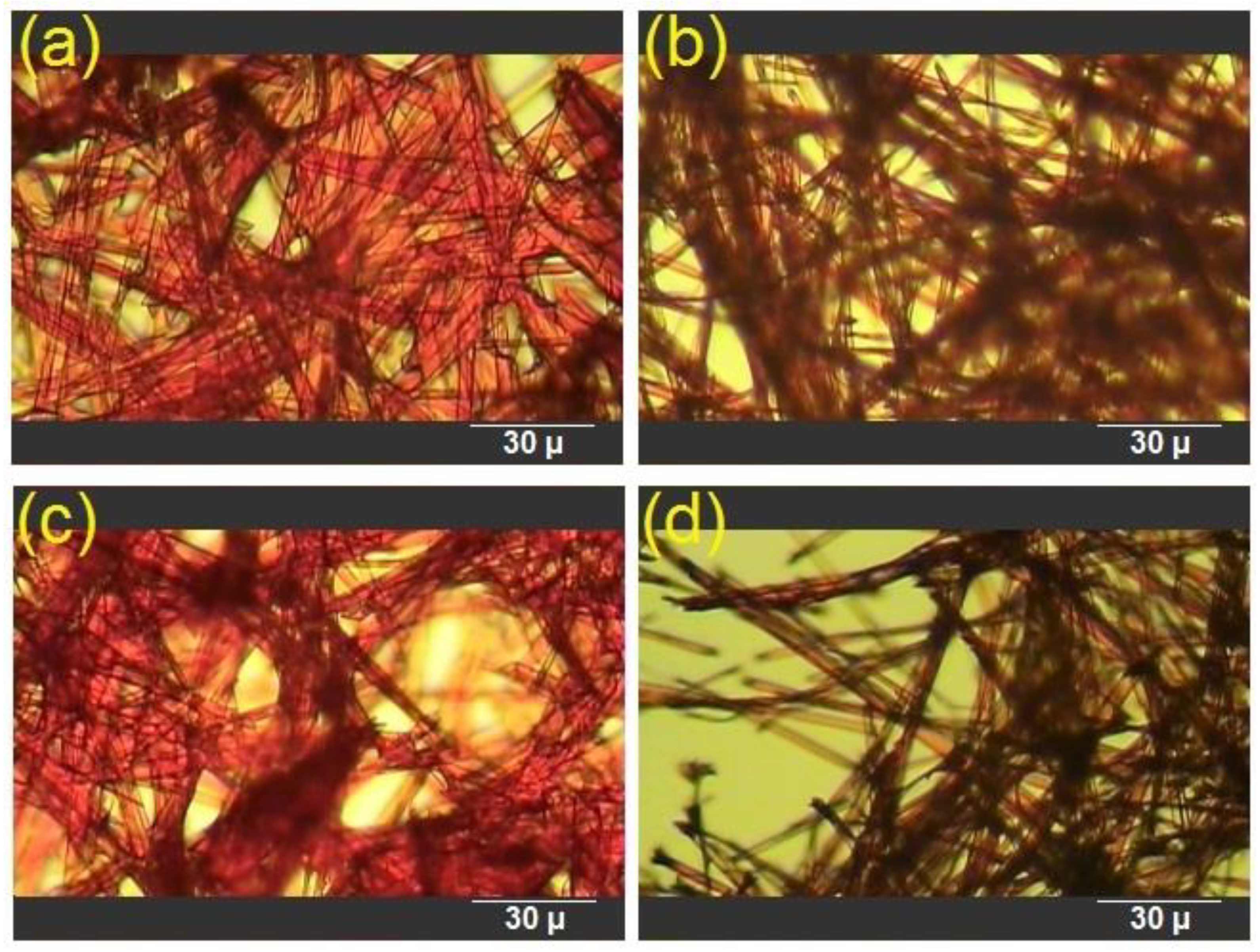
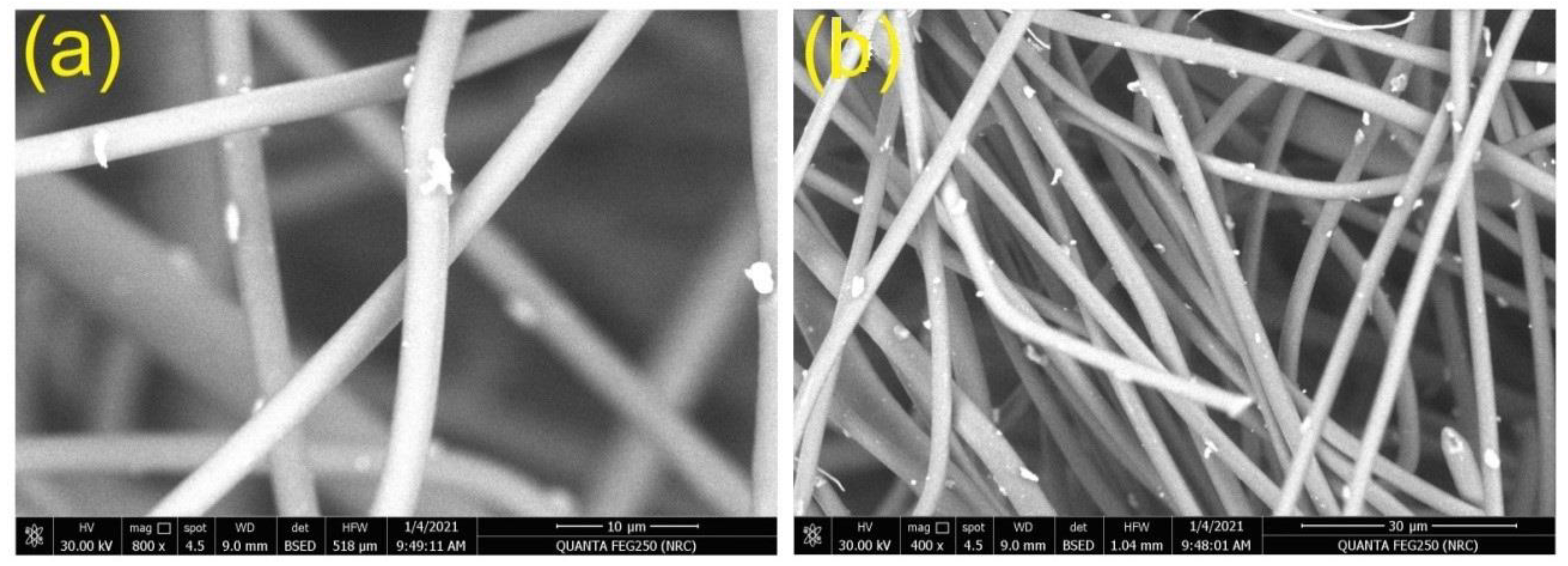
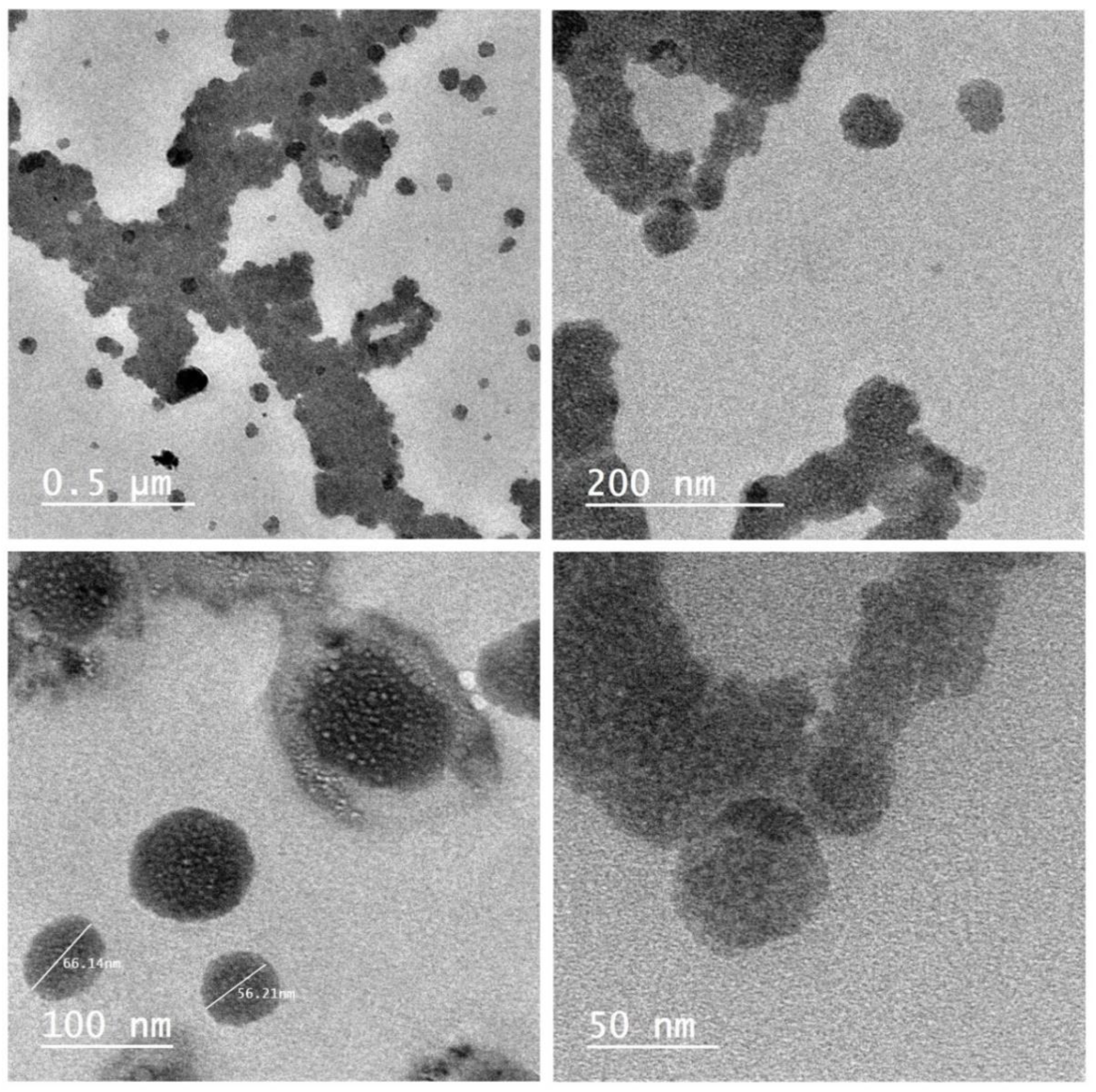
3.2. Morphological Characterization
3.3. Activity of CNF-TCFH Biosensor
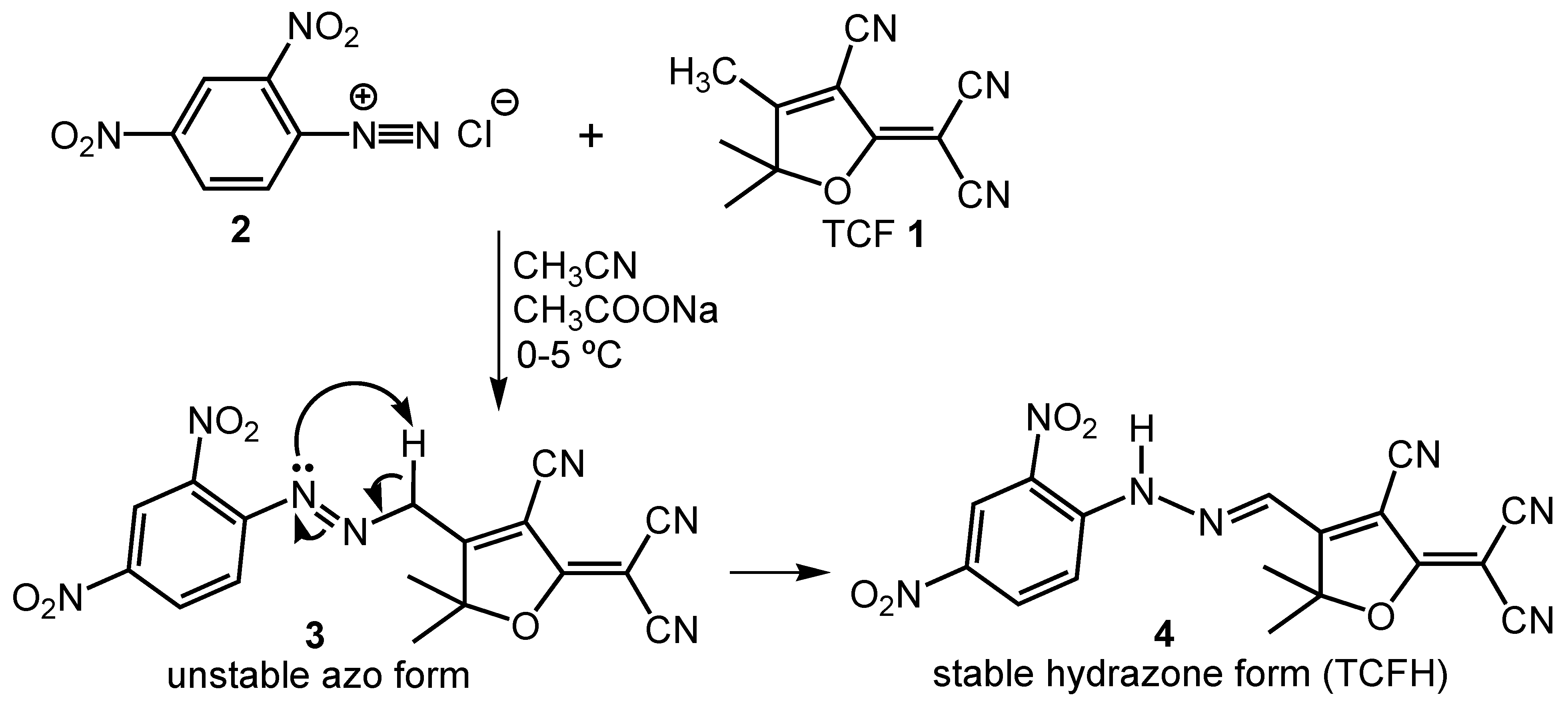
3.4. Mechanism of Detection
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortiz, A.; Covic, A.; Fliser, D.; Fouque, D.; Goldsmith, D.; Kanbay, M.; Mallamaci, F.; Massy, Z.A.; Rossignol, P.; Vanholder, R. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014, 383, 1831–1843. [Google Scholar] [CrossRef]
- Wieringa, F.P.; Broers, N.J.H.; Kooman, J.P.; Van Der Sande, F.M.; Van Hoof, C. Wearable sensors: Can they benefit patients with chronic kidney disease? Expert Rev. Med. Devices 2017, 14, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Le Maout, P.; Wojkiewicz, J.-L.; Redon, N.; Lahuec, C.; Seguin, F.; Dupont, L.; Mikhaylov, S.; Noskov, Y.; Ogurtsov, N.; Pud, A. Polyaniline nanocomposites based sensor array for breath ammonia analysis. Portable e-nose approach to non-invasive diagnosis of chronic kidney disease. Sens. Actuators B Chem. 2018, 274, 616–626. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Liu, R.; Qin, Y.; Qiu, Q.-F.; Chen, Z.; Cheng, S.-B.; Huang, W.-H. Flexible electrochemical urea sensor based on surface molecularly imprinted nanotubes for detection of human sweat. Anal. Chem. 2018, 90, 13081–13087. [Google Scholar] [CrossRef]
- Wang, K.-H.; Hsieh, J.-C.; Chen, C.-C.; Zan, H.-W.; Meng, H.-F.; Kuo, S.-Y.; Nguyễn, M.T.N. A low-cost, portable and easy-operated salivary urea sensor for point-of-care application. Biosens. Bioelectron. 2019, 132, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, A.; Neri, G. Miniaturized bio-and chemical-sensors for point-of-care monitoring of chronic kidney diseases. Sensors 2018, 18, 942. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Jakhar, S.; Narwal, V. Determination of urea with special emphasis on biosensors: A review. Biosens. Bioelectron. 2019, 123, 36–50. [Google Scholar] [CrossRef]
- Sha, R.; Komori, K.; Badhulika, S. Graphene–Polyaniline composite based ultra-sensitive electrochemical sensor for non-enzymatic detection of urea. Electrochim. Acta 2017, 233, 44–51. [Google Scholar] [CrossRef]
- Khattab, T.A.; Fouda, M.M.G.; Abdelrahman, M.S.; Othman, S.I.; Bin-Jumah, M.; Alqaraawi, M.A.; Al Fassam, H.; Allam, A.A. Co-encapsulation of enzyme and tricyanofuran hydrazone into alginate microcapsules incorporated onto cotton fabric as a biosensor for colorimetric recognition of urea. React. Funct. Polym. 2019, 142, 199–206. [Google Scholar] [CrossRef]
- Chen, M.; Wu, W.; Chen, Y.; Pan, Q.; Chen, Y.; Zheng, Z.; Zheng, Y.; Huang, L.; Weng, S. A fluorescent sensor constructed from nitrogen-doped carbon nanodots (N-CDs) for pH detection in synovial fluid and urea determination. RSC Adv. 2018, 8, 41432–41438. [Google Scholar] [CrossRef]
- Nouira, W.; Maaref, A.; Siadat, M.; Errachid, A.; Jaffrezic-Renault, N. Conductometric biosensors based on layer-by-layer coated paramagnetic nanoparticles for urea detection. Sens. Lett. 2011, 9, 2272–2274. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Liu, M.; Wang, Z.; Wang, B.; Li, M.; Li, Y. pH-controlled fluorescence changes in a novel semiconducting polymer dot/pyrogallic acid system and a multifunctional sensing strategy for urea, urease, and pesticides. Anal. Methods 2017, 9, 6669–6674. [Google Scholar] [CrossRef]
- Radwan, E.K.; Kafafy, H.; El-Wakeel, S.T.; Shaheen, T.I.; Gad-Allah, T.A.; El-Kalliny, A.S.; El-Naggar, M.E. Remediation of Cd (II) and reactive red 195 dye in wastewater by nanosized gels of grafted carboxymethyl cellulose. Cellulose 2018, 25, 6645–6660. [Google Scholar] [CrossRef]
- Oh, H.J.; Yeang, B.J.; Park, Y.K.; Choi, H.J.; Kim, J.H.; Kang, Y.S.; Bae, Y.; Kim, J.Y.; Lim, S.J.; Lee, W. Washable colorimetric nanofiber nonwoven for ammonia gas detection. Polymers 2020, 12, 1585. [Google Scholar] [CrossRef]
- Khattab, T.A.; El-Naggar, M.E.; Abdelrahman, M.S.; Aldalbahi, A.; Hatshan, M.R. Simple Development of Novel Reversible Colorimetric Thermometer Using Urea Organogel Embedded with Thermochromic Hydrazone Chromophore. Chemosensors 2020, 8, 132. [Google Scholar] [CrossRef]
- Park, Y.K.; Oh, B.M.; Jo, A.R.; Han, J.H.; Lim, J.Y.; Oh, H.J.; Lim, S.J.; Kim, J.H.; Lee, W.S. Fabrication of colorimetric textile sensor based on rhodamine dye for acidic gas detection. Polymers 2020, 12, 431. [Google Scholar] [CrossRef]
- Park, Y.K.; Oh, H.J.; Bae, J.H.; Lim, J.Y.; Lee, H.D.; Hong, S.I.; Son, H.S.; Kim, J.H.; Lim, S.J.; Lee, W. Colorimetric Textile Sensor for the Simultaneous Detection of NH3 and HCl Gases. Polymers 2020, 12, 2595. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Askim, J.R.; Suslick, K.S. The optoelectronic nose: Colorimetric and fluorometric sensor arrays. Chem. Rev. 2018, 119, 231–292. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wang, J.; Li, Y.-P.; Sun, X.-B.; Wang, J.-P.; Wang, X.-X.; Zhang, J.; You, H.-D.; Yu, G.-F.; Long, Y.-Z. Facile Preparation of Highly Stretchable TPU/Ag Nanowire Strain Sensor with Spring-Like Configuration. Polymers 2020, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Ho, D.; Chiu, G.N.C.; Leong, D.T.; Pastorin, G.; Chow, E.K. Clinical applications of carbon nanomaterials in diagnostics and therapy. Adv. Mater. 2018, 30, 1802368. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, A.M.; El-Naggar, M.E.; Hudson, S.M.; Rojas, O.J. Fabrication and characterization of bactericidal thiol-chitosan and chitosan iodoacetamide nanofibres. Int. J. Biol. Macromol. 2017, 94, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, S.; El-Naggar, M.E. Wound dressing properties of cationized cotton fabric treated with carrageenan/cyclodextrin hydrogel loaded with honey bee propolis extract. Int. J. Biol. Macromol. 2019, 133, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Adnan, A.S.; Yahya, E.B.; Olaiya, N.G.; Safrida, S.; Hossain, M.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.K.; Oyekanmi, A.A. A Review on plant cellulose nanofibre-based aerogels for biomedical applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef]
- Menon, M.P.; Selvakumar, R.; Ramakrishna, S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites–A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef] [PubMed]
- Hebeish, A.; El-Rafie, M.H.; Rabie, A.M.; El-Sheikh, M.A.; El-Naggar, M.E. Ultra-microstructural features of perborate oxidized starch. J. Appl. Polym. Sci. 2014, 131, 40170. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; El-Naggar, M.E.; Elsherbiny, D.A.; Ali, S.; Abdel-Aziz, M.S.; Abd-Elmoneam, Y.K. Antibacterial carrageenan/cellulose-nanocrystal system loaded with silver nanoparticles, prepared via solid-state technique. J. Environ. Chem. Eng. 2020, 8, 104276. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Radwan, E.K.; El-Wakeel, S.T.; Kafafy, H.; Gad-Allah, T.A.; El-Kalliny, A.S.; Shaheen, T.I. Synthesis, characterization and adsorption properties of microcrystalline cellulose based nanogel for dyes and heavy metals removal. Int. J. Biol. Macromol. 2018, 113, 248–258. [Google Scholar] [CrossRef]
- Wang, X.; Sun, F.; Yin, G.; Wang, Y.; Liu, B.; Dong, M. Tactile-sensing based on flexible PVDF nanofibers via electrospinning: A review. Sensors 2018, 18, 330. [Google Scholar] [CrossRef]
- Baji, A.; Agarwal, K.; Oopath, S.V. Emerging developments in the use of electrospun fibers and membranes for protective clothing applications. Polymers 2020, 12, 492. [Google Scholar] [CrossRef]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer–Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Sharaf, S.; El-Naggar, M.E. Eco-friendly technology for preparation, characterization and promotion of honey bee propolis extract loaded cellulose acetate nanofibers in medical domains. Cellulose 2018, 25, 5195–5204. [Google Scholar] [CrossRef]
- Gaminian, H.; Montazer, M. Decorating silver nanoparticles on electrospun cellulose nanofibers through a facile method by dopamine and ultraviolet irradiation. Cellulose 2017, 24, 3179–3190. [Google Scholar] [CrossRef]
- Liu, H.; Hsieh, Y. Surface methacrylation and graft copolymerization of ultrafine cellulose fibers. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 953–964. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Electrospinning of ultrafine cellulose acetate fibers: Studies of a new solvent system and deacetylation of ultrafine cellulose acetate fibers. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 5–11. [Google Scholar] [CrossRef]
- Liu, H.; Hsieh, Y. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2119–2129. [Google Scholar] [CrossRef]
- Zheng, W.; Zheng, W.; Shi, C.; Wang, X. Cylindrical-electrode-assisted solution blowing for nanofiber spinning. J. Appl. Polym. Sci. 2019, 136, 47087. [Google Scholar] [CrossRef]
- Calisir, M.D.; Kilic, A. A comparative study on SiO2 nanofiber production via two novel non-electrospinning methods: Centrifugal spinning vs solution blowing. Mater. Lett. 2020, 258, 126751. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Recent progress in biosensors for environmental monitoring: A review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef]
- Sumner, C.; Krause, S.; Sabot, A.; Turner, K.; McNeil, C.J. Biosensor based on enzyme-catalysed degradation of thin polymer films. Biosens. Bioelectron. 2001, 16, 709–714. [Google Scholar] [CrossRef]
- Ertürk, G.; Hedström, M.; Mattiasson, B. A sensitive and real-time assay of trypsin by using molecular imprinting-based capacitive biosensor. Biosens. Bioelectron. 2016, 86, 557–565. [Google Scholar] [CrossRef]
- Barquero-Quirós, M.; Domínguez-Renedo, O.; Alonso-Lomillo, M.A.; Arcos-Martínez, M.J. Biosensor for aluminium (III) based on its inhibition of α-chymotrypsin immobilized on a screen-printed carbon electrode modified with gold nanoparticles. Microchim. Acta 2012, 179, 65–70. [Google Scholar] [CrossRef]
- Albareda-Sirvent, M.; Merkoci, A.; Alegret, S. Configurations used in the design of screen-printed enzymatic biosensors. A review. Sens. Actuators B Chem. 2000, 69, 153–163. [Google Scholar] [CrossRef]
- Rebriiev, A.V.; Starodub, N.F. Enzymatic biosensor based on the ISFET and photopolymeric membrane for the determinaion of urea. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2004, 16, 1891–1895. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, T.-J.; Zhang, D.-W.; Li, H.-Y.; Ma, Y.-R.; Qi, L.-M.; Zhou, Y.-L.; Zhang, X.-X. Amperometric hydrogen peroxide biosensor based on the immobilization of heme proteins on gold nanoparticles–bacteria cellulose nanofibers nanocomposite. Talanta 2011, 84, 71–77. [Google Scholar] [CrossRef]
- Incani, V.; Danumah, C.; Boluk, Y. Nanocomposites of nanocrystalline cellulose for enzyme immobilization. Cellulose 2013, 20, 191–200. [Google Scholar] [CrossRef]
- Lakard, B.; Herlem, G.; Lakard, S.; Antoniou, A.; Fahys, B. Urea potentiometric biosensor based on modified electrodes with urease immobilized on polyethylenimine films. Biosens. Bioelectron. 2004, 19, 1641–1647. [Google Scholar] [CrossRef]
- Retama, J.R.; Lopez-Ruiz, B.; Lopez-Cabarcos, E. Microstructural modifications induced by the entrapped glucose oxidase in cross-linked polyacrylamide microgels used as glucose sensors. Biomaterials 2003, 24, 2965–2973. [Google Scholar] [CrossRef]
- McShane, M.J. Potential for glucose monitoring with nanoengineered fluorescent biosensors. Diabetes Technol. Ther. 2002, 4, 533–538. [Google Scholar] [CrossRef]
- Tamaddon, F.; Arab, D. Urease covalently immobilized on cotton-derived nanocellulose-dialdehyde for urea detection and urea-based multicomponent synthesis of tetrahydro-pyrazolopyridines in water. RSC Adv. 2019, 9, 41893–41902. [Google Scholar] [CrossRef]
- Khattab, T.A. From chromic switchable hydrazones to smart materials. Mater. Chem. Phys. 2020, 254, 123456. [Google Scholar] [CrossRef]
- Abdelrahman, M.S.; Fouda, M.M.G.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; Khattab, T.A. Development of colorimetric cotton swab using molecular switching hydrazone probe in calcium alginate. J. Mol. Struct. 2020, 1216, 128301. [Google Scholar] [CrossRef]

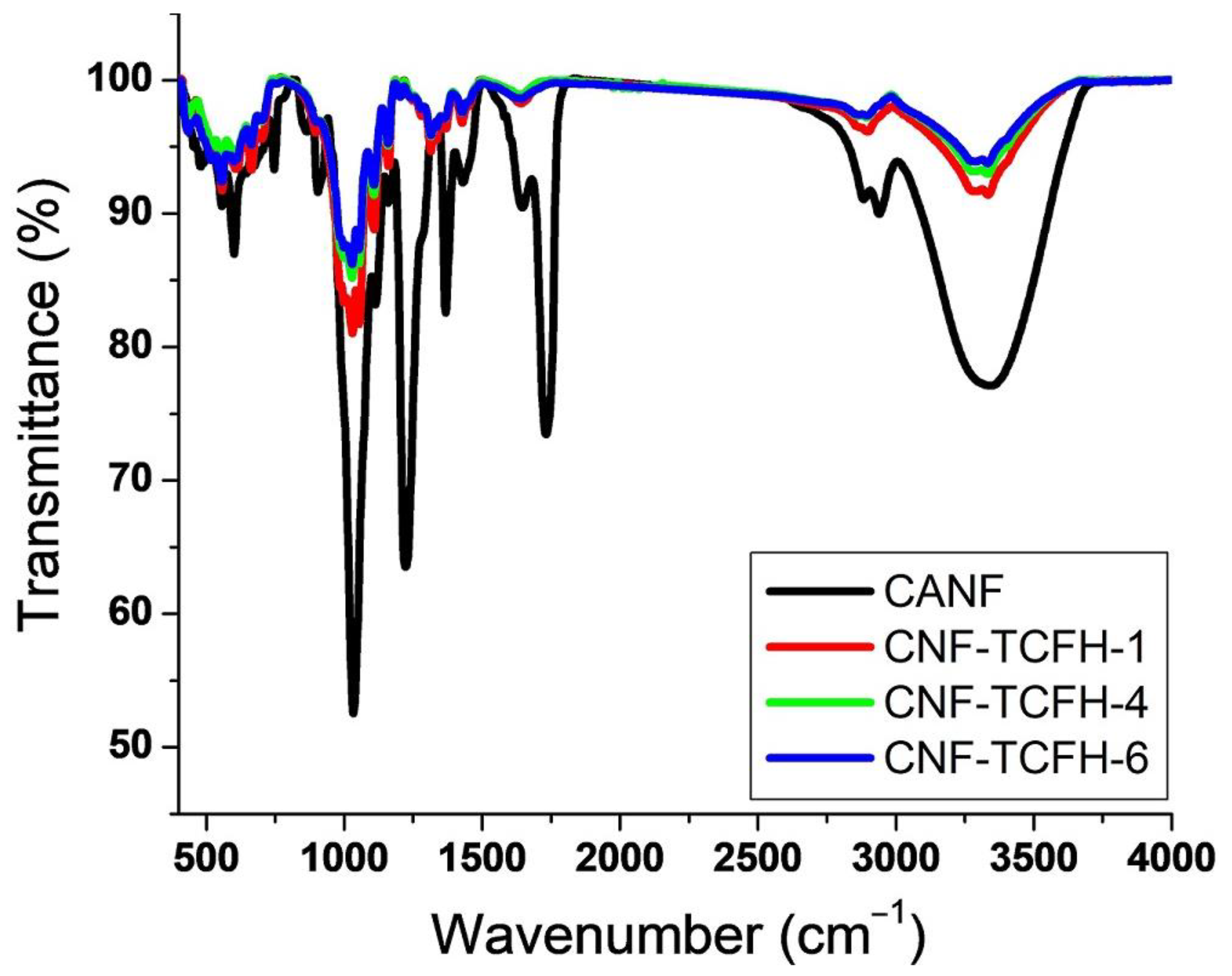

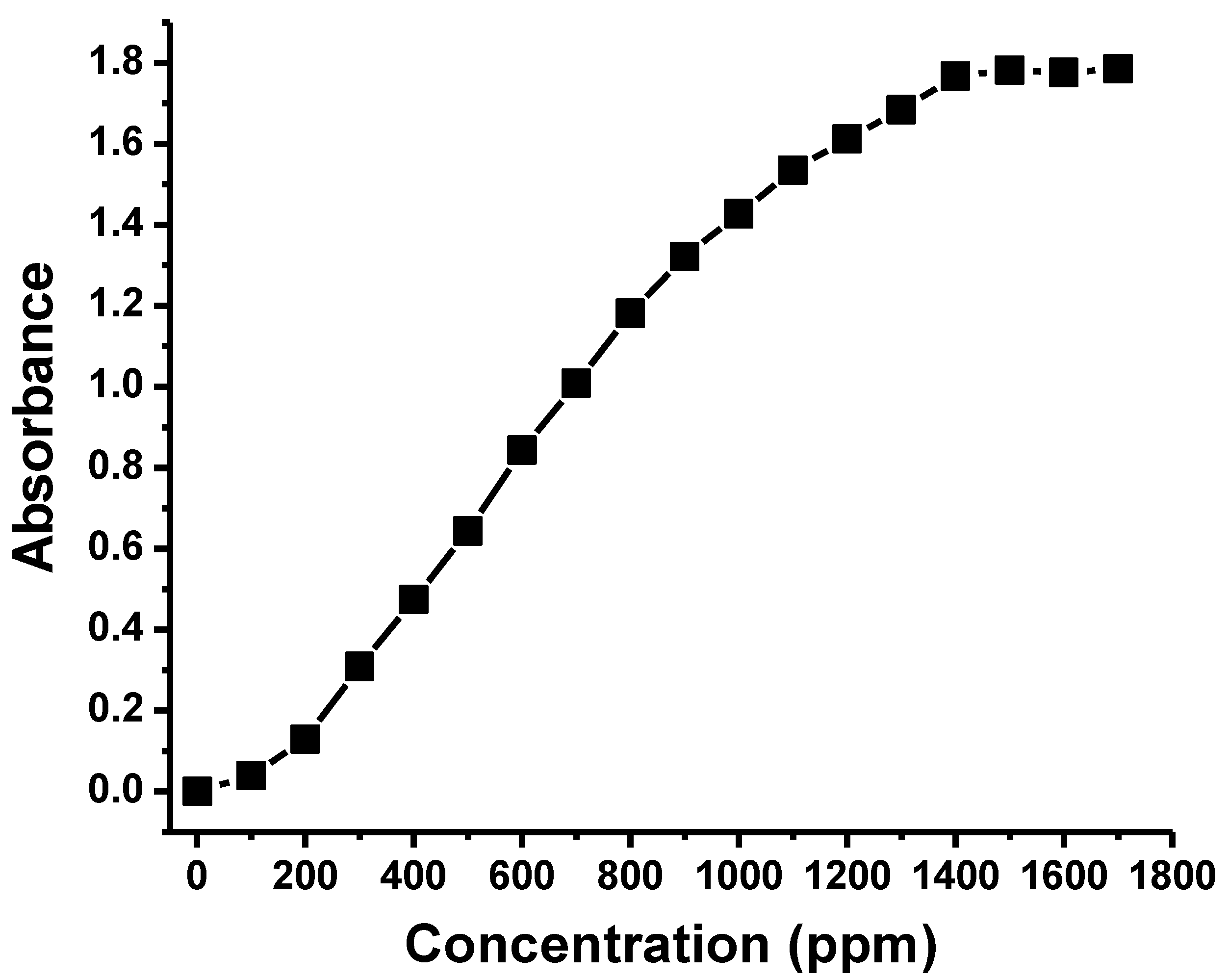
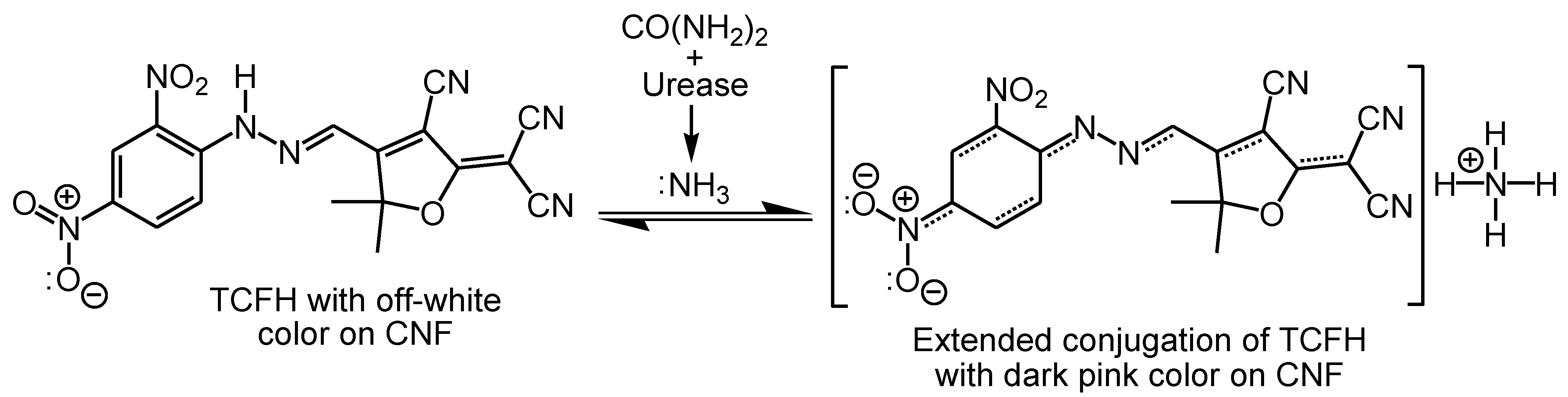
| CNFs | C | O | Ca | Cl | |
|---|---|---|---|---|---|
| CNF | A | 55.72 | 44.28 | 0 | 0 |
| B | 55.41 | 44.59 | 0 | 0 | |
| CNF-TCFH-4 | A | 52.15 | 46.11 | 1.23 | 0.51 |
| B | 52.32 | 46.31 | 0.84 | 0.53 | |
| Sample Code | L | a* | b* | K/S | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B | |
| CNF-TCFH-1 | 74.12 | 61.20 | 4.19 | 5.54 | 10.83 | −4.08 | 3.43 | 3.91 |
| CNF-TCFH-2 | 71.73 | 57.03 | 6.28 | 8.34 | 14.78 | −5.10 | 5.27 | 5.88 |
| CNF-TCFH-3 | 64.19 | 44.45 | 9.22 | 10.27 | 21.61 | −8.74 | 7.18 | 7.68 |
| CNF-TCFH-4 | 63.28 | 44.91 | 9.38 | 13.66 | 21.49 | −9.78 | 8.85 | 9.33 |
| CNF-TCFH-5 | 62.88 | 43.20 | 10.78 | 14.55 | 26.37 | −11.19 | 9.26 | 9.70 |
| CNF-TCFH-6 | 61.49 | 42.45 | 11.24 | 14.97 | 27.62 | −12.65 | 9.79 | 10.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Newehy, M.H.; El-Hamshary, H.; Salem, W.M. Solution Blowing Spinning Technology towards Green Development of Urea Sensor Nanofibers Immobilized with Hydrazone Probe. Polymers 2021, 13, 531. https://doi.org/10.3390/polym13040531
El-Newehy MH, El-Hamshary H, Salem WM. Solution Blowing Spinning Technology towards Green Development of Urea Sensor Nanofibers Immobilized with Hydrazone Probe. Polymers. 2021; 13(4):531. https://doi.org/10.3390/polym13040531
Chicago/Turabian StyleEl-Newehy, Mohamed H., Hany El-Hamshary, and Waheed M. Salem. 2021. "Solution Blowing Spinning Technology towards Green Development of Urea Sensor Nanofibers Immobilized with Hydrazone Probe" Polymers 13, no. 4: 531. https://doi.org/10.3390/polym13040531
APA StyleEl-Newehy, M. H., El-Hamshary, H., & Salem, W. M. (2021). Solution Blowing Spinning Technology towards Green Development of Urea Sensor Nanofibers Immobilized with Hydrazone Probe. Polymers, 13(4), 531. https://doi.org/10.3390/polym13040531








