Thermal Hazard Characteristics of Unsaturated Polyester Resin Mixed with Hardeners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Methods
2.3. Kinetic Analysis
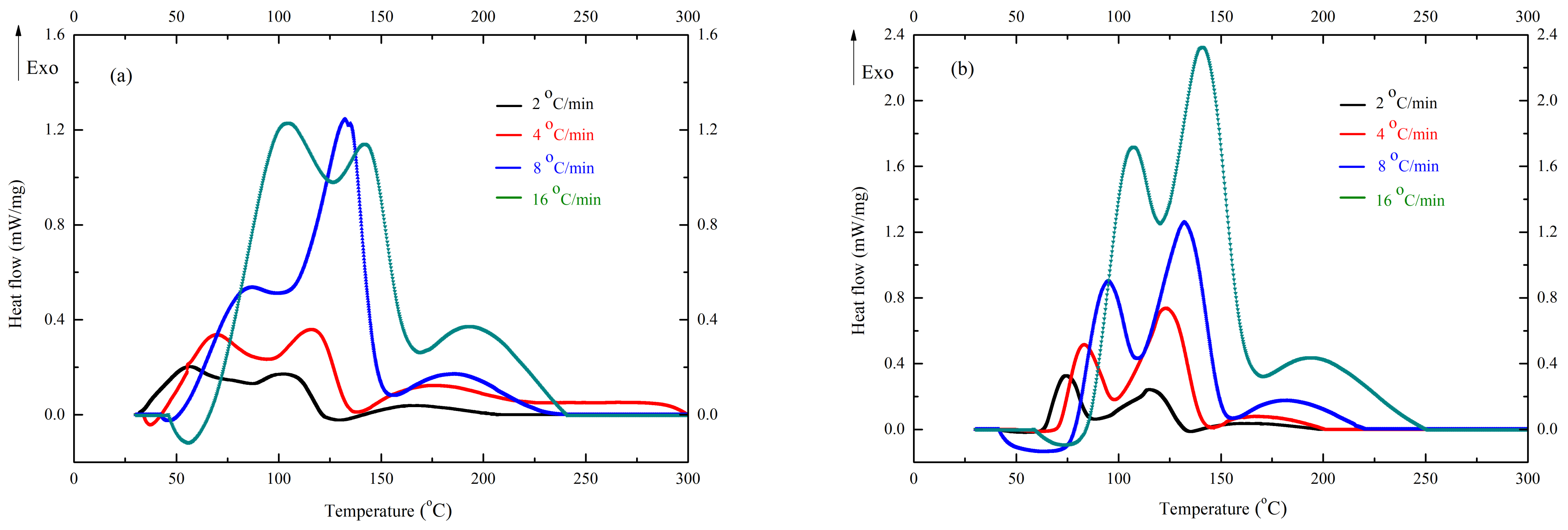
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sheng, H.J.; Wang, X.J.; Pan, Z.G.; Chen, X. Thermodynamics and kinetics of one-step curing process for vinyl ester-unsaturated polyester resin in low shrinkage. J. Therm. Anal. Calorim. 2017, 130, 832–833. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Yang, Y.; Huang, H.C.; Shu, C.M. Inhibitory effects of three chemical dust suppressants on nitrocellulose dust cloud explosion. AIChE J. 2020, 66, e16888. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Ho, S.C.; Huang, A.C.; Shu, C.M. Potential explosion hazard of polyester resin dust formed from a granulation process: Limiting oxygen concentration with different pressures. Appl. Therm. Eng. 2018, 135, 74–82. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Liao, J.Y.; Shu, C.M. Explosion characteristics of chlorodifluoromethane and isobutane at high temperature and pressure using a 20-L apparatus. Int. J. Refrig. 2018, 96, 155–160. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Yang, Y.; Wang, C.; Shu, C.M.; Deng, J. Comparison of the inhibition mechanisms of five types of inhibitors on spontaneous coal combustion. Int. J. Energ. Res. 2018, 42, 1158–1171. [Google Scholar] [CrossRef]
- Yang, Y.; Tsai, Y.T.; Zhang, Y.; Shu, C.M.; Deng, J. Inhibition of spontaneous combustion for different metamorphic degrees of coal by Zn/Mg/Al-CO3-layered double hydroxide. Process Saf. Environ. 2018, 113, 401–412. [Google Scholar] [CrossRef]
- Hong, C.M.; Wang, X.J.; Pan, Z.G.; Zhang, Y.F. Curing thermodynamics and kinetics of unsaturated polyester resin with different chain length of saturated aliphatic binary carboxylic acid. J. Therm. Anal. Calorim. 2015, 122, 427–436. [Google Scholar] [CrossRef]
- Chu, F.K.; Yu, X.J.; Hou, Y.B.; Mu, X.W.; Song, L.; Hu, W.Z. A facile strategy to simultaneously improve the mechanical and fire safety properties of ramie fabric-reinforced unsaturated polyester resin composites. Compos. Part A-Appl. S. 2018, 115, 264–273. [Google Scholar] [CrossRef]
- Xu, X.Q.; Zhou, Q.; Song, N.; Ni, Q.R.; Ni, L.Z. Kinetic analysis of isothermal curing of unsaturated polyester resin catalyzed with tert-butyl peroxybenzoate and cobalt octoate by differential scanning calorimetry. J. Therm. Anal. Calorim. 2017, 129, 843–850. [Google Scholar] [CrossRef]
- Bai, Z.M.; Song, L.; Hu, Y.; Gong, X.L.; Yuen, R.K. Investigation on flame retardancy, combustion and pyrolysis behavior of flame retarded unsaturated polyester resin with a star-shaped phosphorus-containing compound. J. Anal. Appl. Pyrolysis 2014, 105, 317–326. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, J.; Wang, X.L.; Wang, Y.Z. Highly thermostable and durably flame-retardant unsaturated polyester modified by a novel polymeric flame retardant containing Schiff base and spirocyclic structures. Chem. Eng. J. 2018, 344, 419–430. [Google Scholar] [CrossRef]
- Salasinska, K.; Celiński, M.; Barczewski, M.; Leszczyński, M.K.; Kozikowski, P. Fire behavior of flame retarded unsaturated polyester resin with high nitrogen content additives. Polym. Test. 2020, 84, 106379. [Google Scholar] [CrossRef]
- Boulkadid, M.K.; Touidjine, S.; Trache, D.; Belkhiri, S. Analytical methods for the assessment of curing kinetics of polyurethane binders for high-energy composites. Anal. Chem. 2020, 1863768. [Google Scholar] [CrossRef]
- Bessa, W.; Trache, D.; Derradji, M.; Ambar, H.; Tarchoun, A.F.; Benziane, M.; Guedouar, B. Characterization of raw and treated Arundo donax L. cellulosic fibers and their effect on the curing kinetics of bisphenol A-based benzoxazine. Int. J. Biol. Macromol. 2020, 164, 2931–2943. [Google Scholar] [CrossRef]
- Luo, Q.; Liang, D.; Shen, H. Evaluation of self-heating and spontaneous combustion risk of biomass and fishmeal with thermal analysis (DSC-TG) and self-heating substances test experiments. Thermochim. Acta 2016, 635, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, L.; Li, H.; Huang, X.; Huang, L. Characterization of moxa floss combustion by TG/DSC, TG-FTIR and IR. Bioresour. Technol. 2019, 288, 121516. [Google Scholar] [CrossRef]
- Meng, F.; Xue, S.; Amyotte, P.; Li, C.; Yuan, C. Characterization of Ti powders mixed with TiO2 powders: Thermal and kinetic studies. J. Loss Prev. Proc. 2020, 66, 104184. [Google Scholar] [CrossRef]
- Xu, F.X.; Zhang, X.; Zhang, F.; Jiang, L.Q.; Zhao, Z.L.; Li, H.B. TG-FTIR for kinetic evaluation and evolved gas analysis of cellulose with different structures. Fuel 2020, 268, 117365. [Google Scholar] [CrossRef]
- Song, Z.; Li, M.; Pan, Y.; Shu, C.M. A generalized differential method to calculate lumped kinetic triplet of the nth order model for the global one-step heterogeneous reaction using TG data. J. Loss Prev. Proc. 2020, 64, 104094. [Google Scholar] [CrossRef]
- Taylor, S.M.; Fryer, P.J. A numerical study of the use of the kissinger analysis of DSC thermograms to obtain reaction kinetic parameters. Thermoch. Acta 1992, 209, 111–125. [Google Scholar] [CrossRef]
- Liu, L.; Li, M. Curing mechanisms and kinetic analysis of DGEBA cured with a novel imidazole derivative curing agent using DSC techniques. J. App. Polym. Sci. 2010, 117, 3220–3227. [Google Scholar] [CrossRef]
- Yong, H.K.; Mi, J.R.; Han, E.; Kim, K.H.; Yoon, E.S. Hazard prediction of MEKPO decomposition reaction using group contribution method. Theory Appl. Chem. Eng. 2002, 8, 2969–2972. [Google Scholar]
- Chang, Y.; Tsai, Y.T.; Pu, Y.; Pan, W.; Shu, C.M. Evaluation of incompatible hazard for TBPO mixed with inorganic acid or base by thermal analysis technology. Disaster Adv. 2014, 7, 35–39. [Google Scholar]
- Chi, J.H.; Wu, S.H.; Shu, C.M. Thermal explosion analysis of methyl ethyl ketone peroxide by non-isothermal and isothermal calorimetric applications. J. Hazard. Mater. 2009, 171, 1145–1149. [Google Scholar] [CrossRef]
- Tsai, Y.T.; You, M.L.; Qian, X.M.; Shu, C.M. Calorimetric techniques combined with various thermokinetic models to evaluate incompatible hazard of tert-butyl peroxy-2-ethyl hexanoate mixed with metal ions. Ind. Eng. Chem. Res. 2013, 52, 8206–8215. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Yong, L.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stabil. 2008, 93, 90–98. [Google Scholar] [CrossRef]
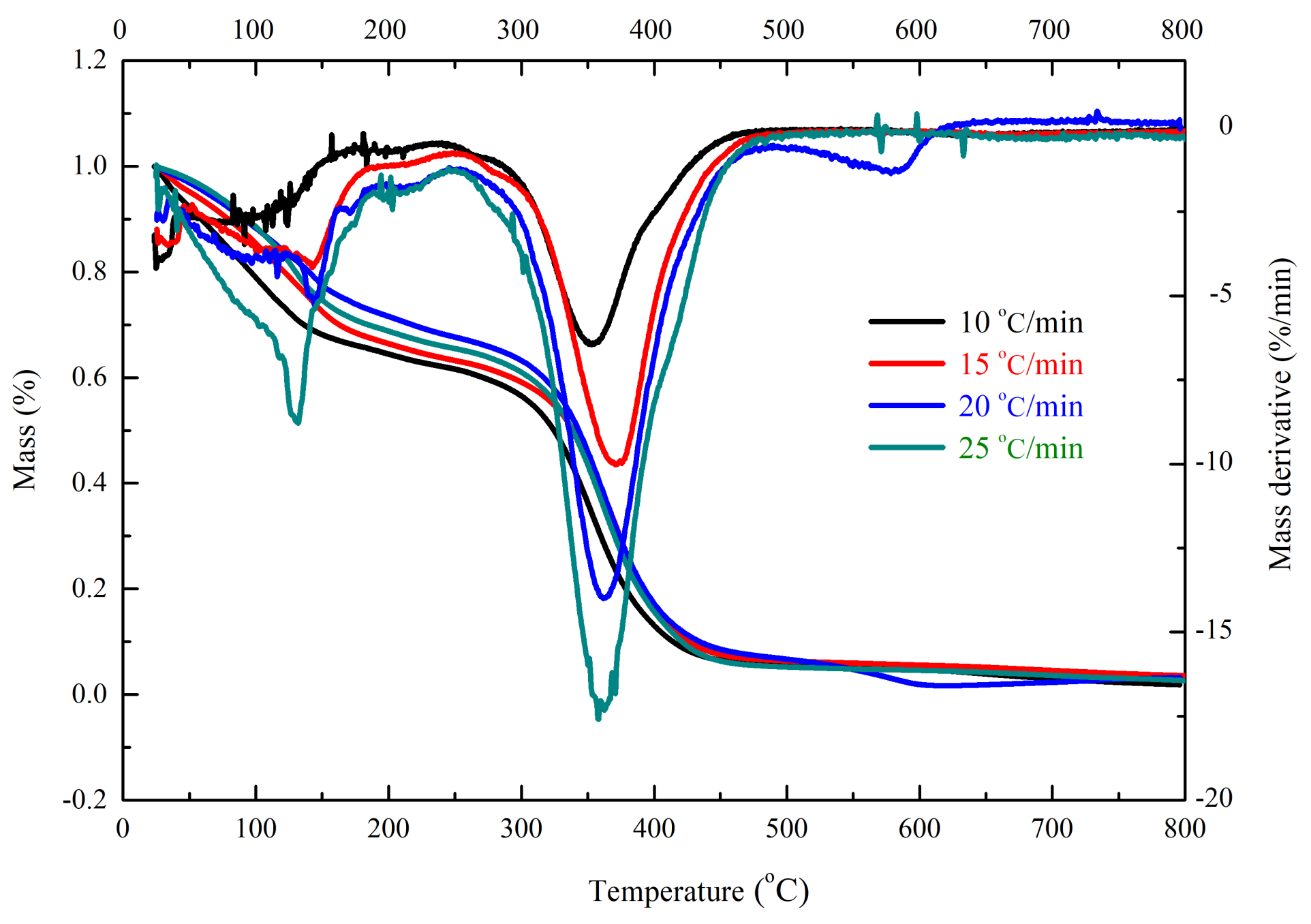
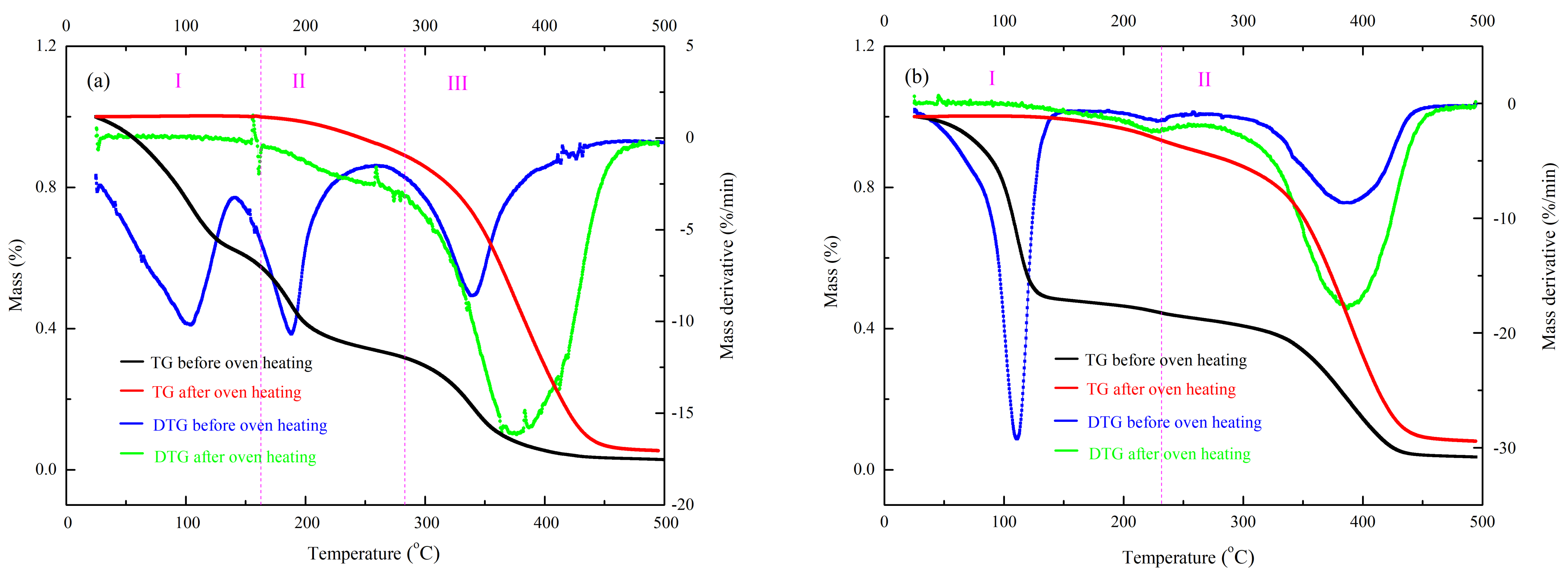


| β (°C/min) | T01 (°C) | Tp1 (°C) | Mass Loss (wt%) | T02 (°C) | Tp2 (°C) | Mass Loss (wt%) | T03 (°C) | Tp3 (°C) | Mass Loss (wt%) | Char Residue (wt%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 30.1 | 128.2 | 36.3 | 263.3 | 354.5 | 61.8 | - | - | - | 1.9 |
| 15 | 33.4 | 128.5 | 34.3 | 264.2 | 371.1 | 62.2 | - | - | - | 3.5 |
| 20 | 37.2 | 131.7 | 28.9 | 286.2 | 362.2 | 65.8 | 510.6 | 583.8 | 2.1 | 3.2 |
| 25 | 40.6 | 141.3 | 30.1 | 289.6 | 363.9 | 67.3 | - | - | - | 2.6 |
| Sample | T01 (°C) | Tp1 (°C) | Mass Loss (wt%) | T02 (°C) | Tp2 (°C) | Mass Loss (wt%) | T03 (°C) | Tp3 (°C) | Mass Loss (wt%) | Char Residue (wt%) |
|---|---|---|---|---|---|---|---|---|---|---|
| UP + MEKPO (before oven heating) | 37.2 | 104.1 | 37.8 | 149.1 | 189.6 | 29.2 | 285.6 | 340.3 | 31.6 | 1.4 |
| UP + MEKPO (after oven heating) | 235.9 | 376.4 | 94.7 | - | - | - | - | - | - | 5.3 |
| UP + TBPO (before oven heating) | 56.8 | 111.1 | 53.7 | 235.9 | 383.9 | 42.7 | - | - | - | 3.6 |
| UP + TBPO (after oven heating) | 213.4 | 396.5 | 91.9 | - | - | - | - | - | - | 8.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, K.; Tsai, Y. Thermal Hazard Characteristics of Unsaturated Polyester Resin Mixed with Hardeners. Polymers 2021, 13, 522. https://doi.org/10.3390/polym13040522
Ren K, Tsai Y. Thermal Hazard Characteristics of Unsaturated Polyester Resin Mixed with Hardeners. Polymers. 2021; 13(4):522. https://doi.org/10.3390/polym13040522
Chicago/Turabian StyleRen, Kewei, and Yunting Tsai. 2021. "Thermal Hazard Characteristics of Unsaturated Polyester Resin Mixed with Hardeners" Polymers 13, no. 4: 522. https://doi.org/10.3390/polym13040522
APA StyleRen, K., & Tsai, Y. (2021). Thermal Hazard Characteristics of Unsaturated Polyester Resin Mixed with Hardeners. Polymers, 13(4), 522. https://doi.org/10.3390/polym13040522