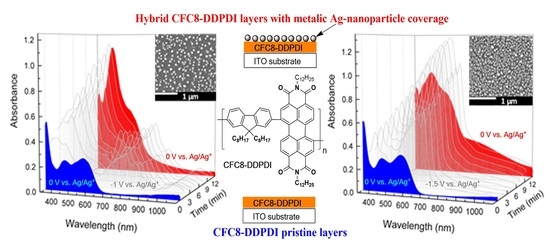
Hybrid Layers of Donor-Acceptor Copolymers with Homogenous Silver Nanoparticle Coverage for Photonic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Layer Preparation
2.2. Methods
3. Results and Discussion
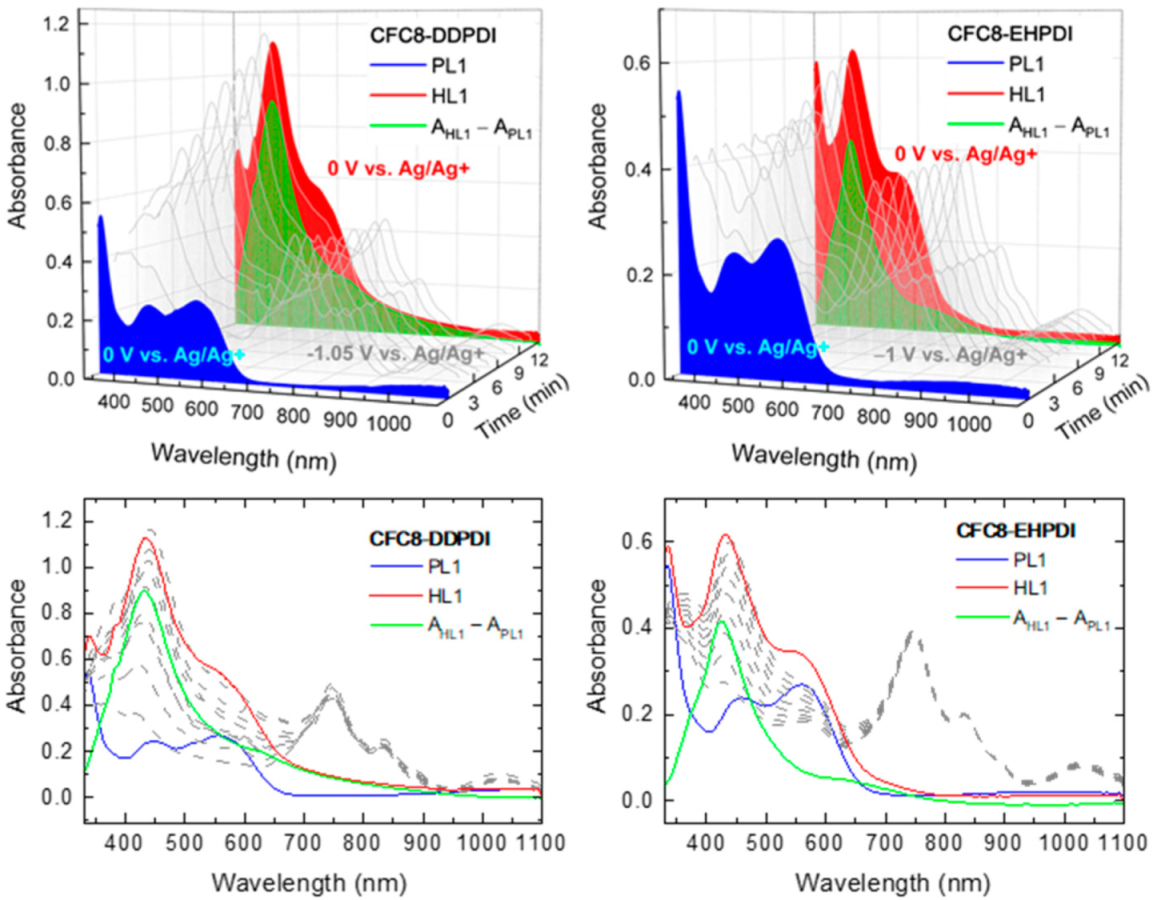
3.1. Hybrid Layer Preparation and Absorption
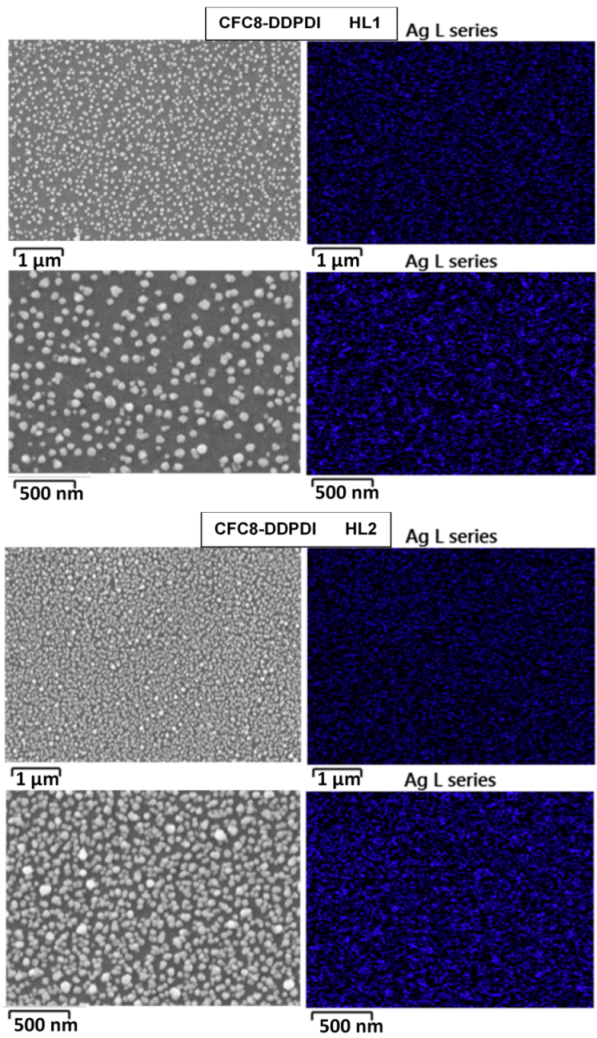
3.2. Scanning Electron Microscopy
3.3. X-ray Photoelectron Spectroscopy
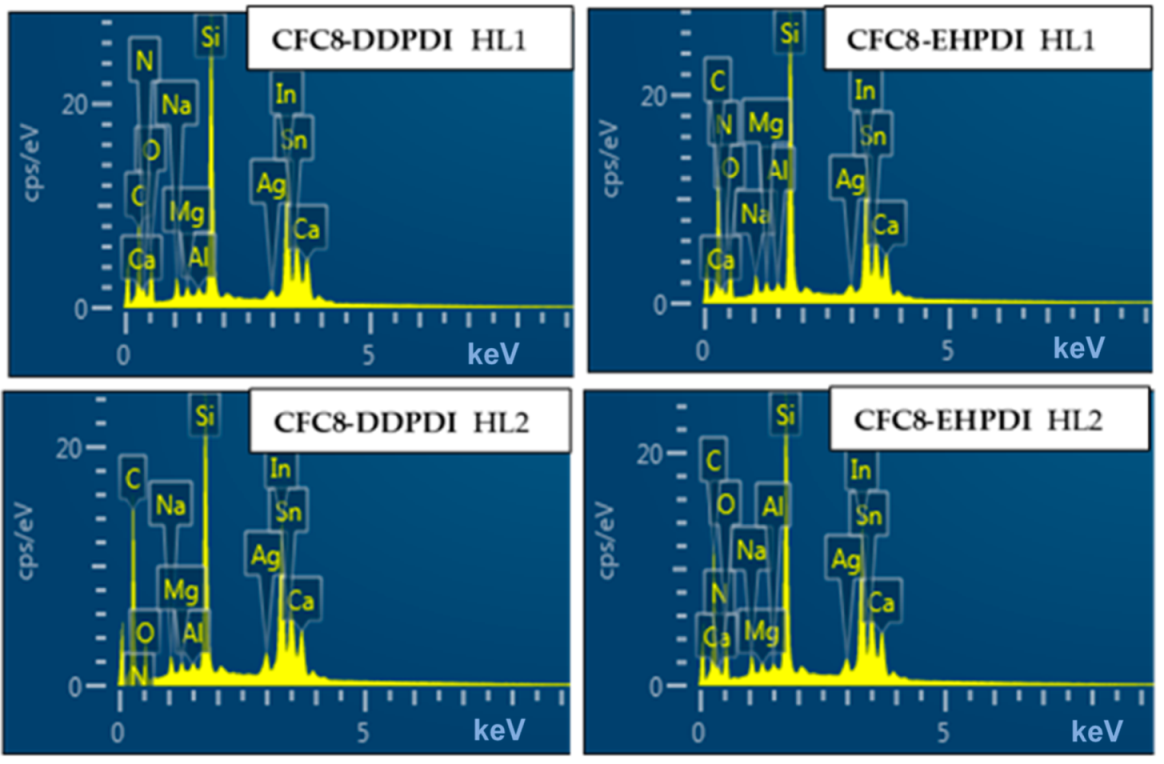
3.4. Energy Dispersive X-ray Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, Q.S.; Zhang, F.J.; Gao, W.; Sun, Q.Q.; Zhang, M.; Yang, C.L.; Zhang, J. High-efficiency and air stable fullerene-free ternary organic solar cells. Nano Energy 2018, 45, 177–183. [Google Scholar] [CrossRef]
- Hou, J.; Inganas, O.; Friend, R.H.; Gao, F. Organic solar cells based on non-fullerene acceptors. Nat. Mater. 2018, 17, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Baumgarten, M.; Müllen, K. Designing π-conjugated polymers for organic electronics. Prog. Polym. Sci. 2013, 38, 1832–1908. [Google Scholar] [CrossRef]
- Lv, X.; Li, W.; Ouyang, M.; Zhang, Y.; Wright, D.S.; Zhang, C. Polymeric electrochromic materials with donor–acceptor structures. J. Mater. Chem. C 2017, 5, 12–28. [Google Scholar] [CrossRef]
- Osaka, I. Semiconducting polymers based on electron-deficient π-building units. Polym. J. 2015, 47, 18–25. [Google Scholar] [CrossRef]
- Wu, J.S.; Cheng, S.W.; Cheng, Y.J.; Hsu, C.S. Donor-acceptor conjugated polymers based on multifused ladder-type arenes for organic solar cells. Chem. Soc. Rev. 2015, 44, 1113–1154. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, J.; Li, Y.; Li, Y. Organic Solar Cell Materials toward Commercialization. Small 2018, 14, 1801793. [Google Scholar] [CrossRef]
- Yuan, J.; Ouyang, J.Y.; Cimrova, V.; Leclerc, M.; Najari, A.; Zou, Y.P. Development of quinoxaline based polymers for photovoltaic applications. J. Mater. Chem. C 2017, 5, 1858–1879. [Google Scholar] [CrossRef]
- Cao, K.; Shen, D.E.; Österholm, A.M.; Kerszulis, J.A.; Reynolds, J.R. Tuning Color, Contrast, and Redox Stability in High Gap Cathodically Coloring Electrochromic Polymers. Macromolecules 2016, 49, 8498–8507. [Google Scholar] [CrossRef]
- Lee, C.; Lee, S.; Kim, G.-U.; Lee, W.; Kim, B.J. Recent Advances, Design Guidelines, and Prospects of All-Polymer Solar Cells. Chem. Rev. 2019, 119, 8028–8086. [Google Scholar] [CrossRef]
- Root, S.E.; Savagatrup, S.; Printz, A.D.; Rodriquez, D.; Lipomi, D.J. Mechanical Properties of Organic Semiconductors for Stretchable, Highly Flexible, and Mechanically Robust Electronics. Chem. Rev. 2017, 117, 6467–6499. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Tan, Z.; Domercq, B.; An, Z.; Zhang, X.; Barlow, S.; Li, Y.; Zhu, D.; Kippelen, B.; Marder, S.R. A high-mobility electron-transport polymer with broad absorption and its use in field-effect transistors and all-polymer solar cells. J. Am. Chem. Soc. 2007, 129, 7246–7247. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Hösel, M.; Dyer, A.L.; Krebs, F.C. Development and Manufacture of Polymer-Based Electrochromic Devices. Adv. Funct. Mater. 2015, 25, 2073–2090. [Google Scholar] [CrossRef]
- Cimrová, V.; Výprachtický, D.; Pokorná, V. Donor-acceptor copolymers containing bithiophene and dithiophenylthienothiadiazole units with fast electrochromic response. J. Mater. Chem. C 2019, 7, 8575–8584. [Google Scholar] [CrossRef]
- Maake, P.J.; Bolokang, A.S.; Arendse, C.J.; Vohra, V.; Iwuoha, E.I.; Motaung, D.E. Metal oxides and noble metals application in organic solar cells. Sol. Energy 2020, 207, 347–366. [Google Scholar] [CrossRef]
- Shamjid, P.; Abhijith, T.; Vivek, P.; Joel, C.S.; Reddy, V.S. Plasmonic effects of Ag nanoparticles for absorption enhancement in polymer solar cells with MoO3 passivation layer. Phys. B Condens. Matter 2019, 560, 174–184. [Google Scholar] [CrossRef]
- Ginting, R.T.; Kaur, S.; Lim, D.K.; Kim, J.M.; Lee, J.H.; Lee, S.H.; Kang, J.W. Plasmonic Effect of Gold Nanostars in Highly Efficient Organic and Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 36111–36118. [Google Scholar] [CrossRef]
- Cheng, Z.K.; Javed, N.; O’Carroll, D.M. Optical and Electrical Properties of Organic Semiconductor Thin Films on Aperiodic Plasmonic Metasurfaces. ACS Appl. Mater. Interfaces 2020, 12, 35579–35587. [Google Scholar] [CrossRef]
- Cimrová, V.; Výprachtický, D.; Pokorná, V.; Babičová, P. Donor–acceptor copolymers with 1,7-regioisomers of N,N′-dialkylperylene-3,4,9,10-tetracarboxydiimide as materials for photonics. J. Mater. Chem. C 2019, 7, 14678–14692. [Google Scholar] [CrossRef]
- Jones, B.A.; Facchetti, A.; Wasielewski, M.R.; Marks, T.J. Tuning orbital energetics in arylene diimide semiconductors. Materials design for ambient stability of n-type charge transport. J. Am. Chem. Soc. 2007, 129, 15259–15278. [Google Scholar] [CrossRef]
- Russ, B.; Robb, M.J.; Brunetti, F.G.; Miller, P.L.; Perry, E.E.; Patel, S.N.; Ho, V.; Chang, W.B.; Urban, J.J.; Chabinyc, M.L.; et al. Power Factor Enhancement in Solution-Processed Organic n-Type Thermoelectrics Through Molecular Design. Adv. Mater. 2014, 26, 3473–3477. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xu, Y.; Wang, X.; Yang, F.; Zhang, A.; Li, C.; Ma, W.; Li, W. Conjugated polymer acceptors based on fused perylene bisimides with a twisted backbone for non-fullerene solar cells. Polym. Chem. 2017, 8, 3300–3306. [Google Scholar] [CrossRef]
- Liu, M.; Yang, J.; Lang, C.; Zhang, Y.; Zhou, E.; Liu, Z.; Guo, F.; Zhao, L. Fused Perylene Diimide-Based Polymeric Acceptors for Efficient All-Polymer Solar Cells. Macromolecules 2017, 50, 7559–7566. [Google Scholar] [CrossRef]
- Sharma, S.; Kolhe, N.B.; Gupta, V.; Bharti, V.; Sharma, A.; Datt, R.; Chand, S.; Asha, S.K. Improved All-Polymer Solar Cell Performance of n-Type Naphthalene Diimide–Bithiophene P(NDI2OD-T2) Copolymer by Incorporation of Perylene Diimide as Coacceptor. Macromolecules 2016, 49, 8113–8125. [Google Scholar] [CrossRef]
- Zhou, E.; Cong, J.; Wei, Q.; Tajima, K.; Yang, C.; Hashimoto, K. All-polymer solar cells from perylene diimide based copolymers: Material design and phase separation control. Angew. Chem. Int. Ed. Engl. 2011, 50, 2799–2803. [Google Scholar] [CrossRef]
- Yin, Z.; Wei, J.; Zheng, Q. Interfacial Materials for Organic Solar Cells: Recent Advances and Perspectives. Adv. Sci. 2016, 3, 1500362. [Google Scholar] [CrossRef]
- Meng, X.; Ho, C.H.Y.; Xiao, S.; Bai, Y.; Zhang, T.; Hu, C.; Lin, H.; Yang, Y.; So, S.K.; Yang, S. Molecular design enabled reduction of interface trap density affords highly efficient and stable perovskite solar cells with over 83% fill factor. Nano Energy 2018, 52, 300–306. [Google Scholar] [CrossRef]
- Sophia, J.; Muralidharan, G. Preparation of vinyl polymer stabilized silver nanospheres for electro-analytical determination of H2O2. Sens. Actuat. B-Chem. 2014, 193, 149–156. [Google Scholar] [CrossRef]
- Abudabbus, M.M.; Jevremovic, I.; Nesovic, K.; Peric-Grujic, A.; Rhee, K.Y.; Miskovic-Stankovic, V. In situ electrochemical synthesis of silver-doped poly(vinyl alcohol)/graphene composite hydrogels and their physico-chemical and thermal properties. Compos. Part B-Eng. 2018, 140, 99–107. [Google Scholar] [CrossRef]
- Laghrib, F.; Ajermoun, N.; Bakasse, M.; Lahrich, S.; El Mhammedi, M.A. Synthesis of silver nanoparticles assisted by chitosan and its application to catalyze the reduction of 4-nitroaniline. Int. J. Biol. Macromol. 2019, 135, 752–759. [Google Scholar] [CrossRef]
- Ponnaiah, S.K.; Periakaruppan, P.; Vellaichamy, B. New Electrochemical Sensor Based on a Silver-Doped Iron Oxide Nanocomposite Coupled with Polyaniline and Its Sensing Application for Picomolar-Level Detection of Uric Acid in Human Blood and Urine Samples. J. Phys. Chem. B 2018, 122, 3037–3046. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.D.; Liu, W.; Zhou, Y.; Yuan, D.; Hu, X.W.; Zhao, W.; Zhou, G.F. Improvement of Electro-Optical Properties of PSLC Devices by Silver Nanowire Doping. Appl. Sci. 2019, 9, 145. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Y.; Sors, T.; Irudayaraj, J.; Ren, W.; Wang, R. Impedimetric detection of bacteria by using a microfluidic chip and silver nanoparticle based signal enhancement. Mikrochim. Acta 2018, 185, 184. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Li, Y.X.; Shahzad, S.A.; Chen, J.; Chen, Y.; Wang, Y.; Yang, M.D.; Yu, C. Fluorescence turn-on detection of glucose via the Ag nanoparticle mediated release of a perylene probe. Chem. Commun. 2015, 51, 6354–6356. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.L.; Zeng, G.M.; Gong, X.M.; Xue, W.J.; Li, J.; Yang, Y.Y.; Zhou, C.Y.; Li, Z.H.; Yan, X.L.; et al. Modifying delafossite silver ferrite with polyaniline: Visible-light-response Z-scheme heterojunction with charge transfer driven by internal electric field. Chem. Eng. J. 2019, 370, 1087–1100. [Google Scholar] [CrossRef]
- Calderon-Jimenez, B.; Johnson, M.E.; Montoro Bustos, A.R.; Murphy, K.E.; Winchester, M.R.; Vega Baudrit, J.R. Silver Nanoparticles: Technological Advances, Societal Impacts, and Metrological Challenges. Front. Chem. 2017, 5, 6. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Fairley, N. CASAXPS, Version 2.3.23; Casa Software Ltd.: BayHouse, UK, 2020. [Google Scholar]
- Tougaard, S. Universality classes of inelastic electron scattering cross-sections. Surf. Interface Anal. 1997, 25, 137–154. [Google Scholar] [CrossRef]
- Slistan-Grijalva, A.; Herrera-Urbina, R.; Rivas-Silva, J.F.; Avalos-Borja, M.; Castillon-Barraza, F.F.; Posada-Amarillas, A. Classical theoretical characterization of the surface plasmon absorption band for silver spherical nanoparticles suspended in water and ethylene glycol. Phys. E 2005, 27, 104–112. [Google Scholar] [CrossRef]
- Chapman, R.; Mulvaney, P. Electro-optical shifts in silver nanoparticle films. Chem. Phys. Lett. 2001, 349, 358–362. [Google Scholar] [CrossRef]
- Ponelyte, S.; Palevicius, A.; Guobiene, A.; Puiso, J.; Prosycevas, I. Investigation of optical properties of Ag: PMMA nanocomposite structures. Proc. of SPIE 2010, 7716, 77161S-1–77161S-10. [Google Scholar]
- Cheon, J.Y.; Kim, S.J.; Park, W.H. Facile Interpretation of Catalytic Reaction between Organic Dye Pollutants and Silver Nanoparticles with Different Shapes. J. Nanomater. 2019, 3257892, 1–8. [Google Scholar] [CrossRef]
- Parnklang, T.; Lertvachirapaiboon, C.; Pienpinijtham, P.; Wongravee, K.; Thammacharoen, C.; Ekgasit, S. H2O2-triggered shape transformation of silver nanospheres to nanoprisms with controllable longitudinal LSPR wavelengths. RSC Adv. 2013, 3, 12886–12894. [Google Scholar] [CrossRef]
- Persson, B.N.J.; Liebsch, A. Optical-Properties of Inhomogeneous-Media. Solid State Commun. 1982, 44, 1637–1640. [Google Scholar] [CrossRef]
- Kreibig, U.; Genzel, L. Optical absorption of small metallic particles. Surf. Sci. 1985, 156, 678–700. [Google Scholar] [CrossRef]
- Quinten, M.; Kreibig, U. Optical properties of aggregates of small metal particles. Surf. Sci. 1986, 172, 557–577. [Google Scholar] [CrossRef]
- Khan, A.U.; Guo, Y.; Chen, X.; Liu, G. Spectral-Selective Plasmonic Polymer Nanocomposites across the Visible and Near-Infrared. ACS Nano 2019, 13, 4255–4266. [Google Scholar] [CrossRef]
- Liu, Y.; Jordan, R.G.; Qiu, S.L. Electronic structures of ordered Ag-Mg alloys. Phys. Rev. B 1994, 49, 4478–4484. [Google Scholar] [CrossRef]
- Tougaard, S. Improved XPS analysis by visual inspection of the survey spectrum. Surf. Interface Anal. 2018, 50, 657–666. [Google Scholar] [CrossRef]
- Tougaard, S. Quantitative x-ray photoelectron spectroscopy: Simple algorithm to determine the amount of atoms in the outermost few nanometers. J. Vac. Sci. Technol. A 2003, 21, 1081–1086. [Google Scholar] [CrossRef]
- Tougaard, S. Algorithm for automatic x-ray photoelectron spectroscopy data processing and x-ray photoelectron spectroscopy imaging. J. Vac. Sci. Technol. A 2005, 23, 741–745. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy. A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Perkin-Elmer Corporation Physical Electronics Division: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Leiro, J.; Minni, E.; Suoninen, E. Study of Plasmon Structure in XPS Spectra of Silver and Gold. J. Phys. F Met. Phys. 1983, 13, 215–221. [Google Scholar] [CrossRef]
- Eckardt, H.; Fritsche, L. Theoretical explanation of the XPS satellite structure of elementary metals: Application to Ag. Solid State Commun. 1985, 54, 405–407. [Google Scholar] [CrossRef]
- Pauly, N.; Yubero, F.; Tougaard, S. Quantitative analysis of satellite structures in XPS spectra of gold and silver. Appl. Surf. Sci. 2016, 383, 317–323. [Google Scholar] [CrossRef]
- Al-Hada, M.; Gregoratti, L.; Amati, M.; Neeb, M. Pristine and oxidised Ag-nanoparticles on free-standing graphene as explored by X-ray photoelectron and Auger spectroscopy. Surf. Sci. 2020, 693, 121533. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiao, J.; Jiang, Z.; Tan, D.; Fu, Q.; Bao, X.; Liu, X.; Jia, J.; Xue, Q. Oxygen adsorption on Ag/Si(111)-7 × 7 surfaces. J. Vac. Sci. Technol. A 2008, 26, 62–67. [Google Scholar] [CrossRef]
- Bukhtiyarov, V.I.; Carley, A.F.; Dollard, L.A.; Roberts, M.W. XPS study of oxygen adsorption on supported silver: Effect of particle size. Surf. Sci. 1997, 381, L605–L608. [Google Scholar] [CrossRef]
- Lopez-Salido, I.; Lim, D.C.; Dietsche, R.; Bertram, N.; Kim, Y.D. Electronic and geometric properties of Au nanoparticles on Highly Ordered Pyrolytic Graphite (HOPG) studied using X-ray Photoelectron Spectroscopy (XPS) and Scanning Tunneling Microscopy (STM). J. Phys. Chem. B 2006, 110, 1128–1136. [Google Scholar] [CrossRef]
- Lopez-Salido, I.; Lim, D.C.; Kim, Y.D. Ag nanoparticles on highly ordered pyrolytic graphite (HOPG) surfaces studied using STM and XPS. Surf. Sci. 2005, 588, 6–18. [Google Scholar] [CrossRef]
- Kim, Y.D.; Wei, T.; Wendt, S.; Goodman, D.W. Ag Adsorption on Various Silica Thin Films. Langmuir 2003, 19, 7929–7932. [Google Scholar] [CrossRef]
- Shin, H.S.; Choi, H.C.; Jung, Y.; Kim, S.B.; Song, H.J.; Shin, H.J. Chemical and size effects of nanocomposites of silver and polyvinyl pyrrolidone determined by X-ray photoemission spectroscopy. Chem. Phys. Lett. 2004, 383, 418–422. [Google Scholar] [CrossRef]
- Sinha, S.; Mukherjee, M. A comparative study about electronic structures at rubrene/Ag and Ag/rubrene interfaces. AIP Adv. 2015, 5, 107204. [Google Scholar] [CrossRef]
- Dolatkhah, A.; Jani, P.; Wilson, L.D. Redox-Responsive Polymer Template as an Advanced Multifunctional Catalyst Support for Silver Nanoparticles. Langmuir 2018, 34, 10560–10568. [Google Scholar] [CrossRef] [PubMed]
- Ferraria, A.M.; Carapeto, A.P.; do Rego, A.M.B. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Egelhoff, W.F. Core-level binding-energy shifts at surfaces and in solids. Surf. Sci. Rep. 1987, 6, 253–415. [Google Scholar] [CrossRef]
- Xu, L.Q.; Wang, L.; Zhang, B.; Lim, C.H.; Chen, Y.; Neoh, K.-G.; Kang, E.-T.; Fu, G.D. Functionalization of reduced graphene oxide nanosheets via stacking interactions with the fluorescent and water-soluble perylene bisimide-containing polymers. Polymer 2011, 52, 2376–2383. [Google Scholar] [CrossRef]
- Ren, L.; Wang, M.; Lu, S.; Pan, L.; Xiong, Z.; Zhang, Z.; Peng, Q.; Li, Y.; Yu, J. Tailoring Thermal Transport Properties of Graphene Paper by Structural Engineering. Sci. Rep. 2019, 9, 4549. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; John Wiley & Sons Ltd.: Chichester, UK, 1992. [Google Scholar]
- Scholz, M.; Schmidt, R.; Krause, S.; Scholl, A.; Reinert, F.; Wurthner, F. Electronic structure of epitaxial thin films of bay-substituted perylene bisimide dyes. Appl. Phys. A 2009, 95, 285–290. [Google Scholar] [CrossRef]
- Zahn, D.R.T.; Gavrila, G.N.; Salvan, G. Electronic and Vibrational Spectroscopies Applied to Organic/Inorganic Interfaces. Chem. Rev. 2007, 107, 1161–1232. [Google Scholar] [CrossRef]
- Scholl, A.; Zou, Y.; Jung, M.; Schmidt, T.; Fink, R.; Umbach, E. Line shapes and satellites in high-resolution x-ray photoelectron spectra of large pi-conjugated organic molecules. J. Chem. Phys. Lett. 2004, 121, 10260–10267. [Google Scholar]
- Emmanouil, K.; Gawrys, P.; Zagorska, M.; Kennou, S. Electronic properties of a perylene bisimide interfaced with gold or aluminum: The influence of the substrate. Microelectron. Eng. 2013, 112, 170–173. [Google Scholar] [CrossRef]
- Erbahar, D.; Susi, T.; Rocquefelte, X.; Bittencourt, C.; Scardamaglia, M.; Blaha, P.; Guttmann, P.; Rotas, G.; Tagmatarchis, N.; Zhu, X.; et al. Spectromicroscopy of C60 and azafullerene C59N: Identifying surface adsorbed water. Sci. Rep. 2016, 6, 35605. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Bluhm, H.; Andersson, K.; Ketteler, G.; Ogasawara, H.; Salmeron, M.; Nilsson, A. In situx-ray photoelectron spectroscopy studies of water on metals and oxides at ambient conditions. J. Phys. Condens. Matter 2008, 20, 184025. [Google Scholar] [CrossRef]
- Salmeron, M. From Surfaces to Interfaces: Ambient Pressure XPS and Beyond. Top. Catal. 2018, 61, 2044–2051. [Google Scholar] [CrossRef]
- Onoe, J.; Takeuchi, K.; Ohno, K.; Kawazoe, Y. X-ray photoelectron spectroscopy of air-exposed C60 films: Origin of the O 1s core peak. J. Vac. Sci. Technol. A 1998, 16, 385–388. [Google Scholar] [CrossRef]
- Borges, B.G.A.L.; Veiga, A.G.; Gioti, M.; Laskarakis, A.; Tzounis, L.; Logothetidis, S.; Rocco, M.L.M. Surface, interface and electronic properties of F8:F8BT polymeric thin films used for organic light-emitting diode applications. Polym. Int. 2018, 67, 691–699. [Google Scholar] [CrossRef]


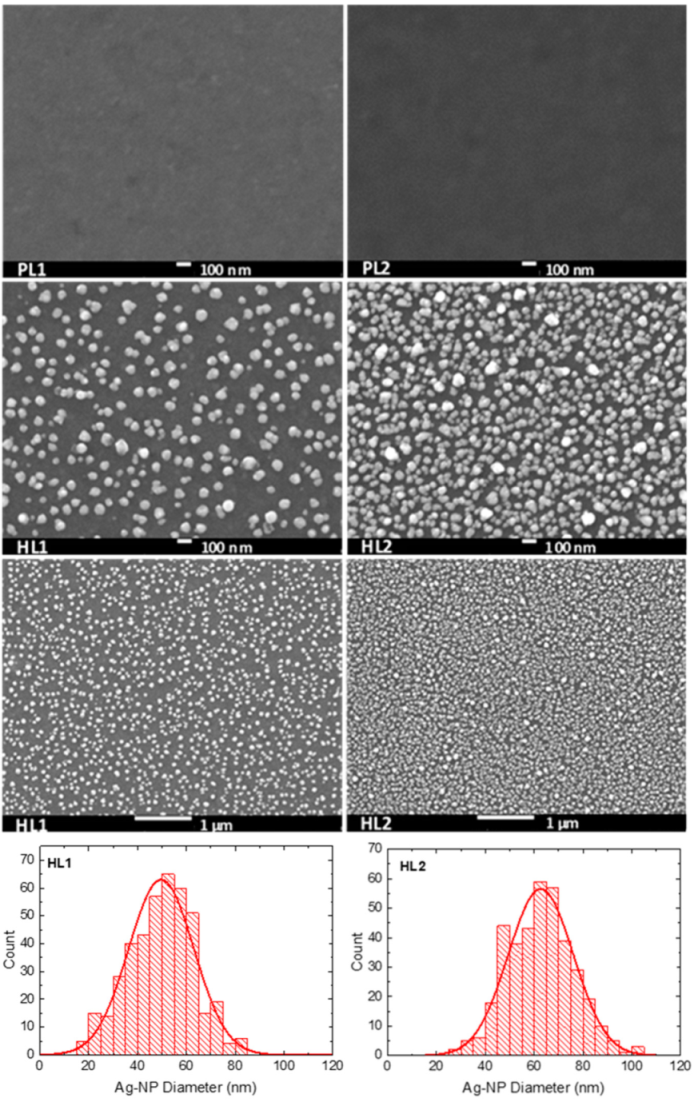
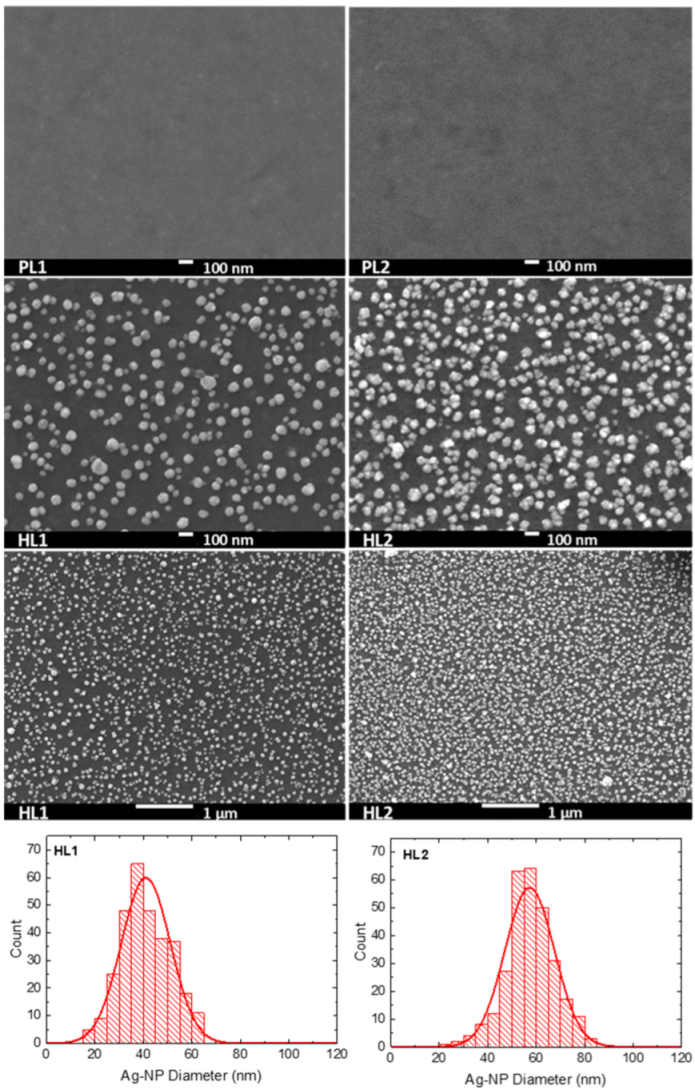
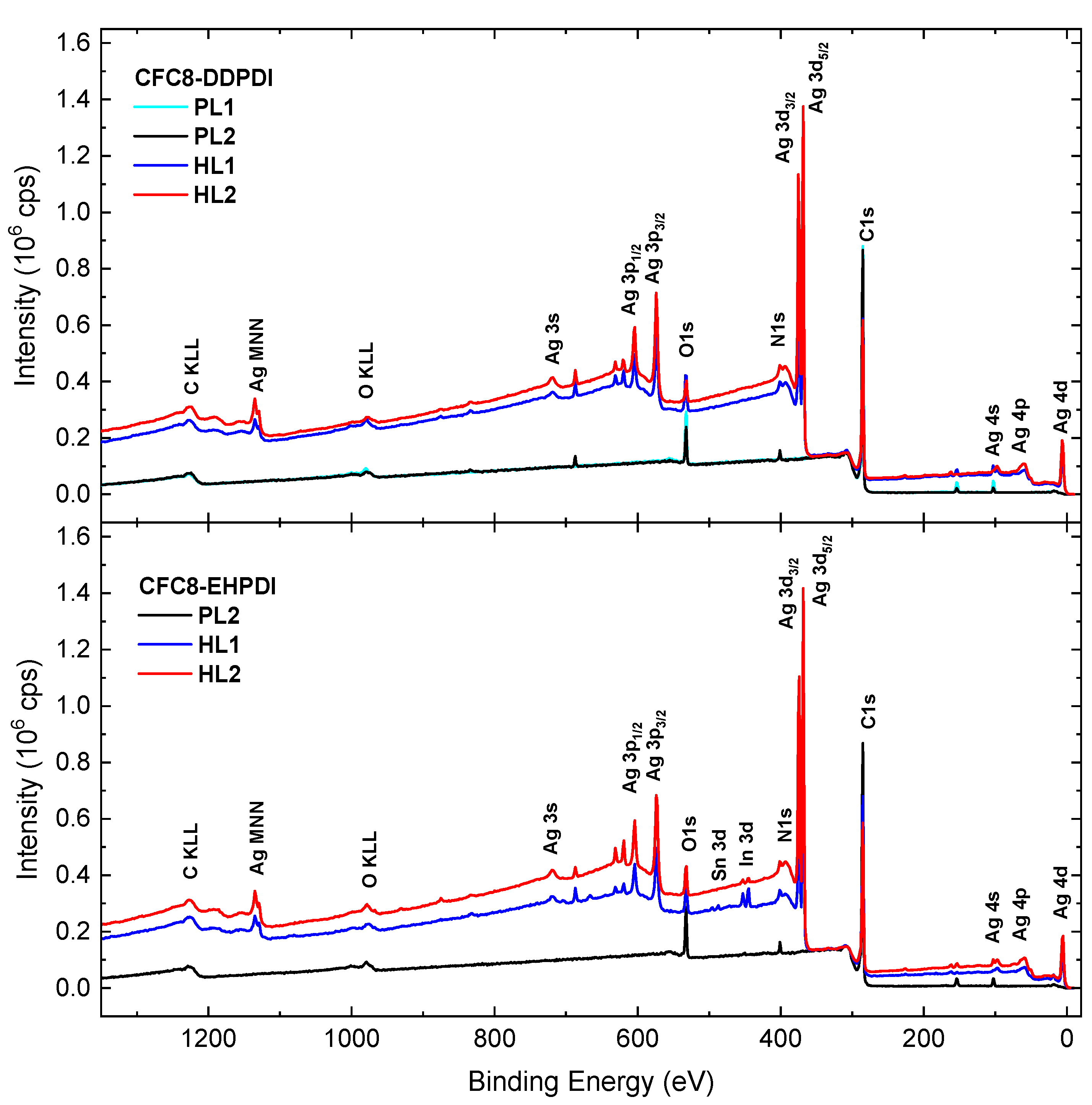




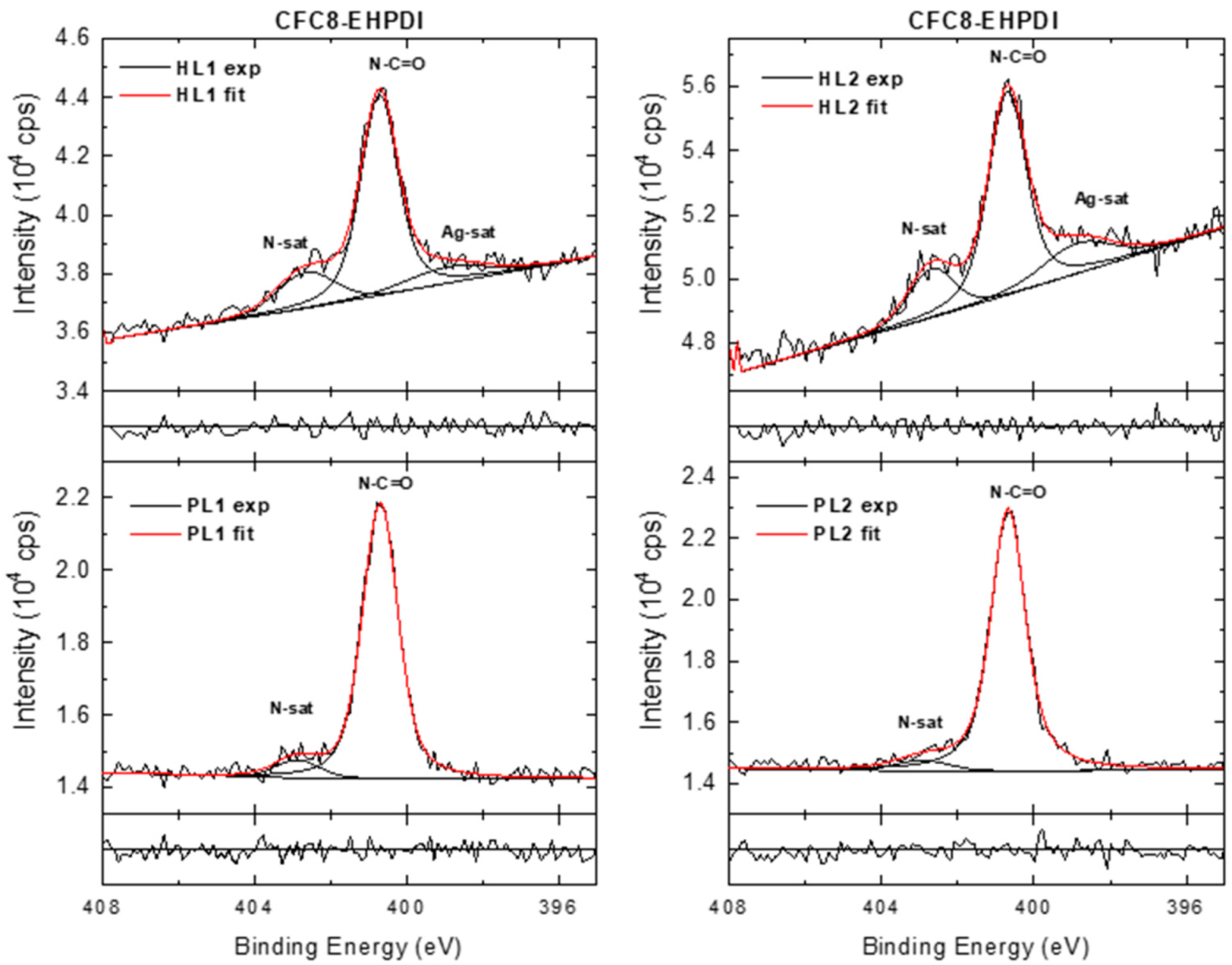
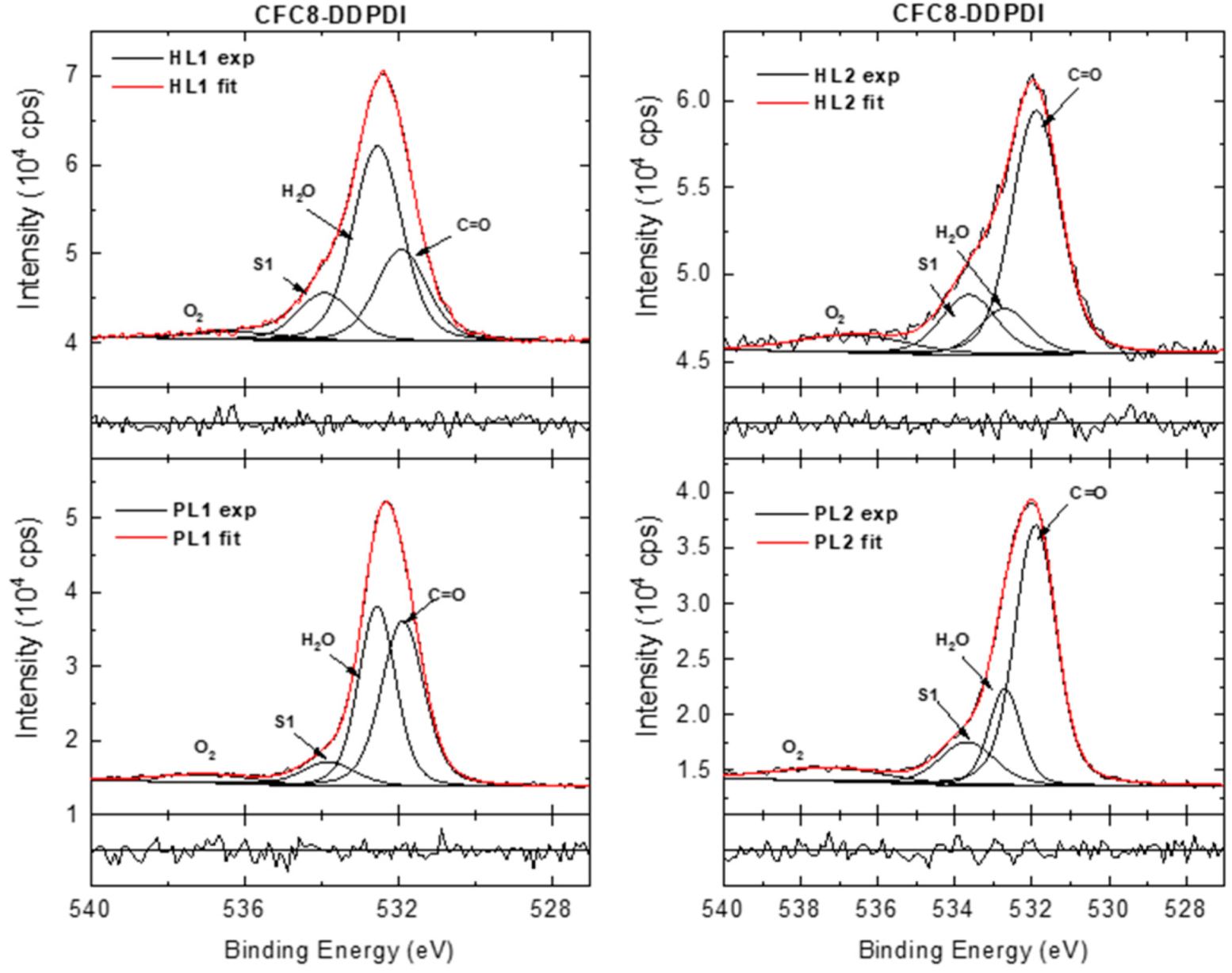
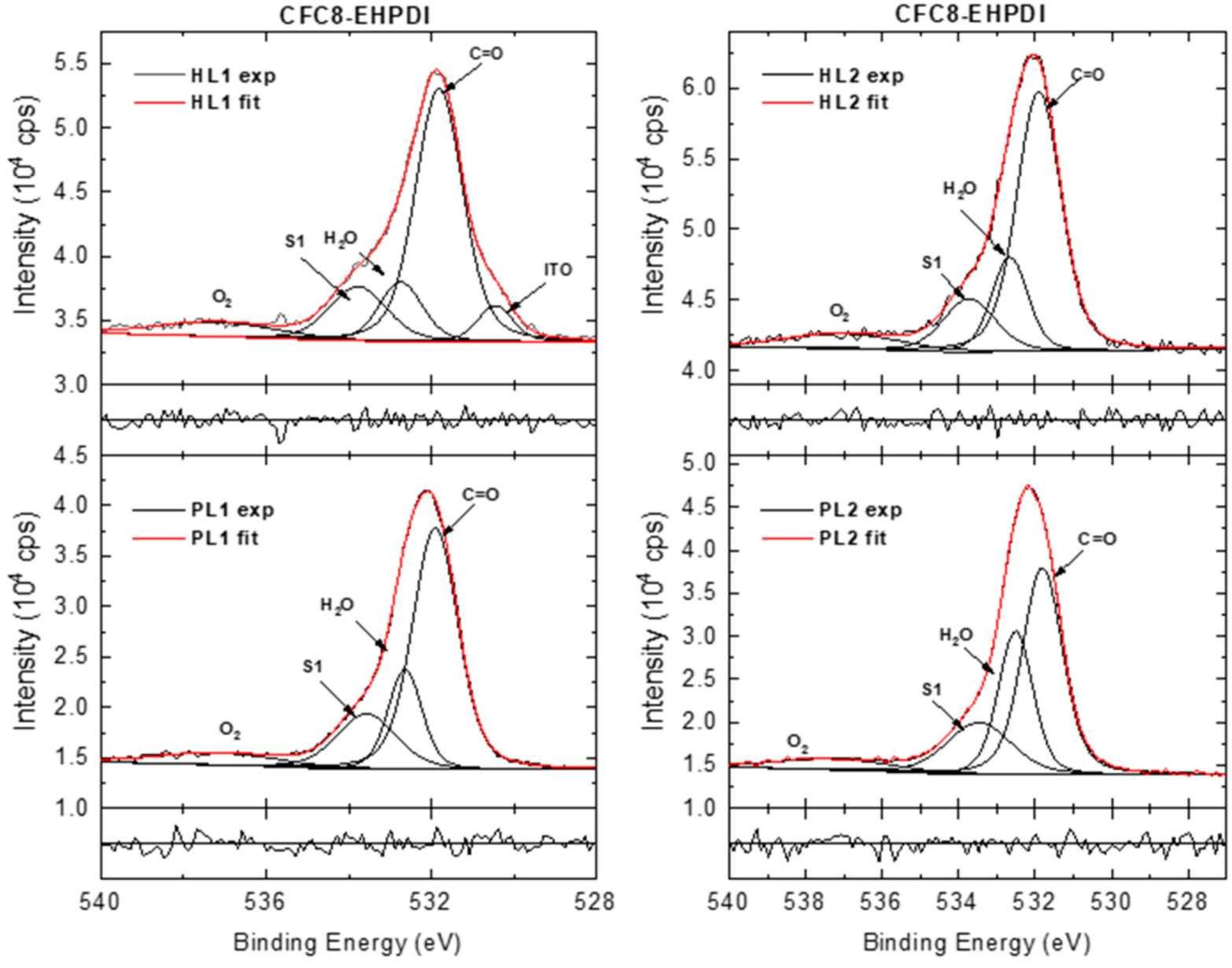

| Copolymer | Layer | λmax (nm) | λdifmax (nm) | d0 (nm) | σ (nm) |
|---|---|---|---|---|---|
| CFC8-DDPDI | PL1 | 336, 447, 556 | |||
| CFC8-DDPDI | PL2 | 334, 445, 557 | |||
| CFC8-DDPDI | HL1 | 340, 433 | 431 | 49.7 | 13.4 |
| CFC8-DDPDI | HL2 | 338, 441 | 439 | 62.7 | 13.4 |
| CFC8-EHPDI | PL1 | 334, 460, 559 | |||
| CFC8-EHPDI | PL2 | 334, 460, 559 | |||
| CFC8-EHPDI | HL1 | 335, 431 | 425 | 41.0 | 10.1 |
| CFC8-EHPDI | HL2 | 336, 472, 546 | 514 | 57.3 | 10.2 |
| Copolymer | Layer | Ag 3d | C 1s | N 1s | O 1s |
|---|---|---|---|---|---|
| CFC8-DDPDI | PL1 | 0 | 89.5 ± 0.4 | 2.3 ± 0.3 | 8.2 ± 0.3 |
| CFC8-DDPDI | HL1 | 15.0 ± 0.2 | 75.2 ± 0.7 | 1.9 ± 0.6 | 7.9 ± 0.6 |
| CFC8-DDPDI | PL2 | 0 | 91.0 ± 0.4 | 2.4 ± 0.3 | 6.6 ± 0.3 |
| CFC8-DDPDI | HL2 | 20.3 ± 0.3 | 72.9 ± 0.7 | 1.9 ± 0.6 | 4.9 ± 0.5 |
| CFC8-EHPDI | PL1 | 0 | 90.1 ± 0.4 | 2.6 ± 0.3 | 7.3 ± 0.3 |
| CFC8-EHPDI | HL1 | 11.7 ± 0.2 | 79.4 ± 0.6 | 2.3 ± 0.5 | 6.6 ± 0.5 |
| CFC8-EHPDI | PL2 | 0 | 89.6 ± 0.4 | 2.6 ± 0.3 | 7.8 ± 0.3 |
| CFC8-EHPDI | HL2 | 19.0 ± 0.3 | 72.0 ± 0.7 | 2.1 ± 0.6 | 6.9 ± 0.6 |
| Copolymer | Layer | Ag at. % | |||||
|---|---|---|---|---|---|---|---|
| CFC8-DDPDI | HL1 | 0.702 | 0.702 | 0.811 | 0.200 | 0.134 | 16.3 |
| CFC8-DDPDI | HL2 | 0.694 | 0.693 | 0.647 | 0.279 | 0.186 | 21.7 |
| CFC8-EHPDI | HL1 | 0.819 | 0.819 | 0.849 | 0.148 | 0.116 | 13.5 |
| CFC8-EHPDI | HL2 | 0.656 | 0.656 | 0.718 | 0.265 | 0.173 | 20.2 |
| Copolymer | Layer | EDX | XPS | EDX | XPS | EDX |
|---|---|---|---|---|---|---|
| Ag Atomic % | ||||||
| CFC8-DDPDI | HL1 | 0.51 | 0.200 | 0.0120 | 0.715 | 0.742 |
| CFC8-DDPDI | HL2 | 1.00 | 0.279 | 0.0162 | 1 | 1 |
| CFC8-EHPDI | HL1 | 0.45 | 0.148 | 0.0082 | 0.529 | 0.505 |
| CFC8-EHPDI | HL2 | 0.83 | 0.265 | 0.0154 | 0.948 | 0.952 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimrová, V.; Eom, S.; Pokorná, V.; Kang, Y.; Výprachtický, D. Hybrid Layers of Donor-Acceptor Copolymers with Homogenous Silver Nanoparticle Coverage for Photonic Applications. Polymers 2021, 13, 439. https://doi.org/10.3390/polym13030439
Cimrová V, Eom S, Pokorná V, Kang Y, Výprachtický D. Hybrid Layers of Donor-Acceptor Copolymers with Homogenous Silver Nanoparticle Coverage for Photonic Applications. Polymers. 2021; 13(3):439. https://doi.org/10.3390/polym13030439
Chicago/Turabian StyleCimrová, Věra, Sangwon Eom, Veronika Pokorná, Youngjong Kang, and Drahomír Výprachtický. 2021. "Hybrid Layers of Donor-Acceptor Copolymers with Homogenous Silver Nanoparticle Coverage for Photonic Applications" Polymers 13, no. 3: 439. https://doi.org/10.3390/polym13030439
APA StyleCimrová, V., Eom, S., Pokorná, V., Kang, Y., & Výprachtický, D. (2021). Hybrid Layers of Donor-Acceptor Copolymers with Homogenous Silver Nanoparticle Coverage for Photonic Applications. Polymers, 13(3), 439. https://doi.org/10.3390/polym13030439