The Impact of AN Contribution on the Thermal Characteristics and Molecular Dynamics of Novel Acrylonitrile–Styrene–Styrene Sodium Sulfonate Terpolymers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
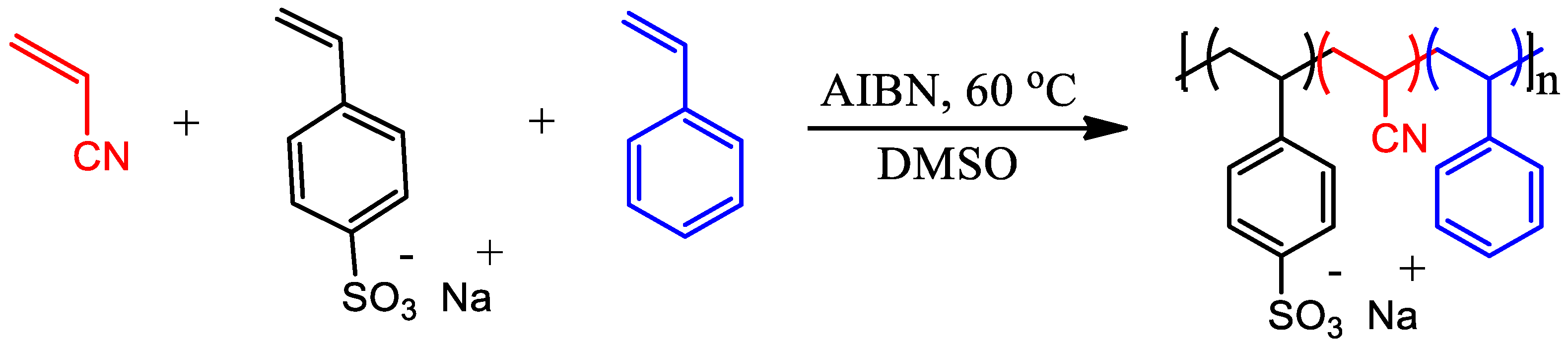
2.2. Terpolymer Synthesis
3. Results and Discussion
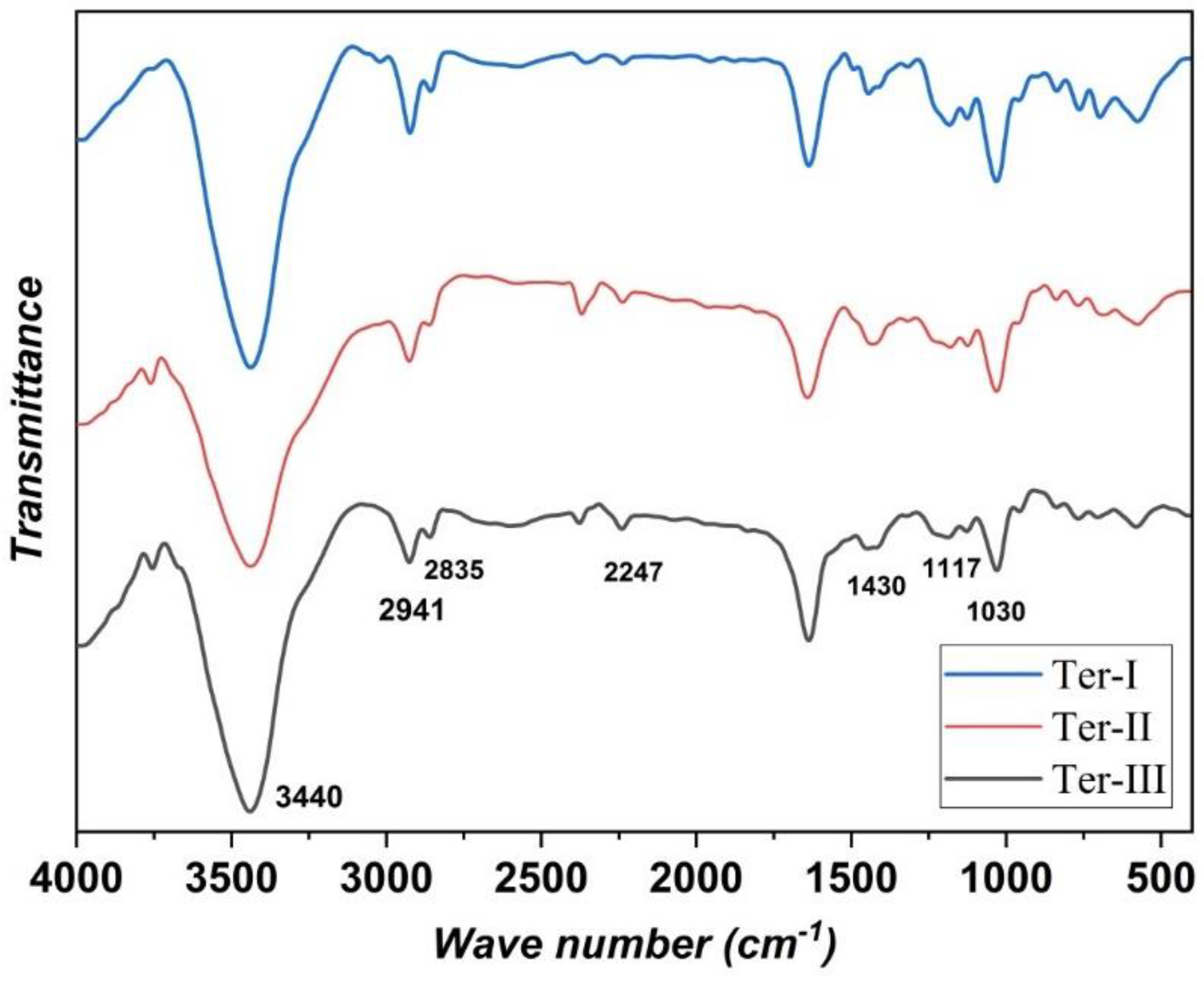
3.1. FTIR and Elemental Analysis
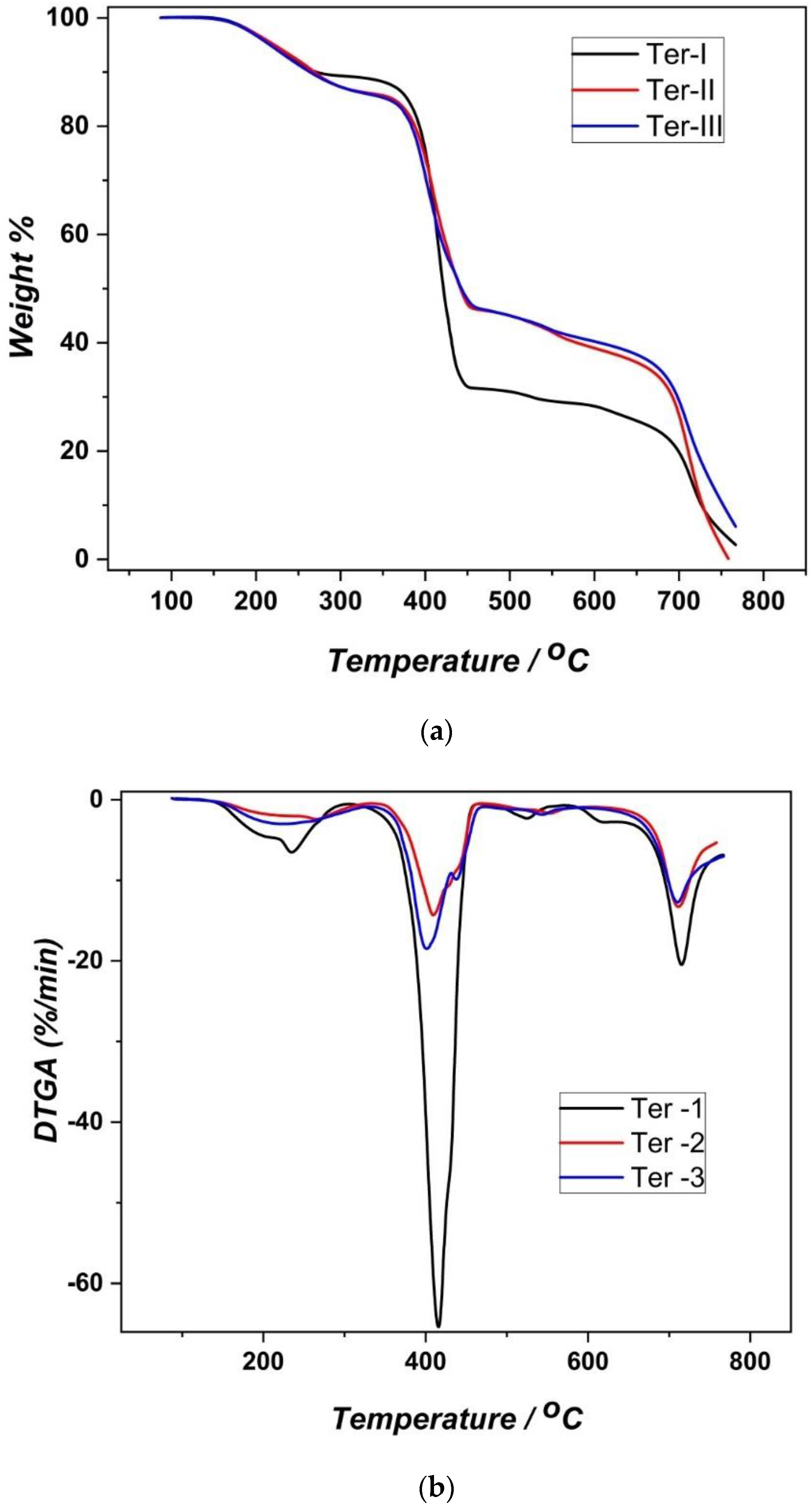
3.2. Thermogravimetric Analysis (TGA)
3.3. X-ray Diffraction (XRD)
3.4. Differential Scanning Calorimetric (DSC) Results
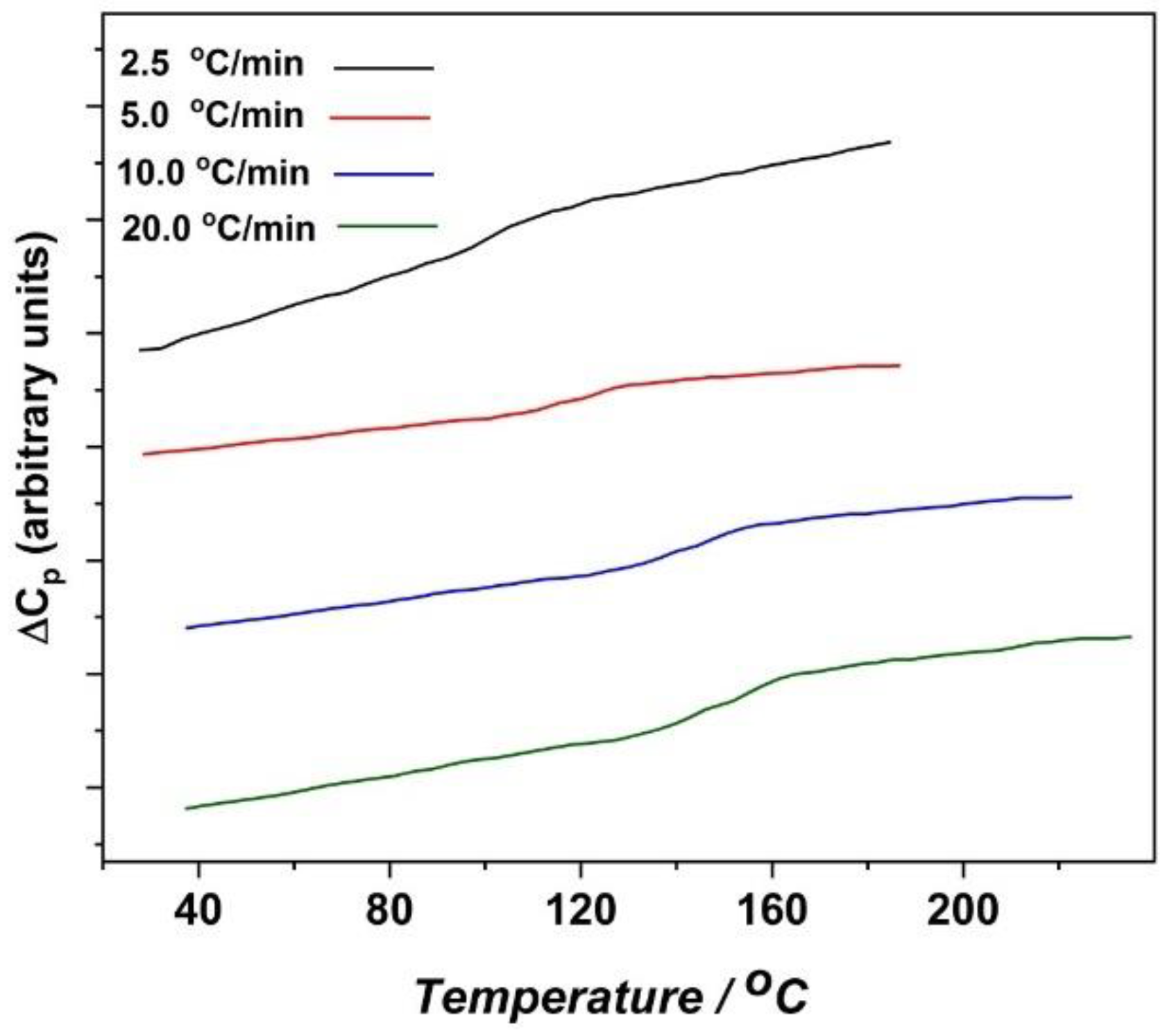
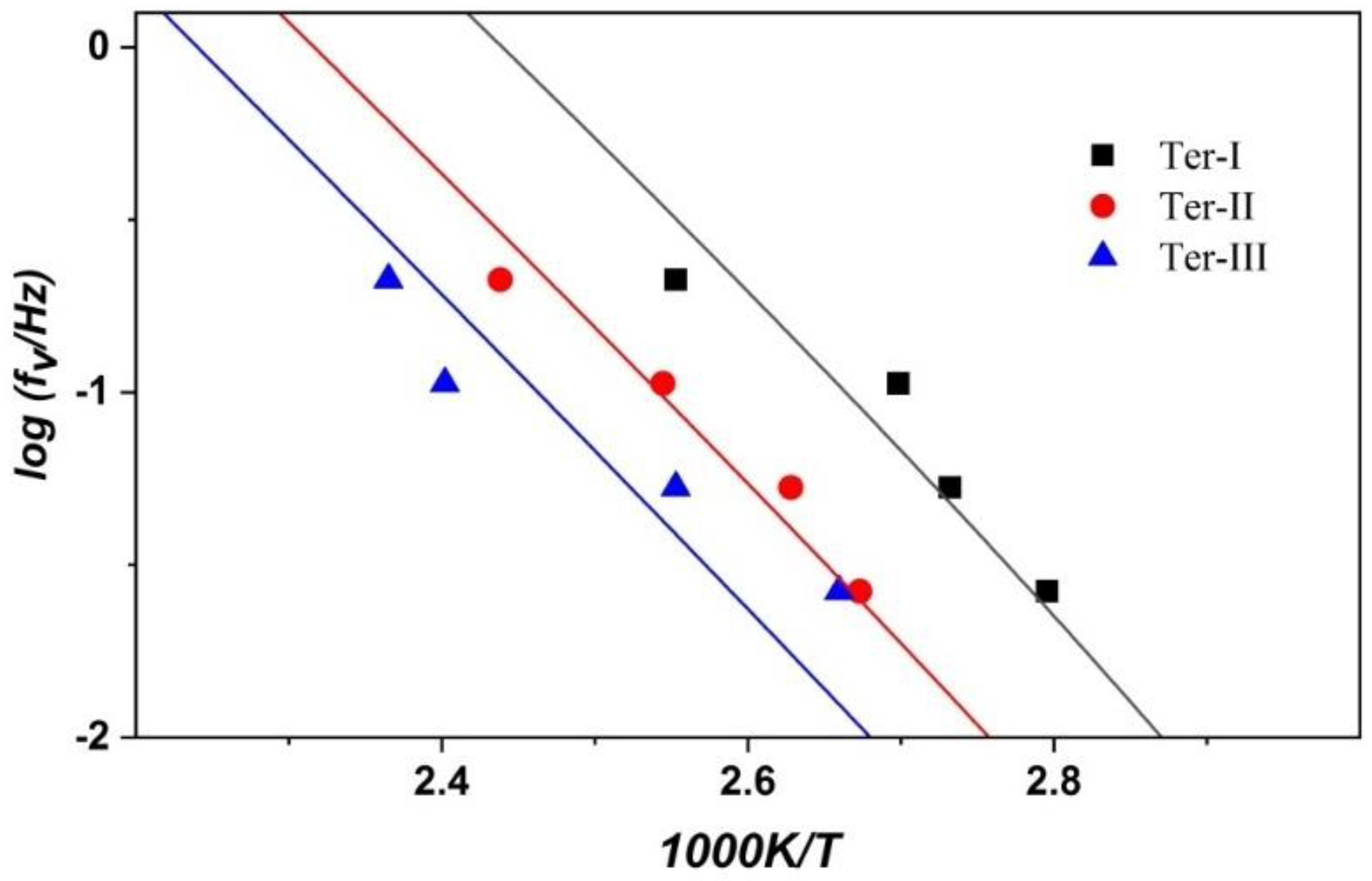
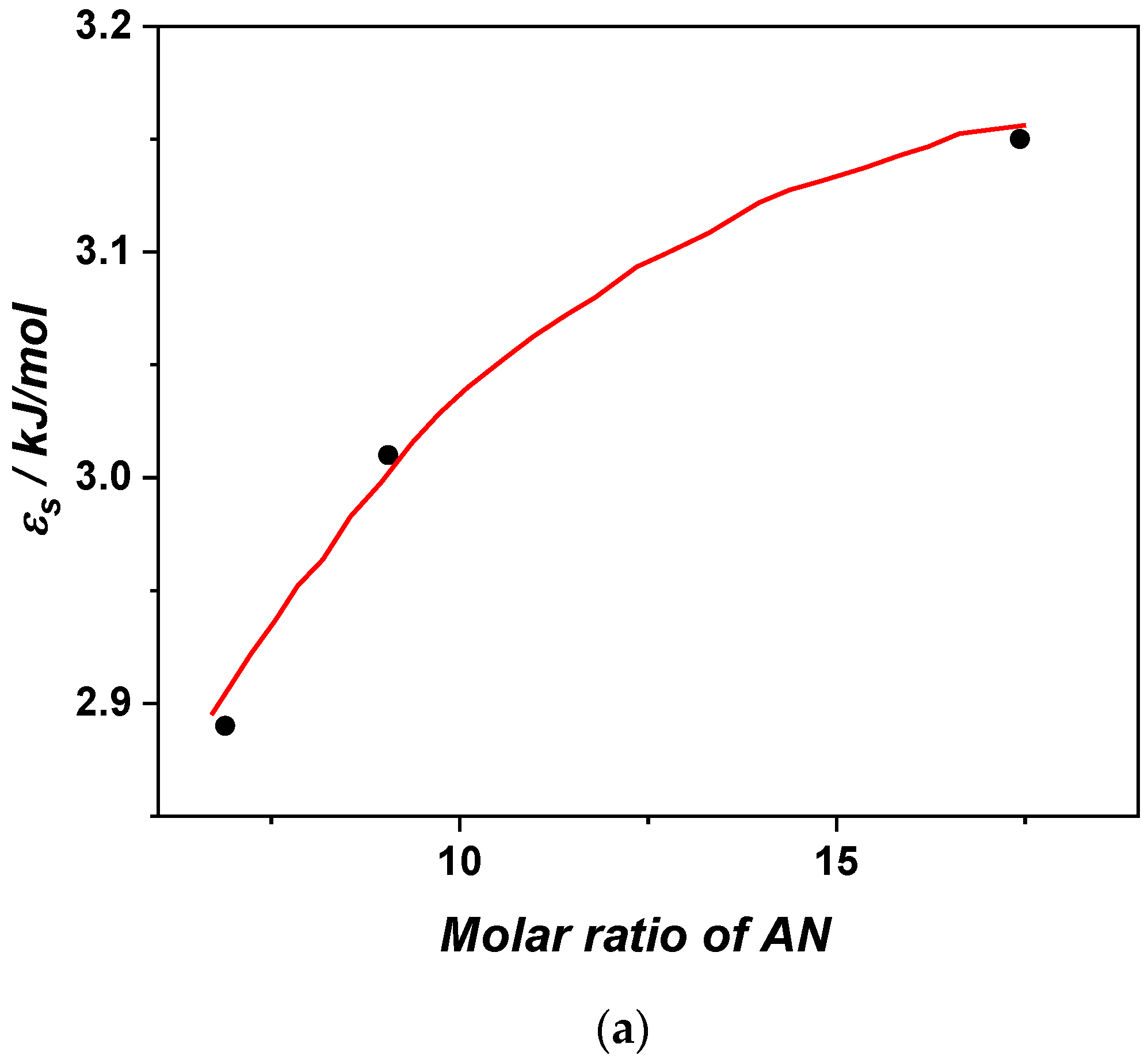
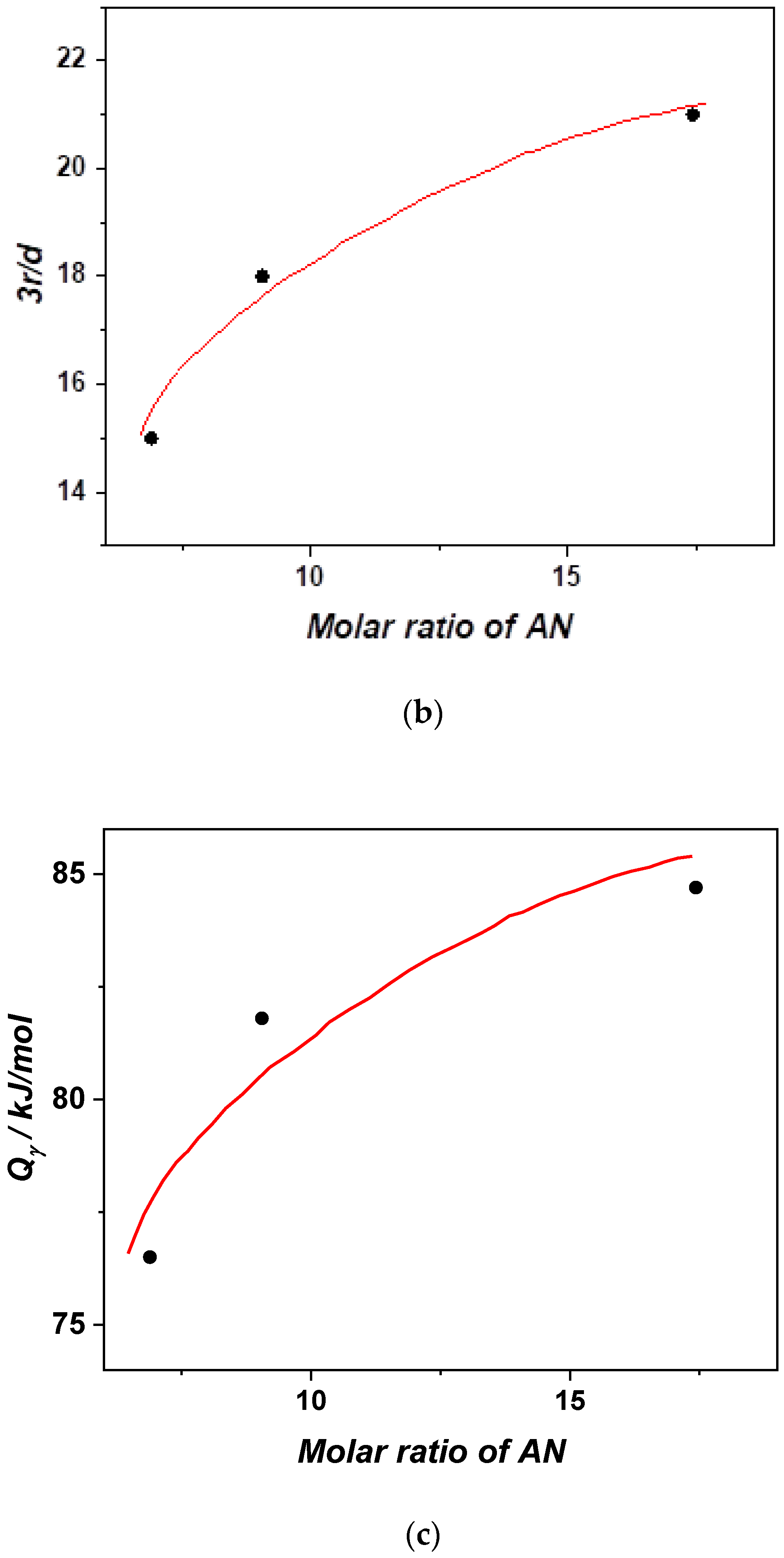
3.5. Activation Curves and the Cooperative Rearranging Regions of the Glass Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanaoka, S.; Ikeda, N.; Tanaka, A.; Yamaoka, H.; Higashimura, T. Cationic copolymerization of indene with styrene derivatives: Synthesis of random copolymers of indene with high molecular weight. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2449–2457. [Google Scholar] [CrossRef]
- Beauchemin, R.C.; Dube’, M.A. Bulk terpolymer composition prediction from copolymer reactivity ratios. Polym. React. Eng. 1999, 7, 485–499. [Google Scholar] [CrossRef]
- Dubé, M.A.; Penlidis, A.A. Systematic approach to the study of multicomponent polymerization kinetics: The butyl acrylate/methyl methacrylate/vinyl acetate example, 2. Bulk (and solution) terpolymerization. Macromol. Chem. Phys. 1995, 196, 1101–1112. [Google Scholar] [CrossRef]
- Dubé, M.A.; Penlidis, A.A. Systematic approach to the study of multicomponent polymerization kinetics of the butyl acrylate/methyl methacrylate/vinyl acetate example: 1. Bulk copolymerization. Polymer 1995, 36, 587. [Google Scholar] [CrossRef]
- Dubé, M.A.; Penlidis, A.A. Systematic approach to the study of multicomponent polymerization kinetics: Butyl acrylate/methyl methacrylate/vinyl acetate. III. Emulsion homopolymerization and copolymerization in a pilot plant reactor. Polym. Int. 1995, 37, 235. [Google Scholar] [CrossRef]
- Dubé, M.A.; Penlidis, A.A.; Reilly, P.M. A systematic approach to the study of multicomponent polymerization kinetics: The butyl acrylate/methyl methacrylate/vinyl acetate example. IV. Optimal Bayesian design of emulsion terpolymerization experiments in a pilot plant reactor. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 811–831. [Google Scholar] [CrossRef]
- Zhongqing, H.; Zhicheng, Z.; Guixi, Z.; Weijun, L. Complex-Radical terpolymerization of styrene, maleic anhydride, and N-vinyl pyrrolidone via γ-ray radiation: Synthesis, characterization, and micellization. Polymer 2005, 46, 12711–12715. [Google Scholar] [CrossRef]
- Braun, D.; Elsässer, H. Free radical terpolymerization of three non-homopolymerizable monomers, 2. Terpolymerization of N-ethylmaleimide, anethol and trans-stilbene. Macromol. Chem. Phys. 2000, 201, 2103. [Google Scholar] [CrossRef]
- Braun, D.; Elsässer, H.; Fengchao, H. Free radical terpolymerization of three non-homopolymerizable monomers. Part III. Europ. Polym. J. 2001, 37, 1779. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, K.; Peng, D.; Wu, H. Synthesis, characterization and hydrolysis of an aliphatic polycarbonate by terpolymerization of carbon dioxide, propylene oxide and maleic anhydride. Polymer 2006, 47, 8453. [Google Scholar] [CrossRef]
- Leamen, M.J.; McManus, N.T.; Penlidis, A. Terpolymerization with depropagation: Modelling the copolymer composition of the methyl methacrylate/alpha-methylstyrene/butyl acrylate system. Chem. Eng. Sci. 2006, 61, 7774–7785. [Google Scholar] [CrossRef]
- Hu, Z.; Tao, M.; Zhang, Z. “Gradient” polymer prepared by complex-radical terpolymerization of styrene, maleic anhydride, and N-vinyl pyrrolidone via gamma ray irradiation by use of a RAFT method. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 307. [Google Scholar] [CrossRef]
- Ribeiro, R.F.; Pardini, L.C.; Alves, N.P.; Brito Júnior, C.A.R. Thermal Stabilization study of polyacrylonitrile fiber obtained by extrusion. Polímeros 2015, 25, 523–530. [Google Scholar] [CrossRef]
- Brijmohan, S.B.; Swier, S.; Weiss, R.A.; Shaw, M.T. Synthesis and characterization of cross-linked sulfonated polystyrene nanoparticles. Ind. Eng. Chem. Res. 2005, 44, 8039–8045. [Google Scholar] [CrossRef]
- Gao, Z.; Kaneko, T.; Hou, D.; Nakada, M. Kinetics of thermal degradation of poly(methyl methacrylate) studied with the assistance of the fractional conversion at the maximum reaction rate. Polym. Deg. Stab. 2004, 84, 399–403. [Google Scholar] [CrossRef]
- Al-Rasheed, H.H.; Mohammady, S.Z.; Dahlous, K.; Siddiqui, M.R.H.; El-Faham, A. Synthesis, characterization, thermal stability and kinetics of thermal degradation of novel polymers based-s-triazine Schiff base. J. Polym. Res. 2020, 27, 10. [Google Scholar] [CrossRef]
- Yu, G.; Liu, C.; Li, G.; Wang, J.; Jian, X. Thermal degradation kinetics of poly(aryl ether sulfone 1,3,5-triazine) containing phthalazinone moieties. Thermochim. Acta 2011, 514, 51–57. [Google Scholar] [CrossRef]
- Lai, X.; Tang, S.; Li, H.; Zeng, X. Flame-Retardant mechanism of a novel polymeric intumescent flame retardant containing caged bicyclic phosphate for polypropylene. Polym. Deg. Stab. 2015, 113, 22–31. [Google Scholar] [CrossRef]
- Yao, Q.; Wilkie, C.A. Thermal degradation of blends of polystyrene and poly(sodium 4-styrenesulfonate) and the copolymer, poly(styrene-co-sodium 4-styrenesulfonate). Polym. Deg. Stab. 1999, 66, 379–384. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Abu-Saied, M.A.; Tamer, T.M.; Youssef, M.E.; Hashem, A.I.; Sabet, M.M. Development of polystyrene based nanoparticles ions exchange resin for water purification applications. Desalination Water Treat. 2015, 57, 14810–14823. [Google Scholar] [CrossRef]
- Watt, I.M. The Principles and Practice of Electron Microscopy; Cambridge University Press: Cambridge, England, 1997. [Google Scholar]
- Kuo, S.W.; Kao, H.C.; Chang, F.C. Thermal behavior and specific interaction in high glass transition temperature PMMA copolymer. Polymer 2003, 44, 6873. [Google Scholar] [CrossRef]
- Pechhold, W.; Grassl, O.; von Soden, W. Dynamic shear compliance of polymer melts and networks in dependence on crosslinking density. Colloid Polym. Sci. 1990, 268, 1089. [Google Scholar] [CrossRef]
- Pechhold, W. Meander model of amorphous polymers. Polym. Bull. 1982, 7, 615–622. [Google Scholar] [CrossRef]
- Donth, E.; Beiner, M.; Reissig, S.; Korus, J.; Garwe, F.; Vieweg, S.; Kahle, S.; Hempel, E.; Schröter, K. Fine structure of the main transition in amorphous polymers: Entanglement spacing and characteristic length of the glass transition. Discussion of examples. Macromolecules 1996, 29, 6589–6600. [Google Scholar] [CrossRef]
- Mohammady, S.Z.; Mansour, A.A.; Stoll, B.; von Soden, W. Effect of crosslinking on block and random copolymers of styrene and butadiene. Macromol. Chem. Phys. 2001, 202, 2732–2741. [Google Scholar] [CrossRef]
- Theobald, S.; Pechhold, W.; Stoll, B. The pressure and temperature dependence of the relaxation processes in poly(methylmethacrylate). Polymer 2001, 42, 289. [Google Scholar] [CrossRef]
- Mohammady, S.Z.; Mansour, A.A.; Knoll, K.; Stoll, B. Detection of the glass relaxation process of the PS-phase in block copolymers. Polymer 2002, 43, 2467–2478. [Google Scholar] [CrossRef]
- Mohammady, S.Z. Molecular dynamics of the glass relaxation process of the soft phase in block copolymers: Effect of molecular architecture. Rheol. Acta 2007, 46, 1109–1119. [Google Scholar] [CrossRef][Green Version]
- Donth, E. The Glass Transition: Relaxation Dynamics in Liquids and Disordered Materials; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Cangialosi, D.; Alegria, A.; Colmenero, J. Route to calculate the length scale for the glass transition in polymers. Phys. Rev. E 2007, 76, 011514. [Google Scholar] [CrossRef]
- Heinrich, W.; Stoll, B. Description of the freezing-in process in poly(vinyl acetate) based on the meander model. Prog. Colloid Polym. Sci. 1988, 78, 37–53. [Google Scholar]
- Mansour, A.A.; Stoll, B. Method of determination of dielectric relaxation characteristics of plasticized polystyrenes on the basis of calorimetric glass temperature. Colloid Polym. Sci. 1993, 271, 834–842. [Google Scholar] [CrossRef]
- Ferry, J.D. Viscoelastic Properties of Polymers, 3rd ed.; John Wiley & Sons: John Wiley, NJ, USA, 1980. [Google Scholar]

| Sample | Terpolymer | Feed Ratio (%) | Yield (%) |
|---|---|---|---|
| Ter-I | AN-St- SSS | 15/70/15 | 73.74 |
| Ter-II | AN-St- SSS | 30/40/30 | 60.21 |
| Ter-III | AN-St- SSS | 60/20/20 | 74.50 |
| Sample | % C | % H | % N | % S | % AN | % St | % SSS |
|---|---|---|---|---|---|---|---|
| Ter-I | 68.29 | 7.21 | 1.82 | 1.95 | 6.9 | 80.6 | 12.5 |
| Ter-II | 57.33 | 5.89 | 2.39 | 1.99 | 9.1 | 78.1 | 12.8 |
| Ter-III | 52.78 | 5.56 | 4.60 | 2.06 | 17.4 | 69.3 | 13.3 |
| Sample | Range (°C) | Peak (°C) | Weight Loss (%) |
|---|---|---|---|
| Ter-I | 130–350 | 235.0 | 11.8 |
| 350–490 | 415.6 | 56.9 | |
| 490–580 | 525.0 | 2.7 | |
| 600–760 | 715.3 | 28.3 | |
| Ter-II | 130–350 | 225.0 | 14.3 |
| 350–490 | 409.2 | 40.3 | |
| 490–580 | 554.0 | 6.0 | |
| 600–760 | 711.8 | 39.0 | |
| Ter-III | 130–350 | 223.0 | 14.7 |
| 350–490 | 401.0 | 39.9 | |
| 490–580 | 543.9 | 5.0 | |
| 600–760 | 709.6 | 40.2 |
| Tg Values (°C) | ||||
|---|---|---|---|---|
| Heating Rate | 20 °C/min | 10 °C/min | 5 °C/min | 2.5 °C/min |
| Ter-I | 118.7 | 97.6 | 93.0 | 84.7 |
| Ter-II | 137.2 | 120.0 | 107.5 | 101.1 |
| Ter-III | 149.8 | 143.3 | 118.6 | 103.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altaleb, H.A.; Al-Enizi, A.M.; El-Hamshary, H.; Mohammady, S.Z. The Impact of AN Contribution on the Thermal Characteristics and Molecular Dynamics of Novel Acrylonitrile–Styrene–Styrene Sodium Sulfonate Terpolymers. Polymers 2021, 13, 420. https://doi.org/10.3390/polym13030420
Altaleb HA, Al-Enizi AM, El-Hamshary H, Mohammady SZ. The Impact of AN Contribution on the Thermal Characteristics and Molecular Dynamics of Novel Acrylonitrile–Styrene–Styrene Sodium Sulfonate Terpolymers. Polymers. 2021; 13(3):420. https://doi.org/10.3390/polym13030420
Chicago/Turabian StyleAltaleb, Hamud A., Abdullah M. Al-Enizi, Hany El-Hamshary, and Sayed Z. Mohammady. 2021. "The Impact of AN Contribution on the Thermal Characteristics and Molecular Dynamics of Novel Acrylonitrile–Styrene–Styrene Sodium Sulfonate Terpolymers" Polymers 13, no. 3: 420. https://doi.org/10.3390/polym13030420
APA StyleAltaleb, H. A., Al-Enizi, A. M., El-Hamshary, H., & Mohammady, S. Z. (2021). The Impact of AN Contribution on the Thermal Characteristics and Molecular Dynamics of Novel Acrylonitrile–Styrene–Styrene Sodium Sulfonate Terpolymers. Polymers, 13(3), 420. https://doi.org/10.3390/polym13030420






