In-Situ Iodine Doping Characteristics of Conductive Polyaniline Film Polymerized by Low-Voltage-Driven Atmospheric Pressure Plasma
Abstract
1. Introduction
2. Materials and Methods
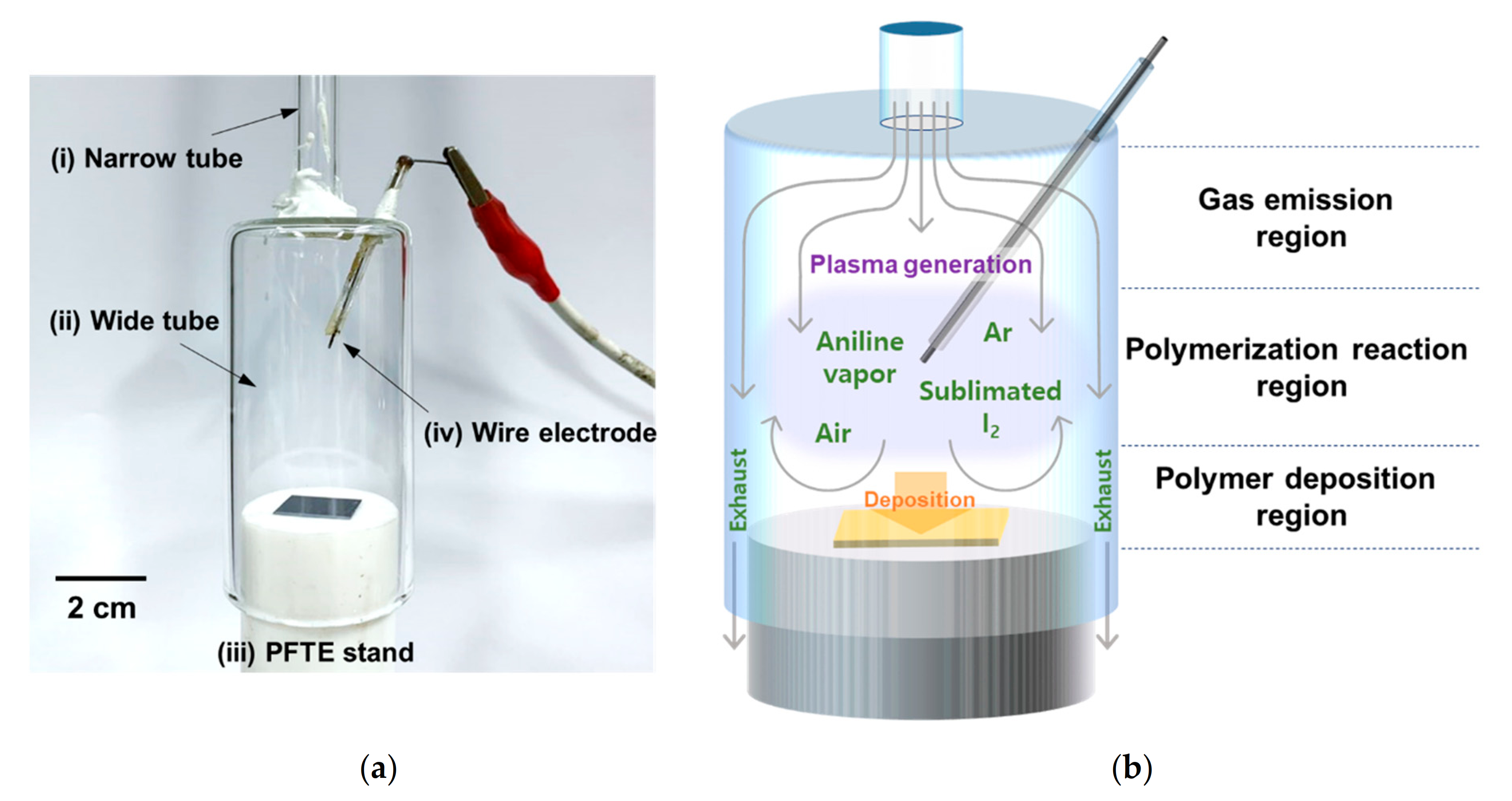
2.1. Preparation of the AP Plasma Reactor Device
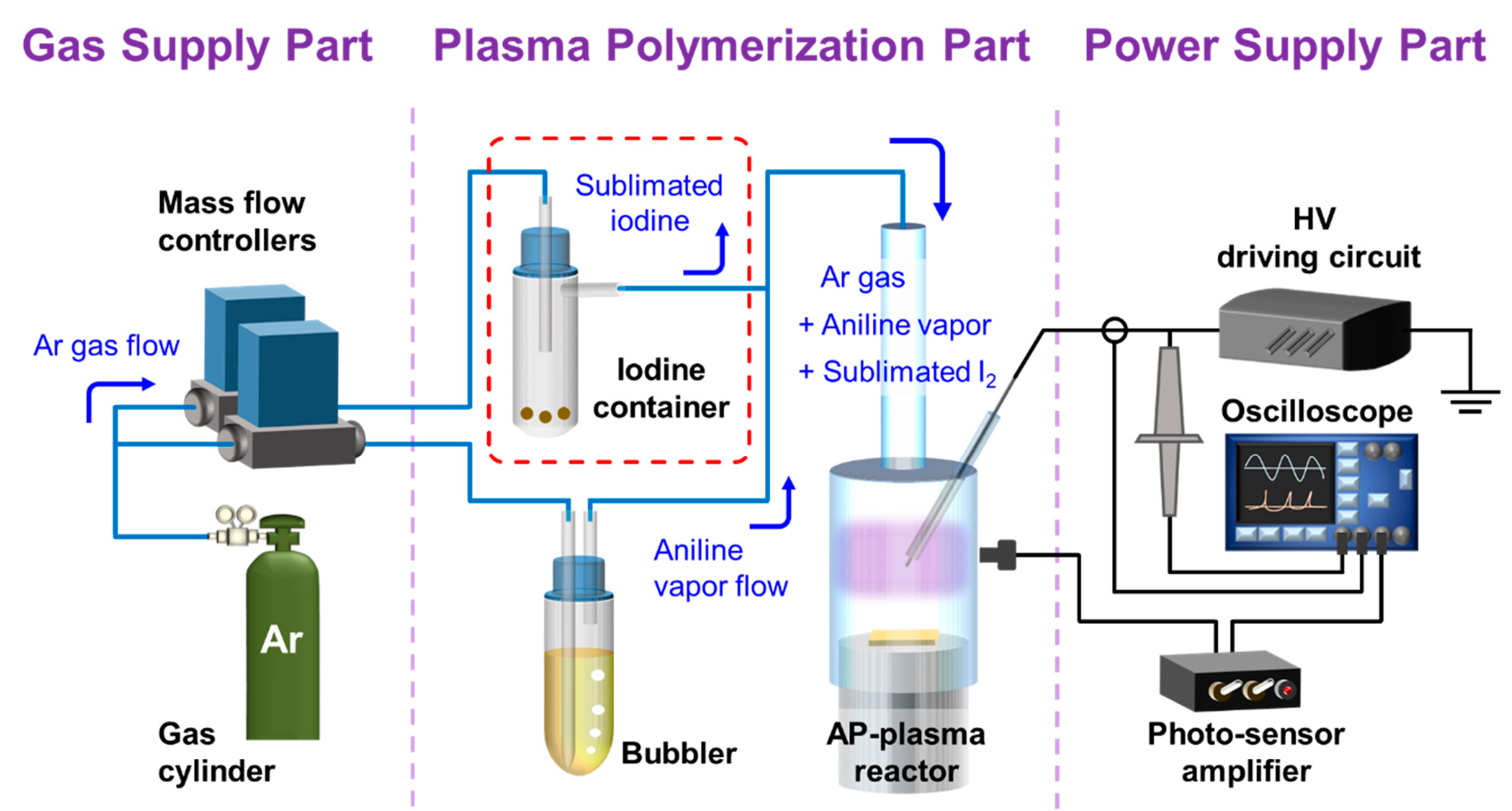
2.2. Entire Assembled System for AP Plasma Polymerization
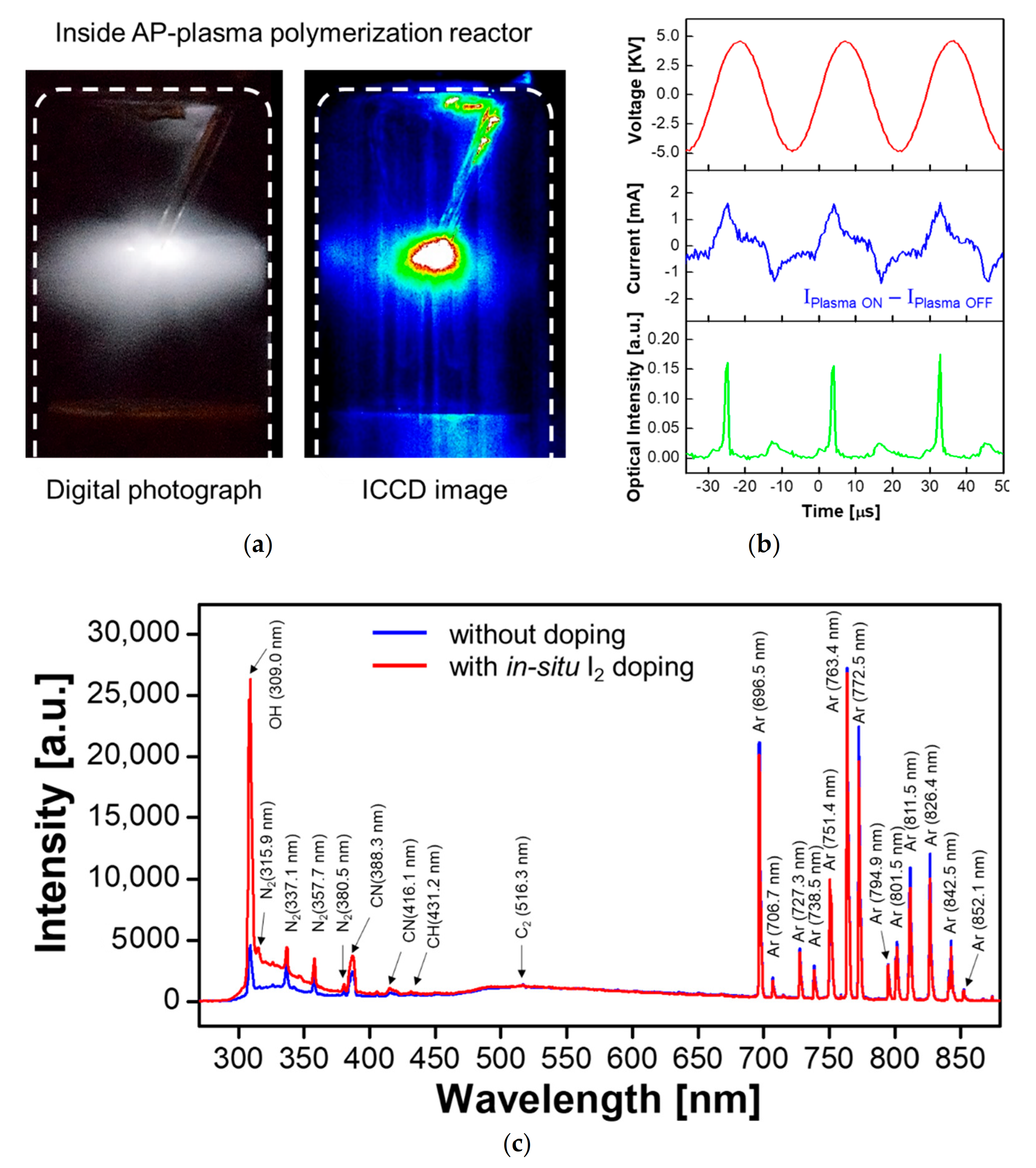
2.3. Characterization of the AP Plasma during Plasma Polymerization
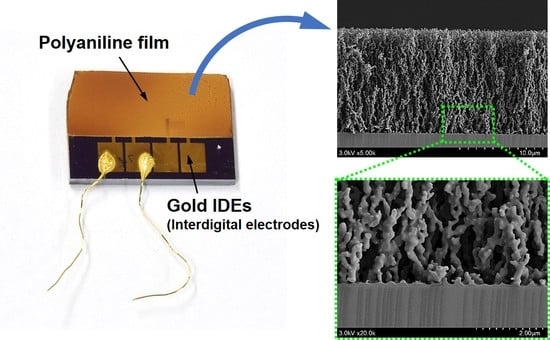
2.4. Characterization of the PANI Films
3. Results and Discussion
3.1. Optical and Electrical Properties of Plasmas Generated by the AP Plasma Reactor
3.2. The AP Plasma Polymerized Aniline Film
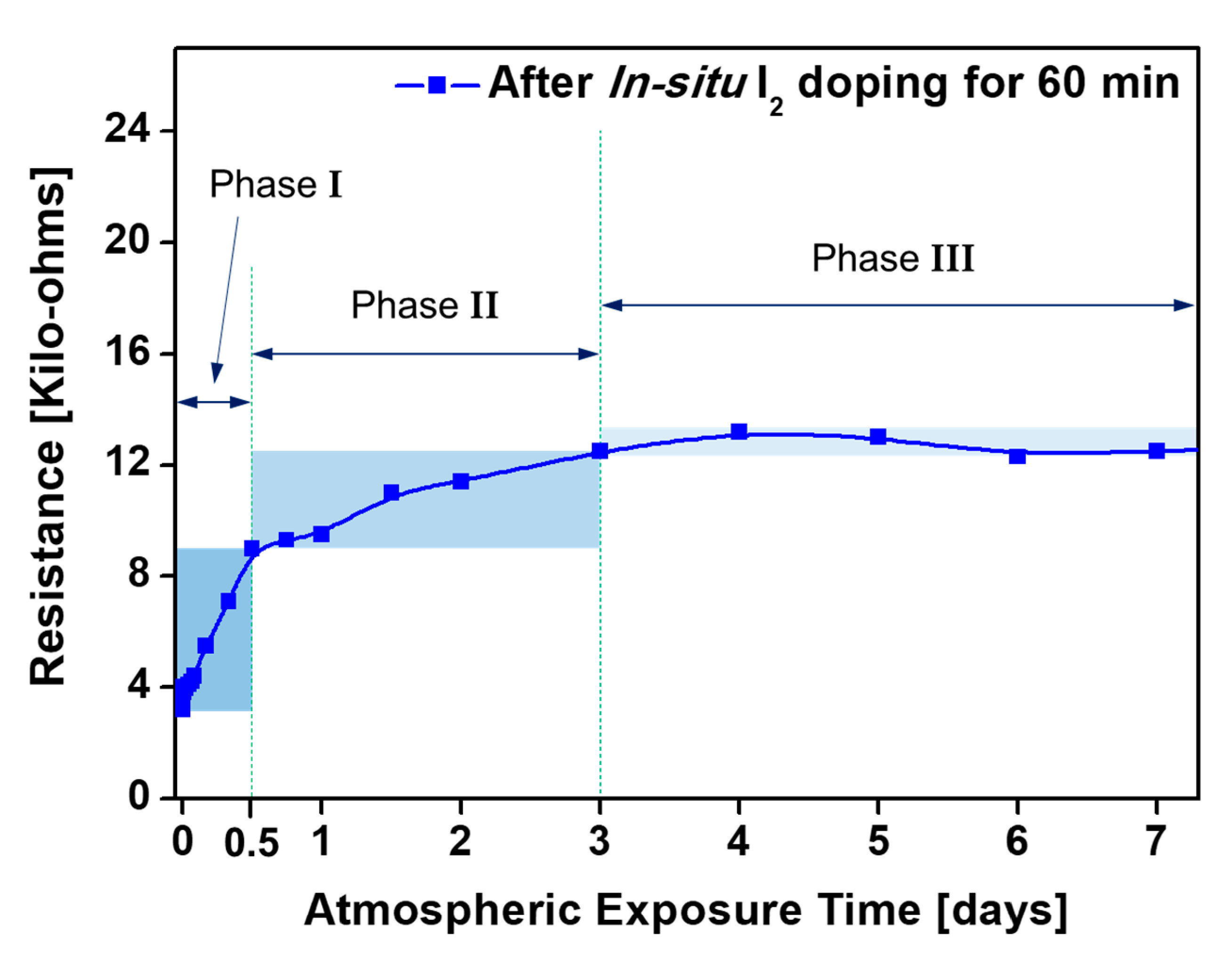
3.3. The In-Situ Iodine Doped PANI Film
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thiry, D.; Konstantinidis, S.; Cornil, J.; Snyders, R. Plasma diagnostics for the low-pressure plasma polymerization process: A critical review. Thin Solid Films 2016, 606, 19–44. [Google Scholar] [CrossRef]
- Teslaru, T.; Topala, I.; Dobromir, M.; Pohoata, V.; Curecheriu, L.; Dumitrascu, N. Polythiophene films obtained by polymerization under atmospheric pressure plasma conditions. Mater. Chem. Phys. 2016, 169, 120–127. [Google Scholar] [CrossRef]
- Cho, S.-H.; Park, Z.-T.; Kim, J.-G.; Boo, J.-H. Physical and optical properties of plasma polymerized thin films deposited by PECVD method. Surf. Coat. Technol. 2003, 174, 1111–1115. [Google Scholar] [CrossRef]
- Friedrich, J. Mechanisms of Plasma Polymerization—Reviewed from a Chemical Point of View. Plasma Process. Polym. 2011, 8, 783–802. [Google Scholar] [CrossRef]
- Vasquez-Ortega, M.; Ortega, M.; Morales, J.; Olayo, M.G.; Cruz, G.J.; Olayo, R. Core–shell polypyrrole nanoparticles ob-tained by atmospheric pressure plasma polymerization. Polym. Int. 2014, 63, 2021–2029. [Google Scholar] [CrossRef]
- Shin, J.-G.; Shin, B.J.; Jung, E.Y.; Park, C.-S.; Kim, J.Y.; Tae, H.-S. Effects of a dielectric barrier discharge (DBD) on characteris-tics of polyaniline nanoparticles synthesized by a solution plasma process with an Ar gas bubble channel. Polymers 2020, 12, 1939. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-G.; Park, C.-S.; Jung, E.-Y.; Shin, B.J.; Tae, H.-S. Synthesis of a polyaniline nanoparticle using a solution plasma pro-cess with an Ar gas bubble channel. Polymers 2019, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Van Deynse, A.; Cools, P.; Leys, C.; De Geyter, N.; Morent, R. Surface activation of polyethylene with an argon atmospheric pressure plasma jet: Influence of applied power and flow rate. Appl. Surf. Sci. 2015, 328, 269–278. [Google Scholar] [CrossRef]
- Petersen, J.; Becker, C.M.; Fouquet, T.; Addiego, F.; Toniazzo, V.; Dinia, A.; Ruch, D. Nano-ordered thin films achieved by soft atmospheric plasma polymerization. RSC Adv. 2013, 3, 4416–4424. [Google Scholar] [CrossRef]
- Kasih, T.P.; Kuroda, S.-I.; Kubota, H. Poly(methyl methacrylate) Films Deposited via Non-Equilibrium Atmospheric Pressure Plasma Polymerization Using Argon as Working Gas. Plasma Process. Polym. 2007, 4, 648–653. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-H.; Choi, E.-H.; Kim, K.-M.; Kim, K.-N. Development of hydrophilic dental wax without surfactant using a non-thermal air atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2014, 47, 235402. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Van Vlierberghe, S.; Dubruel, P.; Leys, C.; Gengembre, L.; Schacht, E.; Payen, E. Deposition of polymethyl methacrylate on polypropylene substrates using an atmospheric pressure dielectric barrier discharge. Prog. Org. Coat. 2009, 64, 230–237. [Google Scholar] [CrossRef]
- Van Vrekhem, S.; Morent, R.; De Geyter, N. Deposition of a PMMA coating with an atmospheric pressure plasma jet. J. Coat. Technol. Res. 2018, 15, 679–690. [Google Scholar] [CrossRef]
- Cools, P.; Sainz-Garcı, E.; Geyter, N.D.; Nikiforov, A.; Blajan, M.; Shimizu, K.; Alba-Elıas, F.; Leys, C.; Morent, R. Influence of DBD inlet geometry on the homogeneity of plasma-polymerized acrylic acid films: The use of a microplasma–electrode in-let configuration. Plasma Process. Polym. 2015, 12, 1153–1163. [Google Scholar] [CrossRef]
- Park, C.-S.; Kim, D.H.; Shin, B.J.; Tae, H.-S. Synthesis and Characterization of Nanofibrous Polyaniline Thin Film Prepared by Novel Atmospheric Pressure Plasma Polymerization Technique. Materials 2016, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-S.; Jung, E.Y.; Kim, D.H.; Cho, B.-G.; Shin, B.J.; Tae, H.-S. TOF-SIMS study on nano size conducting polymer pre-pared by simple atmospheric pressure plasma polymerization technique for display applications. Mol. Cryst. Liq. Cryst. 2017, 651, 16–25. [Google Scholar] [CrossRef]
- Park, C.-S.; Kim, D.Y.; Kim, D.H.; Lee, H.-K.; Shin, B.J.; Tae, H.-S. Humidity-independent conducting polyaniline films syn-thesized using advanced atmospheric pressure plasma polymerization with in-situ iodine doping. Appl. Phys. Lett. 2017, 110, 033502. [Google Scholar] [CrossRef]
- Park, C.-S.; Jung, E.Y.; Kim, D.H.; Kim, D.Y.; Lee, H.-K.; Shin, B.J.; Lee, D.H.; Tae, H.-S. Atmospheric Pressure Plasma Polymerization Synthesis and Characterization of Polyaniline Films Doped with and without Iodine. Materials 2017, 10, 1272. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, C.-S.; Jung, E.Y.; Shin, B.J.; Kim, J.Y.; Bae, G.T.; Jang, H.J.; Cho, B.-G.; Tae, H.-S. Effects of iodine dopant on atmospheric pressure plasma polymerized pyrrole in remote and coupling methods. Mol. Cryst. Liq. Cryst. 2018, 677, 135–142. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, C.-S.; Jung, E.Y.; Kum, D.S.; Kim, J.Y.; Kim, D.; Bae, G.T.; Cho, B.-G.; Shin, B.J.; Lee, N.H.; et al. Experimental study on atmospheric pressure plasma polymerized conducting polymer under coupling and remote conditions. Mol. Cryst. Liq. Cryst. 2018, 663, 108–114. [Google Scholar] [CrossRef]
- Jang, H.J.; Park, C.-S.; Jung, E.Y.; Bae, G.T.; Shin, B.J.; Tae, H.-S. Synthesis and Properties of Thiophene and Aniline Copolymer Using Atmospheric Pressure Plasma Jets Copolymerization Technique. Polymer 2020, 12, 2225. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Vivek, R.; Wang, J.-Y. Polyaniline Nanoparticles: Synthesis, Dispersion and Biomedical Applications. Mini-Rev. Org. Chem. 2017, 14, 56–64. [Google Scholar] [CrossRef]
- Goktas, H.; Demircioglu, Z.; Sel, K.; Gunes, T.; Kaya, I. The optical properties of plasma polymerized polyaniline thin films. Thin Solid Films 2013, 548, 81–85. [Google Scholar] [CrossRef]
- Qin, Q.; Tao, J.; Yang, Y. Preparation and characterization of polyaniline film on stainless steel by elecrochemical polymer-ization as a counter electrode of DSSC. Synth. Met. 2010, 160, 1167–1172. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef]
- Morales, J.; Olayo, M.G.; Cruz, G.J.; Olayo, R. Synthesis by plasma and characterization of bilayer aniline-pyrrole thin films doped with iodine. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1850–1856. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Damaskaite, A.; Plikusiene, I.; Ramanavicius, A.; Ramanaviciene, A. Electrodeposited Gold Nanostructures for the Enhancement of Electrochromic Properties of PANI–PEDOT Film Deposited on Transparent Electrode. Polymer 2020, 12, 2778. [Google Scholar] [CrossRef]
- Gosselin, D.; Gougis, M.; Baque, M.; Navarro, F.P.; Belgacem, M.N.; Chaussy, D.; Bourdat, A.-G.; Mailley, P.; Berthier, J. Screen-Printed Polyaniline-Based Electrodes for the Real-Time Monitoring of Loop-Mediated Isothermal Amplification Reactions. Anal. Chem. 2017, 89, 10124–10128. [Google Scholar] [CrossRef]
- Wang, X.; Deng, J.; Duan, X.; Liu, D.; Guo, J.; Liu, P. Crosslinked polyaniline nanorods with improved electrochemical per-formance as electrode material for supercapacitors. J. Mater. Chem. A 2014, 2, 12323–12329. [Google Scholar] [CrossRef]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Formation and electrochemical evaluation of polyaniline and polypyr-role nanocomposites based on glucose oxidase and gold nanostructures. Polymers 2020, 12, 3026. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive polyaniline-based gas sensors: A mini review. Sens. Actuators B Chem. 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Virji, S.; Huang, J.; Kaner, R.B.; Weiller, B.H. Polyaniline Nanofiber Gas Sensors: Examination of Response Mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Pron, A.; Rannou, P. Processible conjugated polymers: From organic semiconductors to organic metals and superconductors. Prog. Polym. Sci. 2002, 27, 135–190. [Google Scholar] [CrossRef]
- Elmas, S.; Beelders, W.; Nash, J.; Macdonald, T.J.; Jasieniak, M.; Griesser, H.J.; Nann, T. Photo-doping of plasma-deposited polyaniline (PAni). RSC Adv. 2016, 6, 70691–70699. [Google Scholar] [CrossRef]
- Fan, L.; Xu, X. A simple strategy to enhance electrical conductivity of nanotube-conjugate polymer composites via io-dine-doping. RSC Adv. 2015, 5, 78104–78108. [Google Scholar] [CrossRef]
- Takechi, K.; Shiga, T.; Motohiro, T.; Akiyama, T.; Yamada, S.; Nakayama, H.; Kohama, K. Solar cells using iodine-doped polythiophene–porphyrin polymer films. Sol. Energy Mater. Sol. Cells 2006, 90, 1322–1330. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymer 2017, 9, 150. [Google Scholar] [CrossRef]
- Mansuroglu, D.; Uzun-Kaymak, I.U. Investigation into Ex-Situ and In-Situ Iodine Doped Plasma Polymerized Fluo-rene-type Thin Film. In Materials Today: Proceedings; Elsevier: Amsterdam, The Netherlands, 2019; Volume 18, pp. 1955–1963. [Google Scholar]
- Wang, J.; Neoh, K.G.; Kang, E.T. Comparative study of chemically synthesized and plasma polymerized pyrrole and thio-phene thin films. Thin Solid Films 2004, 446, 205–217. [Google Scholar] [CrossRef]
- Silverstein, M.; Visoly-Fisher, I. Plasma polymerized thiophene: Molecular structure and electrical properties. Polymer 2002, 43, 11–20. [Google Scholar] [CrossRef]
- Mathai, C.J.; Saravanan, S.; Anantharaman, M.; Venkitachalam, S.; Jayalekshmi, S. Effect of iodine doping on the bandgap of plasma polymerized aniline thin films. J. Phys. D Appl. Phys. 2002, 35, 2206–2210. [Google Scholar] [CrossRef]
- Kim, T.-W.; Lee, J.-H.; Back, J.-W.; Jung, W.-G.; Kim, J.-Y. Deposition and in-situ plasma doping of plasma-polymerized thi-ophene films using PECVD. Macromol. Res. 2009, 17, 31–36. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V. Effects of Iodine Doping on Optoelectronic and Chemical Properties of Polyterpenol Thin Films. Nanomaterials 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Sajeev, U.S.; Mathai, C.J.; Saravanan, S.; Ashokan, R.R.; Venkatachalam, S.; Anantharaman, M.R. On the optical and electrical properties of rf and a.c. plasma polymerized aniline thin films. Bull. Mater. Sci. 2006, 29, 159–163. [Google Scholar] [CrossRef]
- Kim, D.B.; Rhee, J.K.; Gweon, B.; Moon, S.Y.; Choe, W. Comparative study of atmospheric pressure low and radio frequency microjet plasmas produced in a single electrode configuration. Appl. Phys. Lett. 2007, 91, 151502. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, S.J.; Joh, H.M.; Chung, T.H.; Bae, S.H.; Leem, S.H. Optical and electrical characterization of an atmospheric pressure microplasma jet with a capillary electrode. Phys. Plasmas 2010, 17, 33502. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ballato, J.; Kim, S.-O. Intense and Energetic Atmospheric Pressure Plasma Jet Arrays. Plasma Process. Polym. 2012, 9, 253–260. [Google Scholar] [CrossRef]
- Djurovic, S.; Roberts, J.R.; Sobolewski, M.A.; Olthoff, J.K. Absolute Spatially- and Temporally-Resolved Optical Emission Measurements of rf Glow Discharges in Argon. J. Res. Natl. Inst. Stand. Technol. 1993, 98, 159–180. [Google Scholar] [CrossRef]
- Popescu, S.; Jerby, E.; Meir, Y.; Barkay, Z.; Ashkenazi, D.; Mitchell, J.B.A.; Le Garrec, J.-L.; Narayanan, T. Plasma column and nano-powder generation from solid titanium by localized microwaves in air. J. Appl. Phys. 2015, 118, 023302. [Google Scholar] [CrossRef]
- Kim, H.-J.; Shin, J.-G.; Park, C.-S.; Kum, D.S.; Shin, B.J.; Kim, J.Y.; Park, H.-D.; Choi, M.; Tae, H.-S. In-Liquid Plasma Process for Size- and Shape-Controlled Synthesis of Silver Nanoparticles by Controlling Gas Bubbles in Water. Materials 2018, 11, 891. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, Z.; Xiong, Q.; Tang, Z.; Hu, X.; Pan, Y. An 11cm long atmospheric pressure cold plasma plume for applications of plasma medicine. Appl. Phys. Lett. 2008, 92, 081502. [Google Scholar] [CrossRef]
- Teschke, M.; Kedzierski, J.; Finantu-Dinu, E.G.; Korzec, D.; Engemann, J. High-speed photographs of a dielectric barrier at-mospheric pressure plasma jet. IEEE Trans. Plasma Sci. 2005, 33, 310–311. [Google Scholar] [CrossRef]
- Lia, Q.; Li, J.-T.; Zhu, W.-C.; Zhu, X.-M.; Pu, Y.-K. Effects of gas flow rate on the length of atmospheric pressure nonequilib-rium plasma jets. Appl. Phys. Lett. 2009, 95, 141502. [Google Scholar] [CrossRef]
- Sands, B.L.; Huang, S.K.; Ganguly, B.N. Current scaling in an atmospheric pressure capillary dielectric barrier discharge. Appl. Phys. Lett. 2009, 95, 51502. [Google Scholar] [CrossRef]
- Laroussi, M.; Lu, X. Room-temperature atmospheric pressure plasma plume for biomedical applications. Appl. Phys. Lett. 2005, 87, 113902. [Google Scholar] [CrossRef]
- Ito, T.; Gotoh, K.; Sekimoto, K.; Hamaguchi, S.; Gotou, K. Mass Spectrometry Analyses of Ions Generated by Atmospheric-Pressure Plasma Jets in Ambient Air. Plasma Med. 2015, 5, 283–298. [Google Scholar] [CrossRef]
- Sharma, A.K.; Bhardwaj, P.; Dhawan, S.K.; Sharma, Y. Oxidative synthesis and electrochemical studies of poly(aniline-co-pyrrole)-hybrid carbon nanostructured composite electrode materials for supercapacitor. Adv. Mater. Lett. 2015, 6, 414–420. [Google Scholar] [CrossRef]
- Srinivasan, P.; Gottam, R. Infrared Spectra: Useful technique to identify the conductivity level of emeraldine form of poly-aniline and indication of conductivity measurement either two or four probe technique. Mat. Sci. Res. India 2018, 15, 209–217. [Google Scholar] [CrossRef]
- Botewad, S.N.; Pahurkar, V.G.; Muley, G.G. Fabrication and evaluation of evanescent wave absorption based polyani-line-cladding modified fiber optic urea biosensor. Opt. Fiber Technol. 2018, 40, 8–12. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Liu, Y.; Wang, L.; Gao, C. Enhanced thermoelectric properties of polyaniline/polypyrrole/carbon nano-tube ternary composites by treatment with a secondary dopant using ferric chloride. J. Mater. Chem. C 2020, 8, 528–535. [Google Scholar] [CrossRef]
- Su, N. Improving Electrical Conductivity, Thermal Stability, and Solubility of Polyaniline-Polypyrrole Nanocomposite by Doping with Anionic Spherical Polyelectrolyte Brushes. Nanoscale Res. Lett. 2015, 10, 1–9. [Google Scholar] [CrossRef]

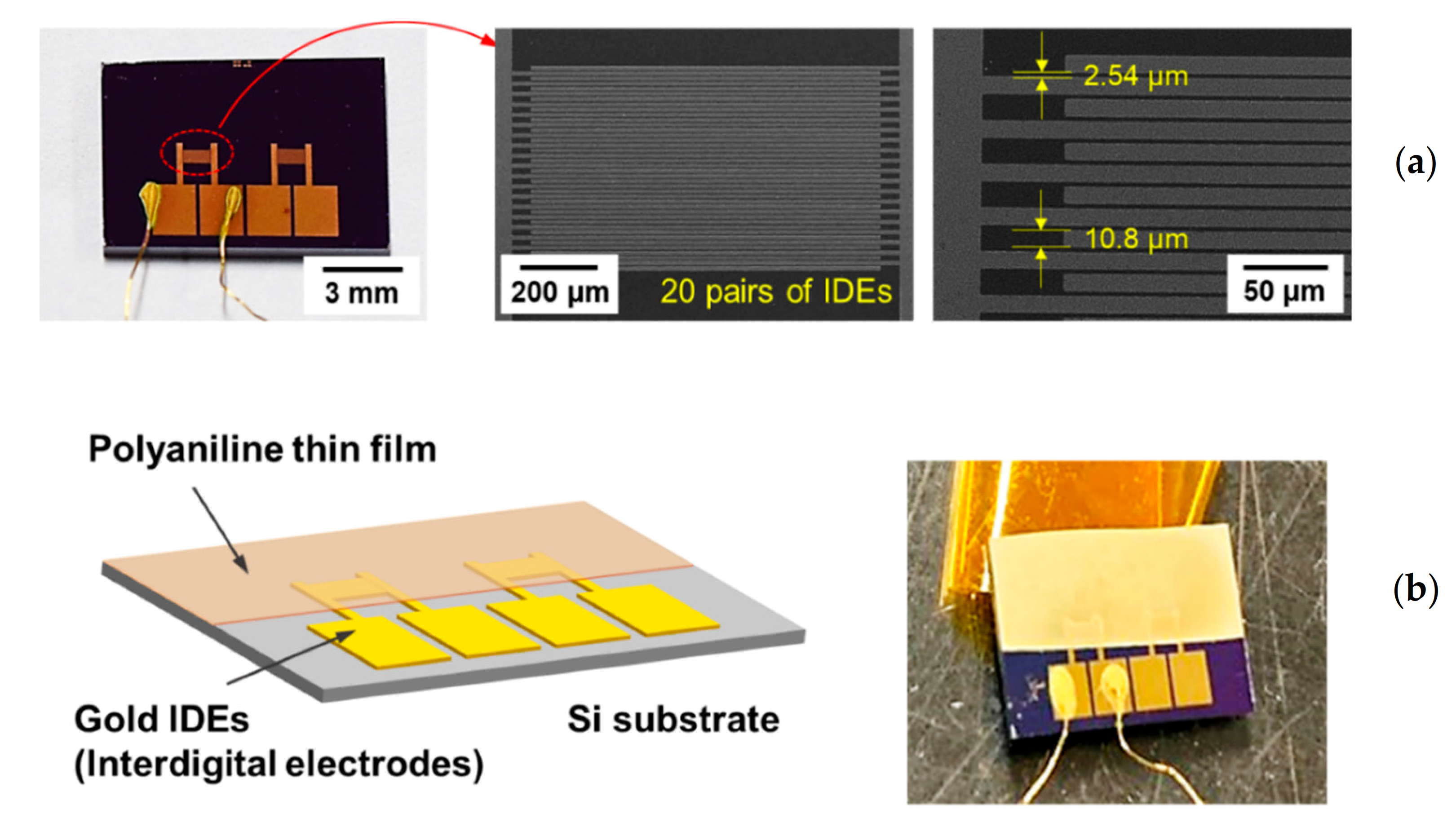
| Experimental Conditions | AP Plasma Reactor | |
|---|---|---|
| Device Configuration | Electrode type | Single electrode |
| Electrode material | Tungsten wire | |
| Inner diameter of narrow glass tube | 6.8 mm | |
| Inner diameter of wide glass tube | 34 mm | |
| Substrate stand material | Polytetrafluoroethylene (PTFE) | |
| Diameter of substrate stand | 30 mm | |
| Driving Conditions | Driving type | AC |
| Voltage waveform | Sinusoidal | |
| Plasma initiation voltage | 2.8 kV | |
| Plasma driving voltage | 5 kV | |
| Driving frequency | 30 kHz | |
| Gas Conditions | Gas type | Ar |
| Gas purity | HP grade (99.999%) | |
| Flow rate for aniline monomer vapor | 500 sccm | |
| Flow rate for sublimated iodine | 500 sccm | |
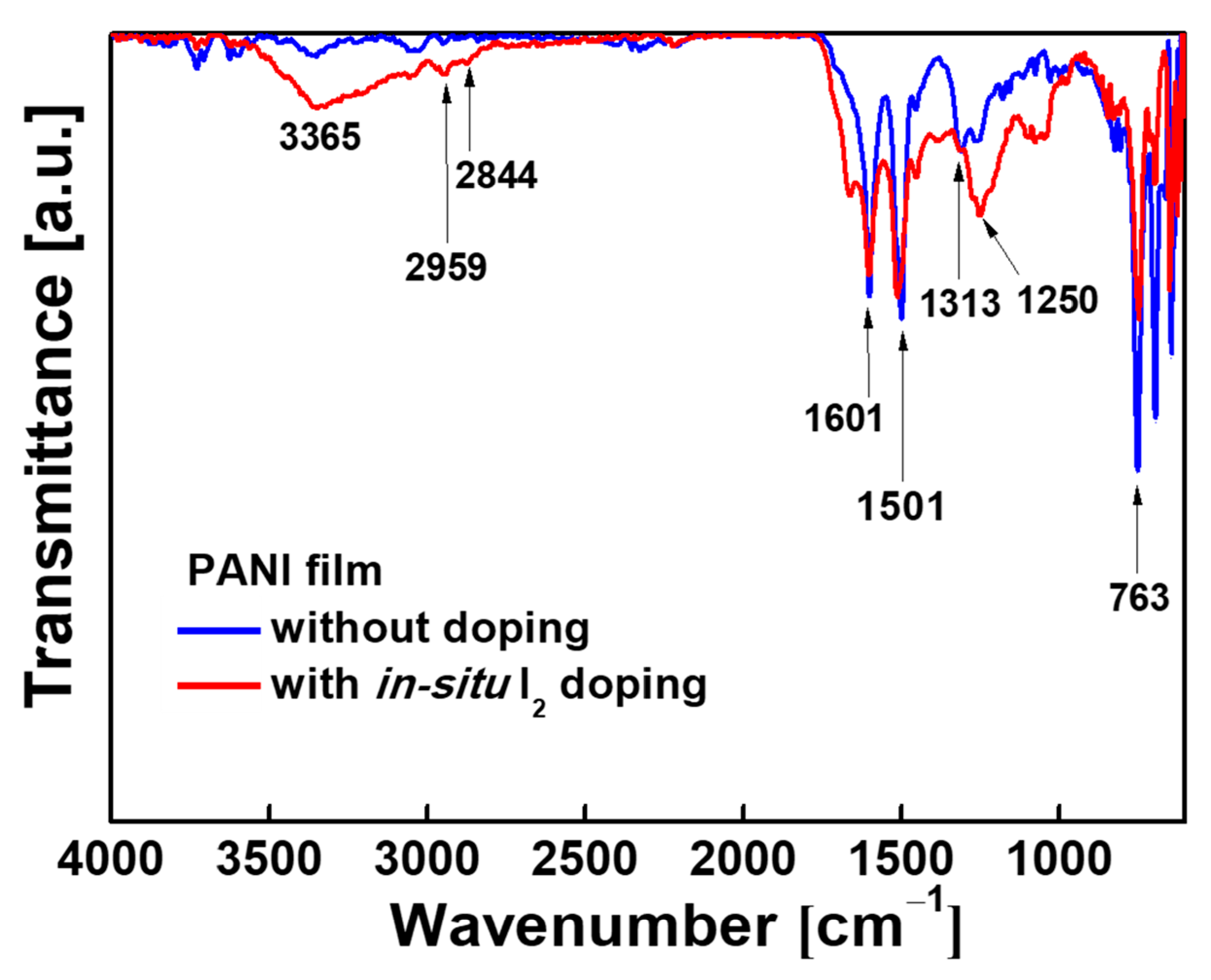
| Wavenumber/cm−1 | Vibration Mode |
|---|---|
| 763 | C-H out of plane bending |
| 1250 | C-N bending |
| 1313 | C-N stretching |
| 1501 | C=C stretching vibrations of the benzenoid |
| 1601 | C=C stretching vibrations of quinoid rings |
| 2844 | C-H asymmetric stretching |
| 2959 | C-H asymmetric stretching |
| 3365 | N-H stretching |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Iqbal, S.; Jang, H.J.; Jung, E.Y.; Bae, G.T.; Park, C.-S.; Tae, H.-S. In-Situ Iodine Doping Characteristics of Conductive Polyaniline Film Polymerized by Low-Voltage-Driven Atmospheric Pressure Plasma. Polymers 2021, 13, 418. https://doi.org/10.3390/polym13030418
Kim JY, Iqbal S, Jang HJ, Jung EY, Bae GT, Park C-S, Tae H-S. In-Situ Iodine Doping Characteristics of Conductive Polyaniline Film Polymerized by Low-Voltage-Driven Atmospheric Pressure Plasma. Polymers. 2021; 13(3):418. https://doi.org/10.3390/polym13030418
Chicago/Turabian StyleKim, Jae Yong, Shahzad Iqbal, Hyo Jun Jang, Eun Young Jung, Gyu Tae Bae, Choon-Sang Park, and Heung-Sik Tae. 2021. "In-Situ Iodine Doping Characteristics of Conductive Polyaniline Film Polymerized by Low-Voltage-Driven Atmospheric Pressure Plasma" Polymers 13, no. 3: 418. https://doi.org/10.3390/polym13030418
APA StyleKim, J. Y., Iqbal, S., Jang, H. J., Jung, E. Y., Bae, G. T., Park, C.-S., & Tae, H.-S. (2021). In-Situ Iodine Doping Characteristics of Conductive Polyaniline Film Polymerized by Low-Voltage-Driven Atmospheric Pressure Plasma. Polymers, 13(3), 418. https://doi.org/10.3390/polym13030418