Development of a Weighted Barite-Free Formate Drilling Mud for Well Construction under Complicated Conditions
Abstract
:1. Introduction
1.1. Relevance of the Work
- Analysis of existing high-density drilling fluids for drilling at unstable intervals and for productive drilling in formations.
- Analysis of application experience for formic acid salt drilling systems and justification of drilling fluid requirements in well construction.
- Development and study of formate-based drilling fluid compositions.
- Assessment of the inhibition effect of systems containing organic salts, polymeric reagents, and calcium carbonate on clay samples.
1.2. Brief Literature Review
1.2.1. Inhibition of Clay Particles
1.2.2. Application of Formate Drilling Muds
2. Methodology and Equipment
3. Investigations and Results
- (1)
- Encapsulation: РHPA encapsulates the clay scales and the wellbore due to the fact that the polyacrylate has too high an affinity to the positive clay edges. Because РHPA has a long polymer chain and a high molecular mass, it bonds to multiple sites along the borehole, i.e., the anionic -COO groups are attracted to the positively charged particle edges, helping to form a protective coating along the borehole that prevents the clay from coming into contact with water. The encapsulation process also prevents water from entering the interlayer structure of the clay.
- (2)
- Increasing the viscosity of the filtrate (thickening of the aqueous phase): This slows down penetration of the liquid into the interlayer structure of the clay.
- (3)
- Adsorption (the taking-up of free water by the polymer): This reduces the amount of water “available” for hydration of the clay [9].
Evaluation of the Inhibiting Capacity of Developed Formate-Based Drilling Fluids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WBF | Water-based fluid |
| HBF | Hydrocarbon-based fluid |
| SBF | Synthetic-based fluid |
| ECD | Equivalent circulation density |
| ROP | Rate of penetration |
| PHPA | Partially hydrolyzed polyacrylamide |
| PV | Plastic viscosity |
| SV | Specific viscosity |
| DSS | Dynamic shear stress |
| SSS | Static shear stress |
References
- Anderson, R.L.; Ratcliffe, I.; Greenwell, H.C.; Williams, P.A.; Cliffe, S.; Coveney, P.V. Clay swelling—A challenge in the oilfield. Earth-Sci. Rev. 2010, 98, 201–216. [Google Scholar] [CrossRef]
- Alcazar, L.A.; Cortes, I.R. Drilling fluids for deepwater fields: An overview. In Recent Insights in Petroleum Science and Engineering; Zoveidavianpoor, M., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Nutskova, M.V.; Rudiaeva, E.Y.; Kuchin, V.N.; Yakovlev, A.A. Investigating of compositions for lost circulation control. In Youth Technical Sessions, Proceedings of the 6th Youth Forum of the World Petroleum Council-Future Leaders Forum, Saint Petersburg, Russia, 23–28 June 2019; CRC Press: London, UK, 2019; pp. 394–398. [Google Scholar]
- Islamov, S.; Grigoriev, A.; Beloglazov, I.; Savchenkov, S.; Gudmestad, O.T. Research risk factors in monitoring well drilling—A case study using machine learning methods. Symmetry 2021, 13, 1293. [Google Scholar] [CrossRef]
- Rogov, E.A. Study of the well near-bottomhole zone permeability during treatment by process fluids. J. Min. Inst. 2020, 242, 169–173. [Google Scholar] [CrossRef]
- Haq, B. Green Enhanced Oil Recovery for Carbonate Reservoirs. Polymers 2021, 13, 3269. [Google Scholar] [CrossRef]
- Tananykhin, D.; Palyanitsina, A.; Rahman, A. Analysis of Production Logging and Well Testing Data to Improve the Development System for Reservoirs with Complex Geological Structure Procedia. Environ. Sci. Eng. Manag. 2020, 7, 629–648. [Google Scholar]
- Blinov, P.A.; Dvoynikov, M.V. Rheological and filtration parameters of the polymer salt drilling fluids based on xanthan gum. J. Eng. Appl. Sci. 2018, 13, 5661–5664. [Google Scholar]
- Rana, A.; Arfaj, M.K.; Saleh, T.A. Advanced developments in shale inhibitors for oil production with low environmental footprints—A review. Fuel 2019, 247, 237–249. [Google Scholar] [CrossRef]
- Pankov, D.Y. Legal aspects of ensuring environmental safety of oil and gas field development at the Arctic continental shelf. Qual. Manag. Oil Gas Ind. 2014, 4, 44–47. [Google Scholar]
- Ilinova, A.; Chanysheva, A. The future of Russian arctic oil and gas projects: Problems of assessing the prospects. J. Mar. Sci. Eng. 2021, 9, 528. [Google Scholar] [CrossRef]
- Pashkevich, M.A.; Petrova, T.A. Assessment of widespread air pollution in the megacity using geographic information systems. J. Min. Inst. 2017, 228, 738. [Google Scholar] [CrossRef]
- Fedosov, R.I.; Nikitin, B.A. Environmentally compatible drilling fluids for drilling and completion of wells at the Arctic shelf. Constr. Oil Gas Wells Land Sea 1999, 4–5, 47–51. [Google Scholar]
- Korelskiy, D.S.; Strizhenok, A.V.; Ismailova, D.V. Development and justification of the method of biotechnological reclaiming of oil-contaminated land. ARPN J. Eng. Appl. Sci. 2020, 15, 342–353. [Google Scholar]
- Balaba, V.I. Environmental requirements to flushing fluids in offshore drilling. Drill. Oil 2010, 2, 54–58. [Google Scholar]
- Buslaev, G.; Morenov, V.; Konyaev, Y.; Kraslawski, A. Reduction of carbon footprint of the production and field transport of high-viscosity oils in the Arctic region. Chem. Eng. Process. Process Intensif. 2021, 159, 108189. [Google Scholar] [CrossRef]
- Pilgun, S.; Aramelev, A. Environmentally Compatible Drilling Fluids. Presented at the SPE Arctic and Extreme Environments Technical Conference and Exhibition, Moscow, Russia, 15–17 October 2013. SPE-166847-MS Paper. [Google Scholar]
- Grigoriev, B.S.; Eliseev, A.A.; Pogarskaya, T.A.; Toropov, E.E. Mathematical modeling of rock crushing and multiphase flow of drilling fluid in well drilling. J. Min. Inst. 2019, 235, 16–23. [Google Scholar] [CrossRef]
- Aghdam, S.K.; Kazemi, A.; Ahmadi, M. A laboratory study of a novel bio-based nonionic surfactant to mitigate clay swelling. Petroleum 2021, 7, 178–187. [Google Scholar] [CrossRef]
- Dvoynikov, M.V.; Kuchin, V.N.; Mintzaev, M.S. Development of viscoelastic systems and technologies for isolating water-bearing horizons with abnormal formation pressures during oil and gas wells drilling. J. Min. Inst. 2021, 247, 1–9. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Kamal, M.S.; Al-Harthi, M. Polymeric and low molecular weight shale inhibitors: A review. Fuel 2019, 251, 187–217. [Google Scholar] [CrossRef]
- Jain, R.; Mahto, V. Evaluation of polyacrylamide/clay composite as a potential drilling fluid additive in inhibitive water based drilling fluid system. J. Pet. Sci. Eng. 2015, 133, 612–621. [Google Scholar] [CrossRef]
- Chudinova, I.V.; Nikolaev, N.I.; Rosentsvet, A.V. Justification on the choice of inhibiting reagents to improve the stability of clay rocks. Oil Eng. 2017, 2, 10–12. [Google Scholar]
- Raupov, I.R.; Shagiakhmetov, A.M. The results of the complex rheological studies of the cross-linked polymer composition and the grounding of its injection volume. Int. J. Civ. Eng. Technol. 2019, 10, 493–509. [Google Scholar]
- De Carvalho Balaban, R.; Vidal, E.L.F.; Borges, M.R. Design of experiments to evaluate clay swelling inhibition by different combinations of organic compounds and inorganic salts for application in water base drilling fluids. Appl. Clay Sci. 2015, 105, 124–130. [Google Scholar] [CrossRef]
- Davarpanah, A. The feasible visual laboratory investigation of formate fluids on the rheological properties of a shale formation. Int. J. Environ. Sci. Technol. 2019, 16, 4783–4792. [Google Scholar] [CrossRef]
- Byrne, M.; Patey, I.; Liz, G.; Downs, J.; Turner, J. Formate Brines: A Comprehensive Evaluation of Their Formation Damage Control Properties under Realistic Reservoir Conditions. Presented at the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 20–21 February 2002. SPE 73766. [Google Scholar]
- Howard, S.K.; Downs, J.D. Formate Fluids Optimize Production Rate. In Proceedings of the AADE 2005 National Technical Conference and Exhibition, Houston, TX, USA, 5–7 April 2005. [Google Scholar]
- Howard, S.K. Formate Brines for Drilling and Completion: State of the Art. Presented at the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 22–25 October 1995. SPE 30498. [Google Scholar]
- Howard, S.K.; Downs, J.D. Formate Brines for HPHT Well Control—New Insights into the Role and Importance of the Carbonate/Bicarbonate Additive Package. Presented at the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 20–22 April 2009. SPE-121550-MS. [Google Scholar]
- Gao, C.H. A Survey of Field Experiences with Formate Drilling Fluid. SPE Drill. Completion 2019, 34, 450–457. [Google Scholar] [CrossRef]
- Ryabtsev, P.; Khomutov, A.; Korolev, V. The First Experience of Formate Based Drilling Fluids Application in Russia. Presented at the SPE Russian Petroleum Technology Conference, Moscow, Russia, 15–17 October 2018. SPE-191503-18RPTC-MS. [Google Scholar]
- Berg, P.C.; Pederson, E.S.; Lauritsen, A.; Behjat, N.; Hagerup-Jenssen, S.; Howard, S.K.; Olsvik, G.; Downs, J.D.; Turner, J.; Harris, M. Drilling and Completing High-Angle Wells in High-Density, Cesium Formate Brine—The Kvitebjorn Experience, 2004–2006. SPE Drill. Completion 2009, 24, 15–24. [Google Scholar] [CrossRef]
- Van Oort, E.; Ahmad, M.; Spencer, R.; Lagacy, N. ROP Enhancement in Shales through Osmotic Processes. Presented at the SPE/IADC Drilling Conference and Exhibition, London, UK, 17–19 March 2015. SPE/IADC-173138-MS Paper. [Google Scholar]
- Cabot Specialty Fluids. Formate Technical Manual. Section B11 Compatibility with Shale. Version 1–02/10; Cabot Corporation: Boston, MA, USA, 2010. [Google Scholar]
- API RP 13B-1. Recommended Practice for Field Testing Water-Based Drilling Fluids, 2nd ed.; Addendum 1 May 2000; API Publishing Services: Washington, DC, USA, 2000. [Google Scholar]
- API RP 13D. Rheology and Hydraulics of Oil-Well Drilling Fluids; Norm of American Petroleum Institute: Washington, DC, USA, 2006. [Google Scholar]
- Hemphill, T.; Pileharvi, A.; Campos, W. Yield power law model more accurately predicts drilling fluid rheology. Oil Gas J. 1993, 91, 45–50. [Google Scholar]
- Novikov, V.S. Criteria of inhibiting properties of drilling fluids. Oil Ind. 1999, 6, 11–15. [Google Scholar]
- Litvinenko, V.S.; Nikolaev, N.I. Development of the weighted biopolimer drilling mud for workover. J. Min. Inst. 2012, 199, 375. [Google Scholar]
- Ulyasheva, N.M.; Logachev, Y.u.L.; Voronik, A.M.; Khodenko, D.V. Features design technology deepening and drilling fluids during well construction in lithified clay rocks. Neftyanoe Khozyaystvo—Oil Ind. 2016, 4, 82–86. [Google Scholar]
- Clark, R.K. Polyacrylamide/Potassium-Chloride Mud for Drilling Water Sensitive Shales. J. Pet. Technol. 1976, 28, 719–727. [Google Scholar] [CrossRef]
- Sharafutdinova, R.Z.; Bliznyukov, Y.V. Investigation of clay rocks instability when using inhibited drilling muds. Constr. Oil Gas Wells Land Sea 2010, 7, 31–33. [Google Scholar]

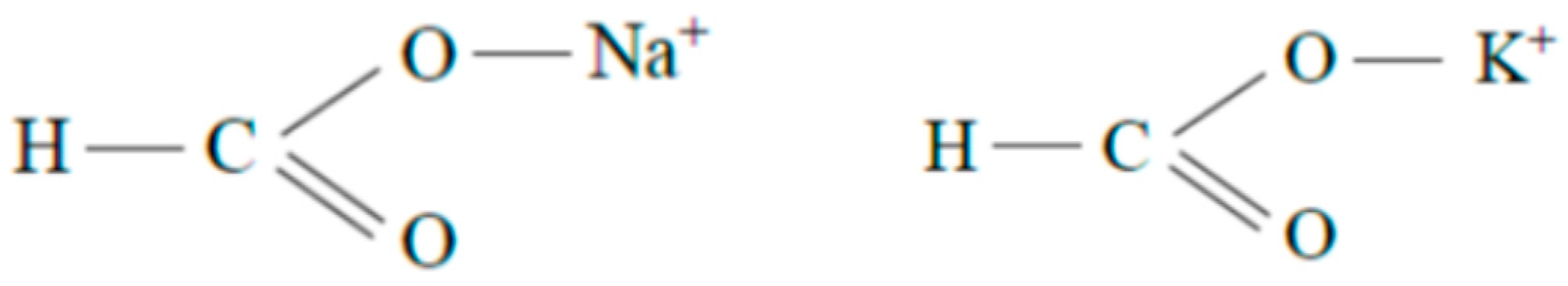
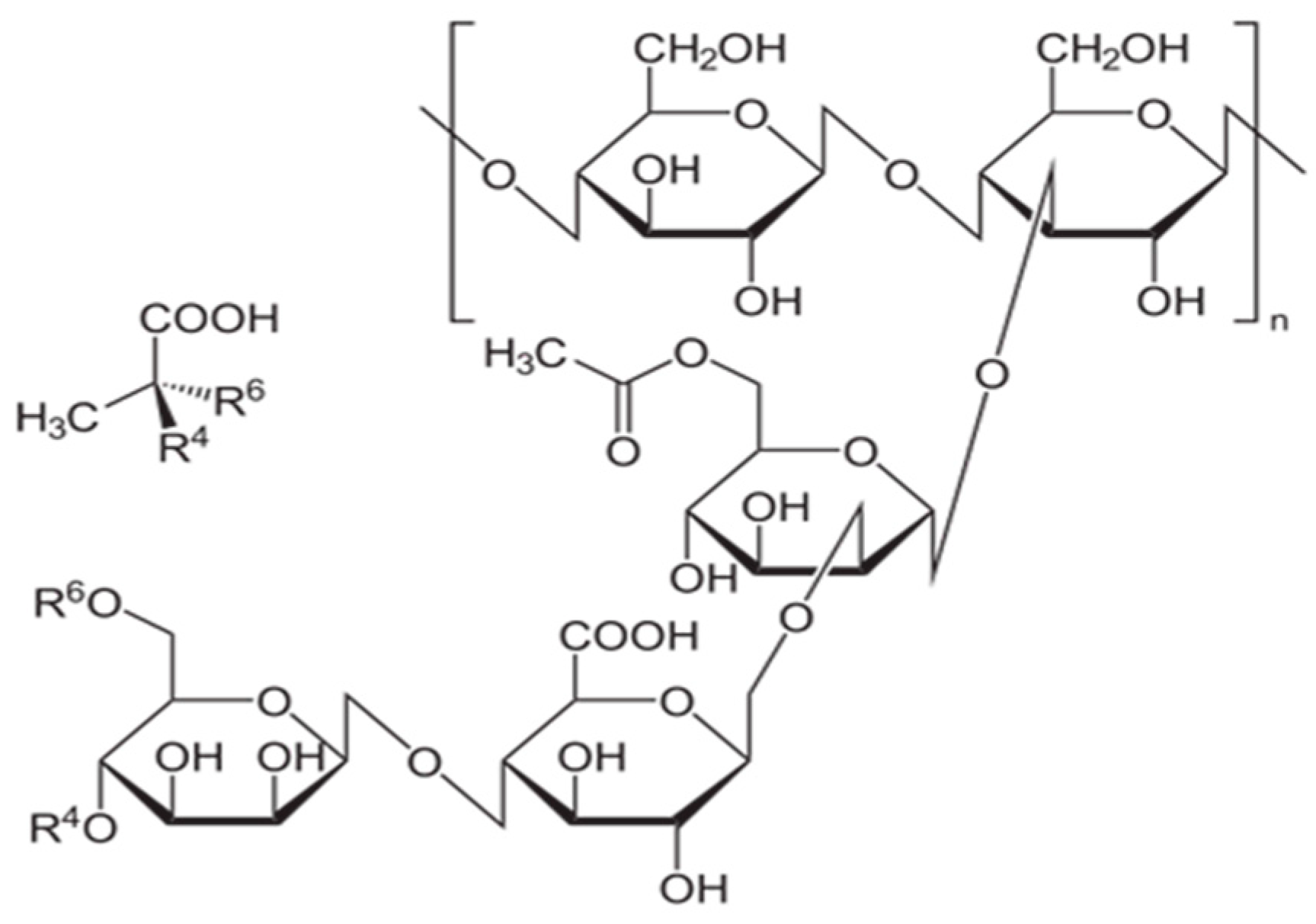
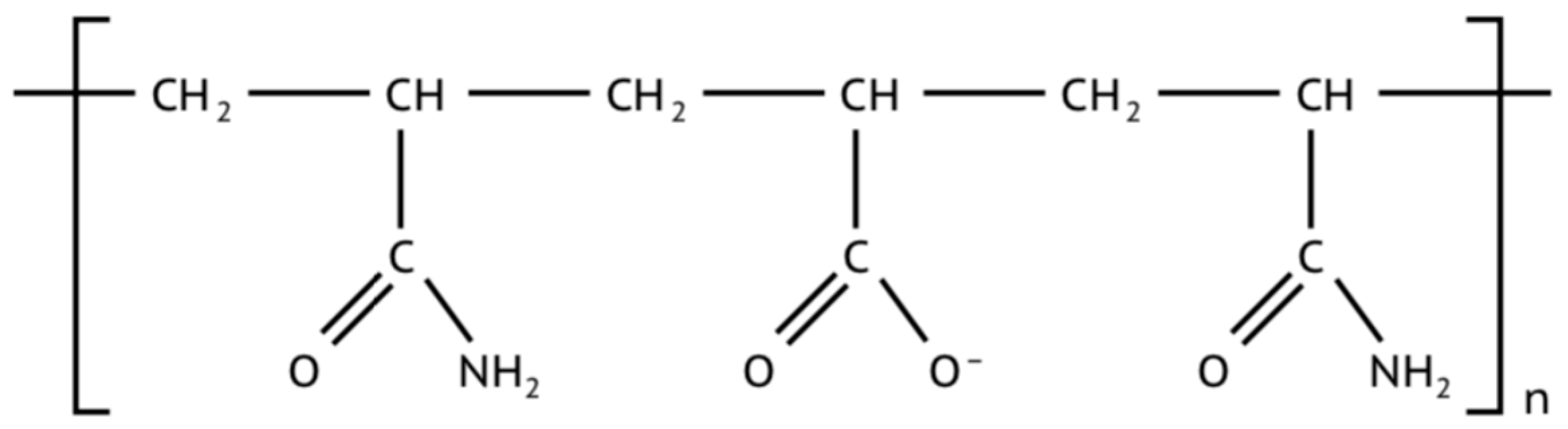

| Function of Drilling Fluid | Corresponding Property of Drilling Fluid |
|---|---|
| Removing the cuttings from under the bit, transporting it to the wellhead | Dynamic shear stress, apparent viscosity, flow velocity, static shear stress |
| Preventing fluid influx into the wellbore | Density |
| Maintaining the wellbore in a stable condition | Density, interaction with clays (inhibition) |
| Cooling and lubrication of drill string and bit | Density, flow velocity |
| Energy transfer from pumps to downhole motors, turbo-drills and displacement motors | Flow velocity, density, viscosity |
| Parameter | Base Mud |
|---|---|
| Density, g/cm3 | 1.45 |
| Specific viscosity, s/quarter | 42 |
| 600 rpm | 49 |
| 300 rpm | 30 |
| 200 rpm | 24 |
| 100 rpm | 15 |
| 6 rpm | 5 |
| 3 rpm | 3 |
| Plastic viscosity, mPa∙s | 19 |
| DSS, Pa | 5.3 |
| SSS (10 s/10 min), Pa | 2.4/3.8 |
| Filtration, mL/30 min | 3.8 |
| рН | 10 |
| Parameter | Solution “РНРА 12” | Solution “РНРА 15” | Solution “РНРА 20” | Solution “РНРА 27” |
|---|---|---|---|---|
| Density, g/cm3 | 1.45 | 1.45 | 1.45 | 1.45 |
| Specific viscosity, s/quarter | 40 | 40 | 41 | 42 |
| 600 rpm | 43 | 46 | 49 | 52 |
| 300 rpm | 27 | 30 | 33 | 36 |
| 200 rpm | 21 | 24 | 26 | 2 |
| 100 rpm | 14 | 17 | 18 | 17 |
| 6 rpm | 5 | 5 | 5 | 5 |
| 3 rpm | 3 | 3 | 3 | 3 |
| Plastic viscosity, mPa∙s | 16 | 16 | 16 | 16 |
| DSS, Pa | 5.3 | 6.7 | 8.1 | 9.6 |
| SSS (10 s/10 min), Pa | 2.4/3.8 | 2.4/3.8 | 2.4/3.8 | 2.4/3.8 |
| Filtration, mL/30 min | 3 | 2.7 | 2.6 | 2.2 |
| рН | 10 | 10 | 10 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morenov, V.; Leusheva, E.; Liu, T. Development of a Weighted Barite-Free Formate Drilling Mud for Well Construction under Complicated Conditions. Polymers 2021, 13, 4457. https://doi.org/10.3390/polym13244457
Morenov V, Leusheva E, Liu T. Development of a Weighted Barite-Free Formate Drilling Mud for Well Construction under Complicated Conditions. Polymers. 2021; 13(24):4457. https://doi.org/10.3390/polym13244457
Chicago/Turabian StyleMorenov, Valentin, Ekaterina Leusheva, and Tianle Liu. 2021. "Development of a Weighted Barite-Free Formate Drilling Mud for Well Construction under Complicated Conditions" Polymers 13, no. 24: 4457. https://doi.org/10.3390/polym13244457
APA StyleMorenov, V., Leusheva, E., & Liu, T. (2021). Development of a Weighted Barite-Free Formate Drilling Mud for Well Construction under Complicated Conditions. Polymers, 13(24), 4457. https://doi.org/10.3390/polym13244457








