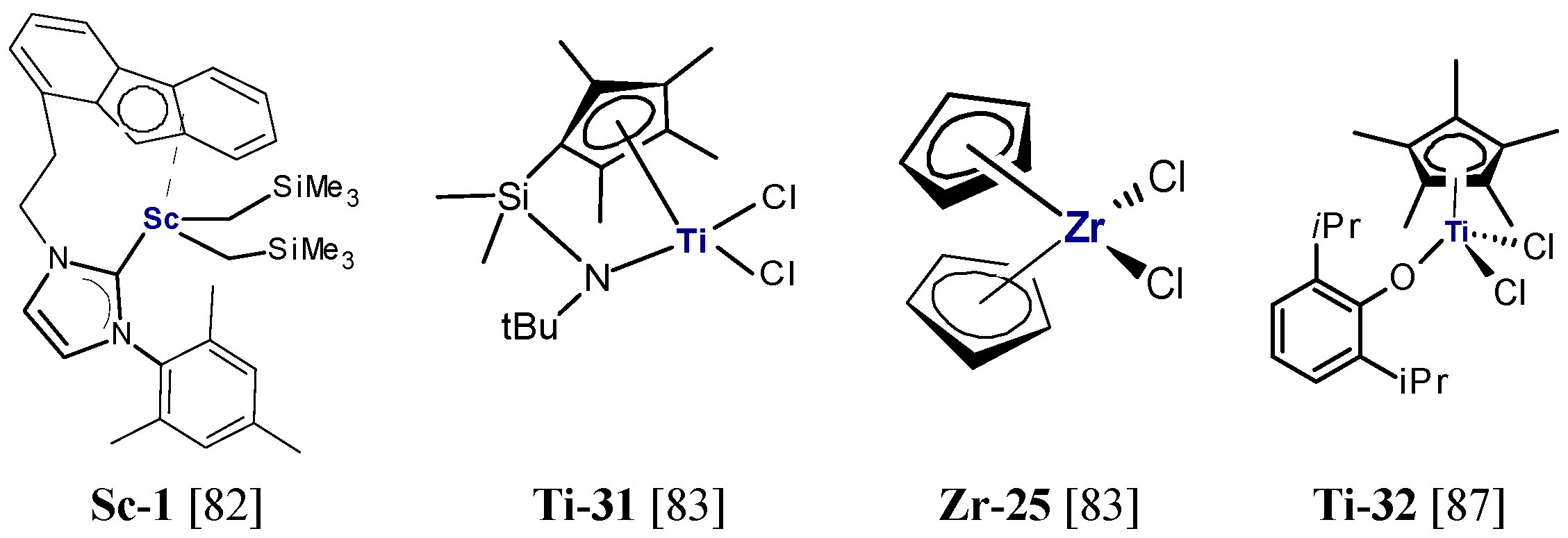
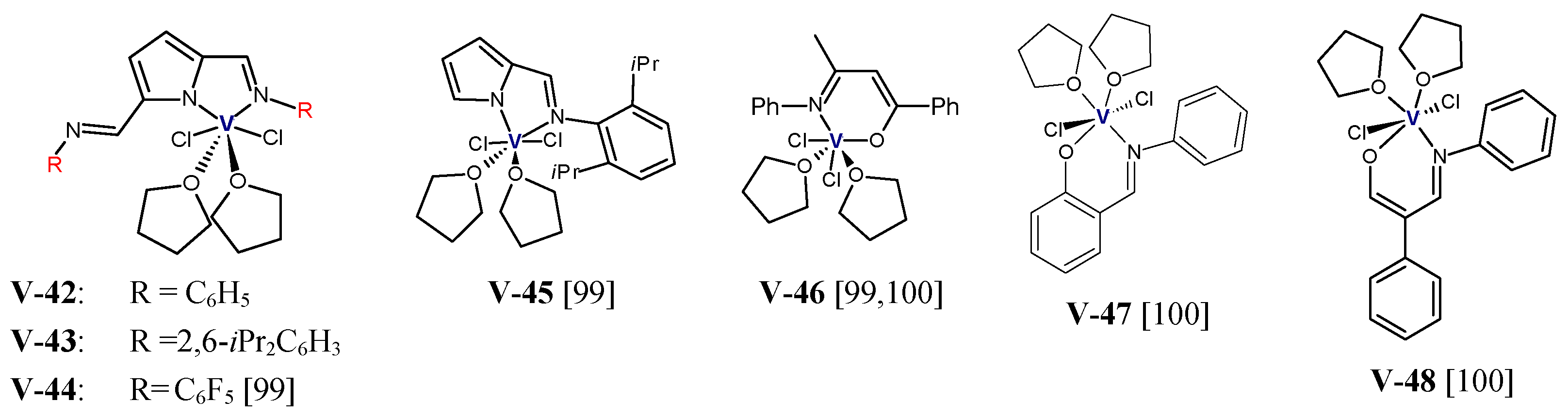
2.1.1. Complexes of Group 4 Transition Metals
The post-metallocene catalysts, which are based on the complexes of group 4 transition metals, have been extensively examined in copolymerization of ethylene with 1-olefins. It is not possible to present the catalytic performance of all the groups of the investigated complexes within this short review. Hence, the survey is limited only to the most representative catalysts producing copolymers with different macromolecular structures.
The titanium and zirconium complexes with tetradentate ligands make one of the groups most often used in olefin copolymerization. S.E. Reybuck et al. [
29] compared the effects of the symmetry of complexes, steric bulk of the substituent at the aryl ring, and the type of activator on the ethylene/1-hexene copolymerization behavior of zirconium diamino-bis(phenolate) complexes
Zr-1–Zr-5 (
Figure 1). The complexes with
Cs symmetry (
Zr-2–
Zr-5), i.e., those with the amino-bis(phenolate) ligands with the pendant donor group, were demonstrated to be more active and to offer a better ability to incorporate comonomer than those with
C2 symmetry (
Zr-1). The copolymer produced by
Zr-2/MMAO (MMAO- modified methylaluminoxane) at X
E/X
H = 0.11 (mol/mol) contained 44.9 mol% 1-hexene, while the copolymer synthesized with
Zr-1/MMAO at the same feed ratio of comonomers contained only 8.7 mol% of 1-hexene. The symmetry of the complex also affected the distribution of comonomer. The product of the reactivity ratios for copolymerization with
Zr-1 was higher than 1 (r
er
h = 2.6) and for copolymerization with
Zr-2 below 1 (r
er
h = 0.18), which suggested the presence of blocky and slightly alternating sequences of the comonomer units, respectively. It was also shown that the 1-hexene incorporation ability of the
Cs symmetric complexes increases in the order of decreasing steric bulk at the 2,4-aryl positions. However, bulky substituents like
tert-butyl groups (
Zr-2) or phenyl groups (
Zr-3) were found to improve the complex activity at the same time. The copolymerization behavior of the complexes was also sensitive to the nature of the activator. Copolymers produced by
Cs-symmetric complexes activated with MMAO had higher 1-hexene incorporation by about 10 mol% than those synthesized with borates as activators. All the copolymers synthesized with the use of
Zr-1–Zr-5 complexes showed monomodal distribution and narrow molecular weight distribution (M
w/M
n = 1.3–1.9), which is indicative for homogeneity of active sites in the catalysts [
29].
The dimeric diamine-bisphenolate complex
Zr-6 activated with Al(
iBu)
3/Ph
3CB(C
6F
5)
4 produced copolymers with very high 1-octene incorporation, up to ≈20 mol% at 0.70 mol/L of comonomer in the feed, with moderate molecular weight and dispersity (M
w = 14,000–42,000 g/mol, M
w/M
n = 2.1–3.2). In addition, the activating effect of the comonomer on the copolymerization yield was observed independently on the comonomer concentration. Its activity was over four times higher in ethylene/1-octene copolymerization than in ethylene homopolymerization [
30]. In contrast, the titanium complexes
Ti-1–
Ti-4 bearing the same type of tetradentate ligand in conjunction with Al(
iBu)
3/Ph
3CB(C
6F
5)
4 exhibited the negative comonomer effect and lower activity than the zirconium catalyst. The comonomer incorporation ability for these catalysts was also lower than for
Zr-6 and it was strictly dependent on the ligand structure. The complexes
Ti-1 and
Ti-4 with the NMe
2 donor group gave copolymers with 9.0 and 12.0 mol% of 1-octene at the comonomer concentration equal to 0.70 mol/L. Interestingly,
Ti-4 produced the ethylene/1-octene copolymer, not only with a high incorporation level, but at the same time with the very high molecular weight and very narrow molecular weight distribution (M
w = 755,000 g/mol, M
w/M
n = 1.7). The introduction of sterically bulkier N(
iPr)
2 pendant donor group in the complexes
Ti-2 and
Ti-3 resulted in lower 1-octene contents: 5.1 mol% and 2.8 mol%, respectively [
30].
Further studies [
31] showed that the presence of AlMe
3 in the feed during the copolymerization catalyzed by
Zr-6/Al(
iBu)
3/Ph
3CB(C
6F
5)
4 lowered the catalyst activity and greatly increased the amount of comonomer content in the copolymer from about 10 to approximately 40 mol% for 1-octene and 31 mol% for 1-hexene when the AlMe
3/Zr molar ratio was equal to 100/1. The increase in the AlMe
3/Zr molar ratio to 150:1 resulted in the production of ethylene/1-octene copolymer with the comonomer content of 65 mol%. The presence of AlMe
3 significantly affected the microstructure of the produced copolymers which possessed both blocky and alternating sequences, and decreased the molecular weight of the produced copolymers. However, other alkylaluminium compounds, AlEt
3 and Al(
iBu)
3, changed molecular weight of copolymers but did not cause any important changes in the catalytic activity, composition, or microstructure of the copolymerization products. Hence, only AlMe
3 interacts with the active sites to modify their catalytic properties [
31].
Titanium catalyst
Ti-5/MAO (MAO-methylaluminoxane), based on a complex with tetradentate salan type ligand, was investigated in ethylene/1-octene copolymerization as well. At the comonomer concentration of 0.73 mol/L, it gave the copolymer with moderate incorporation (3.7 mol%) and its activity was low (3.7 kg/(mol
Ti·h)) [
32]. Similarly, low activity and moderate incorporation ability were exhibited in ethylene/1-octene copolymerization by the titanium catalyst
Ti-6/MAO, which was based on the tetradentate salen type ligand [
33]. At the highest investigated comonomer concentration, 1.340 mol/L, the copolymer with 5.5 mol% of 1-octene was obtained and the catalytic activity was 14.7 kg/(mol
Ti·0.5 h). The copolymers produced by
Ti-6 were characterized by very high molecular weights (M
w = 1023 × 10
3–1545 × 10
3 g/mol) and very broad molecular weight distributions.
Among the large number of group 4 post-metallocene catalysts reported for ethylene copolymerization, are those based on complexes with tridentate ligands. That group covers
Ti-7 and
Zr-7 complexes bearing the bisphenolate ligand with the pyridinediyl linker which were tested in ethylene/1-octene copolymerization [
34]. Both complexes in conjunction with MAO turned out ineffective in that process. The titanium catalyst exhibited very low, 3 kg/(mol
Ti·h), activity and
Zr-7/MAO gave the copolymer which contained only 0.5 mol% of comonomer, despite low ethylene pressure (1 atm) and high concentration of 1-olefin (~50% vol.) in the feed. In contrast, the titanium complexes
Ti-8–Ti-10 bearing the tridentate monoanionic [ONX] (X = O, S) ligands, after activation with MMAO, showed much better capability to copolymerize ethylene and 1-hexene [
35,
36]. The complex
Ti-9 with S in the sidearm was both highly active (387 kg/[mol
Ti·h·atm]) and gave a copolymer with the very high 1-hexene content (30 mol%). The corresponding complex with an O-donor as the sidearm (
Ti-8) under the same reaction conditions produced copolymers with lower activity and comonomer incorporation equal to 23 kg/(mol
Ti·h·atm) and 14.1 mol%, respectively. The excellent copolymerization behavior showed the complex
Ti-10 with sulfoxide group [
36] which produced copolymer with incorporation levels as high as 38.8 mol% and the activity of 320 kg/(mol
Ti·h·atm). These results show that pendant donor group in phenoxy-imine ligand can easily tune the activity of catalyst and its capability for copolymerization. Further investigations showed that substituent on the sidearm also influenced strongly the copolymerization behavior of the complexes. In contrast to
Ti-10, complex
Ti-11, due to the steric effects of the isopropyl group, produced copolymer with very low comonomer content (0.6 mol%) [
36]. Those results demonstrate that the catalytic properties of
Ti-8–
Ti-11 can be well correlated with the steric and electronic properties of the side arm donor group [
35].
When the zirconium
Zr-8 and titanium
Ti-12 complexes bearing potentially tridentate phenoxy-imine ligands were employed in ethylene/1-octene copolymerization in the presence of MMAO, their activities were very high (≈12,300 kg/[mol
Zr·h]) and moderate (≈450 kg/[mol
Ti·h]). However, their comonomer incorporation abilities were very low (up to 0.1 mol%) and low (up to 1.5 mol%), respectively [
37]. When activated with Al(
iBu)
3/Ph
3CB(C
6F
5)
4, these complexes were able to produce copolymers with higher comonomer contents (1.6 and 3.8 mol%, respectively), yet their activities were much lower. Interestingly, the produced copolymers showed broad chemical composition distributions and very broad molecular weight distributions, as well as high molecular weights irrespective of the comonomer concentration in the feed. At the 1-octene concentration of 0.58 mol/L,
Zr-8 produced copolymers with M
w = 440,000 g/mol and M
w/M
n = 116 (trimodal distribution), whereas
Ti-12 gave the product with M
w = 690,000 g/mol and M
w/M
n = 26.5 (bimodal distribution) [
37].
Y. Gao et al. [
38] studied ethylene/1-octene copolymerization with the use of phenoxy-imine complexes of zirconium (
Zr-9–
Zr-12). In contrast to other studies, they did not focus on modification of ancillary ligand structure, but on changing the type of monodentate σ-ligands. The synthesized complexes had chloride, methyl, benzyl, and dimethylamine as labile ligands. Such modification of precatalyst
Zr-9–
Zr-12 was found to have considerable effect on their copolymerization behavior. Poly(ethylene-
co-1-octene) produced by
Zr-9/AlMe
3/Ph
3CB(C
6F
5)
4 contained 7.2 mol% of comonomer. Under the same conditions (5 mL of 1-octene, 4 atm of ethylene, 40 °C, Zr/AlMe
3 = 40), complexes with benzyl and methyl σ-ligands,
Zr-10 and
Zr-11, gave copolymers with much lower 1-olefin incorporation, ≤1 mol%. Then, the complex
Zr-12 with the chloride labile ligand turned out inactive in copolymerization of ethylene and 1-octene. The comparison of the copolymerization results for the bis-FI and mono-FI complexes
Zr-9 and
Zr-13 revealed that both complexes are capable of producing copolymers with high comonomer incorporation, with 6.3 and 7.2 mol% of 1-octene, respectively, albeit with different activities. The activity of
Zr-9 with two phenoxy-imine ligands was 2846 kg/(mol·h·atm) while that of
Zr-13 was equal to 1812 kg. These two complexes behaved even more similarly at
Zr/AlMe
3 = 120 which could indicate that both those complexes form similar or identical cationic mono-FI-Zr catalytic species [
38].
Recently, dinuclear titanium complex bearing anthracene-briged bisphenoxyimine ligand (
Ti-13) activated with MAO was investigated as a precatalyst for ethylene/1-hexene copolymerization [
39]. However, it was found to exhibit rather low activity and comonomer incorporation ability (303 kg/(mol
Ti·h), 1.8 mol%). In contrast,
C2 symetric dinuclear zirconium catalysts supported by bisphenolate ligands with bulky Si(
iPr)
3 substituent,
Zr-14 and
Zr-15, in conjunction with MAO showed high activity and produced copolymers with good 1-hexene incorporation. At 3 bar of ethylene and 20 mL of comonomer in the feed, their activity and 1-hexene incorporation were equal to 2000 kg/(mol
Zr·h), 22 mol% and 1900 kg/(mol
Zr·h), 30 mol%, respectively. Their monometallic analogues,
Zr-16 and
Zr-17, showed slightly lower incorporation ability (19 mol% and 28 mol%) [
40]. It was also revealed that
Zr-14–Zr-17 incorporate the longer chain 1-olefin, 1-tetradecene, at lower level (3 mol%–10 mol%).
Ethylene and 1-hexene were also copolymerized by hafnium-amine bis(phenolate) complex with tetrahydrofuran pendant arm (
Hf-1), activated by B(C
6F
5) [
41]. The catalyst was able to produce copolymers over a wide range of 1-hexene incorporation, from 25 mol% to 85 mol%, narrow dispersity, and M
n = 5600–47500 g/mol by changing comonomer concentration from 0.1 to 2.5 mol/L and reaction time from 10 to 90 min. However, its activity was modest (5.4–41.6 g/(mmol
Hf·h)). Amido-trihydroquinoline hafnium complexes
Hf-2, Hf-3 and
Hf-4 with Ph
3CB(C
6F
5)
4 as the activator showed both high activity and ability to produce copolymers with high 1-octene content and high molecular weights (224,000–1220,000 g/mol) at high temperature (100 °C). At 1-octene concentration equal to 1 mol/L their activity decreased in the order
Hf-2, 16,200 kg/(mol
Hf·h) >>
Hf-4, 2520 kg/(mol
Hf·h)
> Hf-3, 2340 kg/(mol
Hf·h) and incorporation ability was changed as follows:
Hf-2 (15.6 mol%) >
Hf-3 (7.8 mol%) >
Hf-4 (4.8 mol%), indicating significant substituent steric effect on catalytic properties of hafnium catalysts [
42]. Zirconium complex
Zr-18 bearing the same ligand as complex
Hf-2 was also highly efficient in ethylene/1-octene copolymerization (13,500 kg/(mol
Zr·h), 12.4 mol%) but the exchange of Hf by Ti (
Ti-14) led to catalyst with lower activity and low incorporation ability (2160 kg/(mol
Ti·h), 1.7 mol%) [
42].
Phosphine–amido hafnium and zirconium complexes bearing various substituents at the 2-position of the tetrahydroquinoline framework (R = H, Me,
iPr,
nBu) were evaluated in ethylene/1-octene copolymerization with [HNMe(C
18H
37)
2][B(C
6F
5)
4] as activator and under the following conditions: 1 mol/L of 1-octene, 30 bar of ethylene, 100 °C (initial reaction temperature), and 3 min [
43]. Moderate 1-octene incorporation (7.7 mol%) showed zirconium complex
Zr-19, however its Hf counterpart (
Hf-5) and the other Zr and Hf complexes exhibited low comonomer incorporation (2.1 mol%–3.6 mol%). Activities of complexes fell in the range of 7000–29,000 kg/(mol
M·h), with the highest and lowest activity shown by
Zr-20 and
Hf-5, respectively.
One of the interesting classes of postmetallocene catalysts are catalysts based on pyridylamido hafnium complexes which were shown to have high incorporation ability in ethylene/1-olefin copolymerization at high temperature [
44,
45,
46].
C1-symmetric
tert-butyl substituted pyridylamido hafnium complex
Hf-6 activated with Ph
3CB(C
6F
5)
4/Al(
iBu)
3 was able to incorporate 6.0 mol% of 1-octene and 8.7 mol% of 1-hexene in ethylene/1-olefin copolymerization carried out with the addition of 0.1 mol of comonomer, at 80 °C for 10 min under 10 atm of ethylene. The produced copolymers had broad MWD with bimodal distribution and high molecular weight equal to 262,000 g/mol and 931,000 g/mol (copolymer with 1-octene and 1-hexene, respectively) [
44]. Selected results of ethylene/1-olefin copolymerization with group 4 metal complexes are displayed in
Table 4.
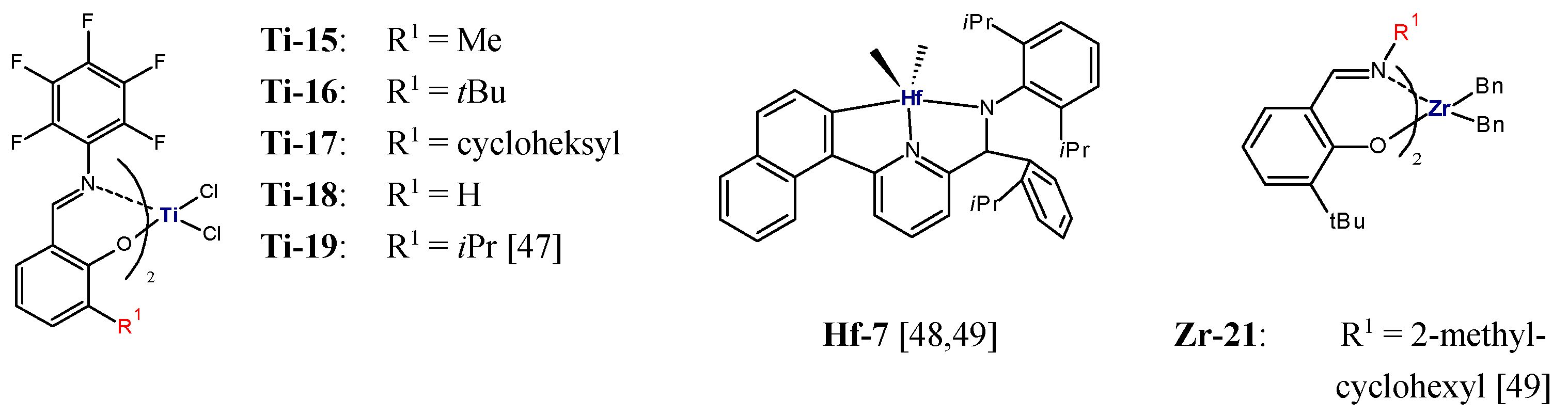
Fluorinated bis(phenoxy-imine)titanium complexes
Ti-15,
Ti-16,
Ti-17,
Ti-18, and
Ti-19 (
Figure 2) activated by MAO were also applied in ethylene/1-olefin copolymerization (1-olefin = 1-hexene, 1-octene, 1-decene) [
47]. All the catalysts were shown to offer high productivity. However, they had significantly different abilities to incorporate 1-olefins; this was dependent on the steric hindrance of the
ortho-substituent. Reduction in the bulkiness of the substituent R enhanced reactivity of the catalysts towards higher 1-olefins.
Ti-18 (R
1 = H) displayed the highest 1-hexene incorporation ability (22.6 mol%), whereas
Ti-16 (R
1 =
tBu) showed the lowest efficiency (3.2 mol%). Moreover, the produced copolymers had very narrow molecular weight distributions (M
w/M
n = 1.07–1.22) which indicated that the living ethylene/1-olefin copolymerization took place in the presence of those catalysts.
The living polymerization which occurs with rapid initiation and negligible chain termination or chain transfer makes it possible to synthesize copolymers with well-defined blocks [
50]. Most of the catalysts displayed the living olefin polymerization characteristics at low temperatures which resulted in low activity and insufficient molecular weights of the polymers [
51,
52]. Bis(phenoxy-imine) titanium catalysts which incorporate fluorine atom(s) ortho to the imine-nitrogen can catalyze the living (co)polymerization of olefins at ambient and elevated temperatures [
51]. In the presence of
Ti-15/MAO, a number of block copolymers of type PE-
block-poly(ethylene-
co-1-hexene) were synthesized using the sequential addition polymerization procedure. Linear polyethylene macromolecules (M
n = 38,300 g/mol, M
w/M
n = 1.11) were obtained initially and then a mixture of ethylene and 1-hexene monomers was fed to obtain another block. The produced block copolymers contained from 3.3 mol% to 15.0 mol% of 1-hexene and were characterized by high molecular weights (M
n = 61,500–121,000 g/mol), narrow MWD (M
w/M
n = 1.21–1.31), and melting points from 117 °C to 130 °C [
47]. The block copolymers of that type were shown to possess a good combination of extensibility and toughness compared to the corresponding random copolymers.
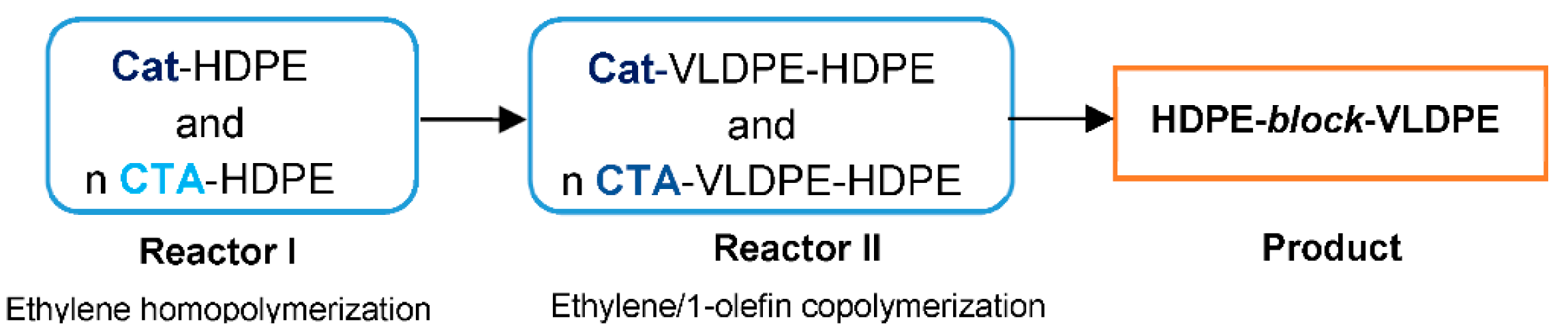
Further, the coordinative chain transfer copolymerization, CCTcoP, of ethylene with 1-olefins was developed. It was aimed at reducing the amounts of transition metal catalysts needed and controlling the molecular weight of polymers. In this approach, the growing polymer chain is capable of transferring from the catalyst to the chain transfer agent (CTA), which is a dormant species in the course of copolymerization [
53]. P.D. Hustad et al. reported the method for the synthesis of block copolymers in the continuous process using CCT
coP, with
Hf-7/MAO as a catalyst (
Figure 2) and ZnEt
2 as CTA [
48]. Two reactors were employed in the process (
Figure 3). Ethylene homopolymerization took place in reactor I, while copolymerization of ethylene and 1-olefin was carried out in reactor II. When the HDPE-
block-VLDPE copolymers obtained with CTA were compared with the products synthesized with no CTA (physical blend obtained in a dual-reactor), it was found that the copolymer produced with the use of CTA showed higher molecular weight, narrower MWD (M
n = 44.5 kg/mol, M
w/M
n = 1.67), and somewhat lower melting point (T
m = 122 °C) than the product obtained with no use of CTA (M
n = 25.9 kg/mol, M
w/M
n = 4.42, T
m = 126 °C). In that type of copolymerization, both the block composition and the comonomer content in each block can be easily tailored by changing the production rate in reactor I and reactor II, and by feed composition, respectively [
48].
Block copolymers with novel architecture, showing excellent elastomeric properties, were introduced by Arriola et al. [
49]. Linear multiblock ethylene/1-octene copolymers, with sequential crystallizable segments (low 1-octene content) and amorphous segments (high 1-octene content) were synthesized via chain shuttling polymerization. That type of polymerization involves two catalysts with different comonomer incorporation abilities and a chain shuttling agent (CSA) which is able to pass the growing polymer chain between catalytic sites so that different parts of a single polymer molecule grow on different catalysts [
49,
53]. The pyridylamide complex
Hf-7, with the high ability to incorporate 1-octene, and the bis(phenoxy-imine) complex
Zr-21 (
Figure 2), with low incorporation ability, combined with ZnEt
2 as a CSA, made it possible to obtained multiblock copolymers which were characterized by very low densities (0.879–0.883 g/cm
3) and high melting points (T
m = 120–124 °C) at the same time. Moreover, unlike the copolymer prepared without ZnEt
2, which was bimodal with M
w/M
n = 13.8, they show monomodal and narrow distributions of molecular weights (M
w/M
n = 1.98–3.22) [
49].
2.1.2. Complexes of Vanadium
The vanadium catalysts for the olefin polymerization have been known since the 1950s and such classical Ziegler–Natta ones possess some interesting properties, which made them applicable in the commercial production of ethylene/propylene/diene and ethylene/cyclic olefins copolymers [
54]. Although metallocene-type vanadium catalysts did not attract much attention of the researchers, the vanadium catalysts based on complexes with multidentate ligands started drawing considerably more and more interest as their catalytic performance, including thermal stability, can be modified by the use of specific multidentate ligands. The detailed review of the vanadium catalysts, which were employed in the olefin polymerization processes within the last decade, was published by A. M. F. Phillips et al. in Coordination Chemistry Reviews in 2020 [
55], and earlier works were summarized by S. Gambarotta in Coordination Chemistry Reviews in 2003 [
56]. Here, we summarize some interesting developments of the vanadium complexes for ethylene/1-olefin copolymerization.
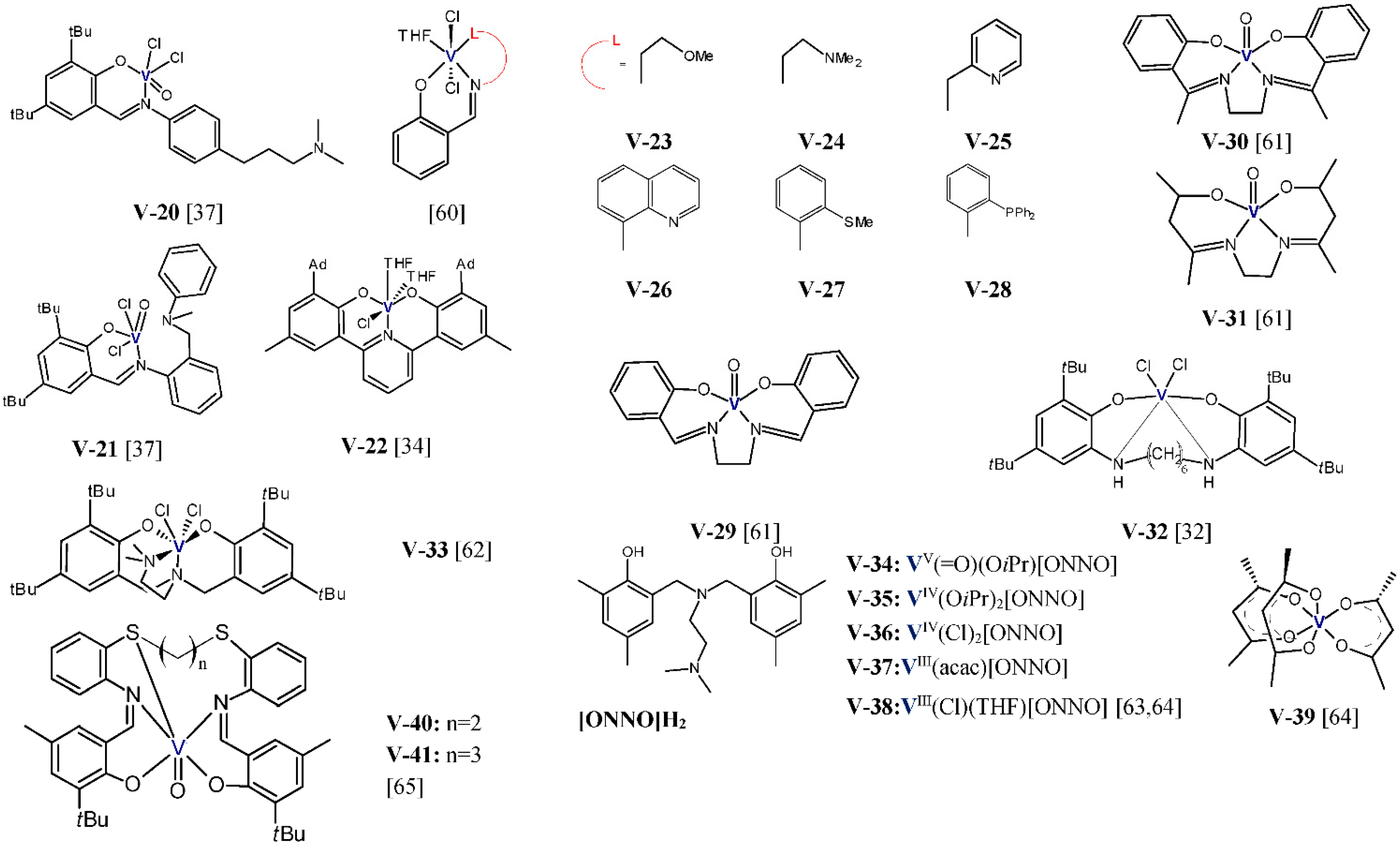
Y.S. Li et al. studied the performance of vanadium(III) complexes (
Figure 4) bearing bidentate ligands with [O,P], [N,N], and [O,O] donor atoms in ethylene/1-hexene copolymerization [
57,
58,
59]. Et
2AlCl and Cl
3CCOOEt (ETA) were used as the activator and the reactivator, respectively. The copolymerizations were carried out at 1 atm of ethylene, 1-hexene concentration equal to 0.2 mol/L, and at 25 °C for 5 or 10 min. The complexes
V-1–
V-5 showed high activities, 4.08–7.44 kg/(mmol
V·h), and they produced copolymers with comonomer content between 2.22–4.20 mol% and with very narrow molecular weight distribution (M
w/M
n ≤ 1.9). For the complexes with electron donating imino groups, the 1-hexene incorporation ability was shown to increase in line with the decreasing steric hindrance at the 2,6-aryl positions (
V-3 <
V-2,
V-4 <
V-1). The presence of the electron withdrawing group in the complex decreased the 1-hexene incorporation (
V-1 >
V-5). In addition, the properties of the resultant copolymers can be controlled over a wide range by changing the reaction parameters. At 75 °C and at 1-hexene initial concentration of 0.6 mol/L, the
V-3/Et
2AlCl system was found to produce the copolymer with 1-hexene incorporation as high as 16 mol% [
57]. In general, the complexes
V-6–
V-12 and
V-13–
V-19 exhibited good activities and incorporation ability in the range of 1.08-5.34 kg/(mmol
V·h), 2.34–5.21 mol% [
58], 0.84–4.56 kg/(mmol
V·h), and 2.33–3.75 mol% [
59], respectively. Nevertheless, their copolymerization behavior is influenced by the nature of substituents in the ligands. The increasing steric hindrance on the aryloxy moiety of the phenoxy-phosphine ligand was shown to improve the catalytic activity (
V-13 <
V-15 <
V-16, and
V-17 <
V-18). However, the increased steric bulk of the ligand in
V-19 slightly lowered the catalytic activity for copolymerization (
V-18 >
V-19). In addition, the electron-withdrawing effect of the F-substituted phenoxy-phosphine ligand influenced the catalytic performance unfavorably [
59]. Moreover, the steric hindrance of the ligand and the electron-withdrawing effect of the ligand was also unfavorable for 1-hexene insertion.
The vanadium complexes
V-20 and
V-21 (
Figure 5), activated either with EtAlCl
2 or with Et
2AlCl, were also examined in ethylene/1-octene copolymerization [
37]. The copolymerization tests were carried out in the presence of ETA at 60 °C, for 20 min, under 5 bar ethylene, and in the presence of 2, 5, and 6.5 mL 1-octene. Both these complexes in conjunction with EtAlCl
2 exhibited high catalytic activities (66,400–10,400 kg/(mol
V·h)) and, taking into account the high pressure of ethylene and low comonomer concentration, they offered acceptable incorporation ability (1.3–3.6 mol%). The produced copolymers were characterized by high molecular weights and low MWD (M
w = 380,000–120,000 g/mol, M
w/M
n ≤ 2). When the activator was changed for Et
2AlCl, both complexes were less active and they produced copolymers with lower comonomer contents and high dispersity (M
w/M
n = 6.4–7.9).
S.R. Golisz [
34] reported the use of the vanadium complex
V-22 with the tridentate bis(phenolate) ligand with the pyridinediyl linker, which was analogous to the complexes
Ti-7 and
Zr-7, in copolymerization of ethylene and 1-octene.
V-22/MAO exhibited low activity (50 kg/[mol
v⋅h]) and moderate incorporation ability. The copolymer obtained at high 1-octene/ethylene ratio contained 6.6 mol% of comonomer; still, it was much higher than for the use of group 4 catalysts under the same conditions. J-Q. Wu et al. [
60] studied the performance of the vanadium(III) complexes
V-23–
V-28 bearing tridentate phenoxy-imine ligands with different pendant donor groups, in copolymerization of ethylene with 1-hexene. At the same copolymerization conditions: 0.27 mol/L 1-hexene, 1 bar ethylene, at 25 °C, with Et
2AlCl as an activator, and ETA as a reactivator, the studied complexes were characterized by different activities, from 0.3 kg/(mmol
V·h·bar) up to 3.61 kg/(mmol
V·h·bar), with the figures changing as follows:
V-23 <
V-27 <
V-24 <
V-26 <
V-28 <
V-25. Hence, the results were dependent both on the pendant donor and on the electronic effect of the backbone of the ligands. All the catalysts produced copolymers with moderate comonomer contents, 2.3–3.2 mol%, with high molecular weights, 41,800–93,800 g/mol, and with unimodal molecular weight distributions (M
w/M
n = 1.9–2.9). The most active complex,
V-25, produced the copolymer with 3 mol% of 1-hexene and its content in the copolymer increased to 12.9 mol% when the comonomer concentration was raised to 1.35 mol/L. Moreover, enhanced catalytic activity was observed for the catalyst
V-25 with the increasing polymerization temperature up to 50 °C, which is indicative for its good thermal stability.
The oxovanadium(V) complexes (
V-29–
V-31) with tetradentate Schiff base ligands were also used in copolymerization of ethylene with 1-olefins as precatalysts. Those complexes, when activated with EtAlCl
2, make it possible to produce poly(ethylene-
co-1-octene) with the comonomer content from 5.26 to 6.25 mol% and melting points ~112 °C, at the comonomer concentration of 0.40 mol/L. The copolymer with the highest 1-octene content was obtained with the use of
V-29 and it was found to contain isolated comonomer units only [
61]. The discussed complexes offered low catalytic activities in copolymerization, from 2.6 kg/(mol
V·h) to 18.1 kg/(mol
V·h), and it grew up in the following line:
V-29 <
V-30 <
V-31. Higher activities in ethylene/1-octene copolymerization, from 1193.5 to 197.6 kg/(mol
V·h), at the comonomer concentrations changing from 0.20 to 0.73 mol/L, were exhibited by the vanadium catalyst
V-32/EtAlCl
2 which was based on the complex with the tetradentate salan-type ligand. However, no neat copolymer was formed at higher comonomer concentrations, but the mixtures of products were obtained [
32].
The copolymerization process of ethylene with 1-octene was also explored for three different comonomer concentrations: 0.19, 0.38, and 0.70 mol/L, with the use of the vanadium(IV) complex
V-33 with the diamino-bis(phenolate) ligand, which was activated by EtAlCl
2 [
62]. Catalyst activity fell within 350–120 kg/(mol
V·h) and it decreased for the increasing comonomer concentrations. The comonomer incorporation was equal to 4.1 mol% at the lowest comonomer concentration and it increased to 9.1 mol% for 0.70 mol/L. At this comonomer concentration, however, a by-product was formed as a colorless oily liquid in addition to the solid copolymer. It should also be noted that the activator considerably affected the copolymerization process. When the activator was changed for Al(
iBu)
3/Ph
3CB(C
6F
5)
4, some lowering of the catalytic activity and considerably lower 1-octene incorporation was observed. The obtained copolymer showed a high molecular weight (M
w ~ 1 × 10
6 g/mol) and narrow MWD (M
w/M
n = 1.4) [
62].
Lorber et al. employed the vanadium(III-V) complexes
V-34–
V-38 bearing similar diamine-bis(phenolate) ligands in the ethylene/1-hexene copolymerization reaction [
63,
64]. The copolymerizations were conducted in the presence of the EtAlCl
2 activator, under 2 bars ethylene, and with 8 mmol 1-hexene. Although
V-38 did not produce any copolymer, the complexes
V-34–
V-37 gave the products which contained 3.5–7.4 mol% comonomer, with the activity from 12 to 120 kg/(mol
V·h). The V(III) complex
V-37 exhibited both the highest activity and incorporation ability. When the catalytic properties were compared for
V-37/EtAlCl
2 and for the classical-type system based on vanadium acetylacetonate,
V-39/EtAlCl
2, those catalysts were found to have similar comonomer incorporation abilities: they produced copolymers with 7.4 mol% and 7.5 mol% comonomer. However, the distinguishing feature of the copolymer synthesized with
V-39 was its very high dispersity (M
w/M
n = 225) versus the copolymer produced with
V-37 (M
w/M
n = 1.7). The catalytic performance of
V-37 in copolymerization of ethylene with 1-hexene and ethylene with 1-octene was additionally compared in [
64]. It was found that, under the same conditions, the copolymers with higher amounts of comonomer units were created in copolymerization of ethylene with 1-octene (8.0–12.0 mol% vs. 7.4–10.6 mol%).
Vanadium complexes bearing ligands featuring sulfur, nitrogen, and oxygen donors were screened for the copolymerization of ethylene and 1-hexene in the presence of dimethylaluminum chloride as an activator and ETA as reactivator by Homden et al. [
65]. Copolymerizations were performed at 25 °C for 30 min under 1 bar of ethylene and with different comonomer concentrations. It was found that
V-40 exhibited the highest incorporation ability (11.3 mol% at 4000 equv of 1-hexene) and the highest activity, 1190 kg/(mol
V·h), showed
V-41. Selected results of ethylene/1-olefin copolymerization with vanadium complexes were displayed in
Table 5.
As results from the review of the literature reports on copolymerization of ethylene with 1-olefins show, it is hard to directly compare the performance of the complexes which are used in this process due to the different copolymerization conditions adopted for the experiments, and, in particular, ethylene pressure and 1-olefin concentration values. The studied complexes contained ligands with various denticity and with different donor atoms, and their catalytic performance in copolymerization may be significantly modified, not only by ligand backbone, but also by the type of substituents on the aromatic rings (the effects of their steric and electron donor properties need to be considered). Since the living polymerization catalysts and new coordination polymerization techniques were developed, the group 4 metal complexes made it possible to obtain block copolymers and multiblock copolymers which, so far, could not be produced with the use of the vanadium catalysts. It should also be noted that when the vanadium complexes and the titanium/zirconium complexes with the ligands of the same structure are used in copolymerization, the former usually exhibit higher comonomer incorporation ability and produce more uniform copolymers. However, it should also be stressed that when ethylene/1-octene copolymerization with vanadium complexes activated by EtAlCl2 is conducted with no reactivator, higher comonomer concentrations in the reaction medium may yield mixture of products (copolymerization in the presence of V-32 and V-33). This is probably due to the simultaneous presence of various types of active sites formed by reduction of the initial vanadium compound.