Kinetics, Isotherm and Thermodynamic Studies for Efficient Adsorption of Congo Red Dye from Aqueous Solution onto Novel Cyanoguanidine-Modified Chitosan Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
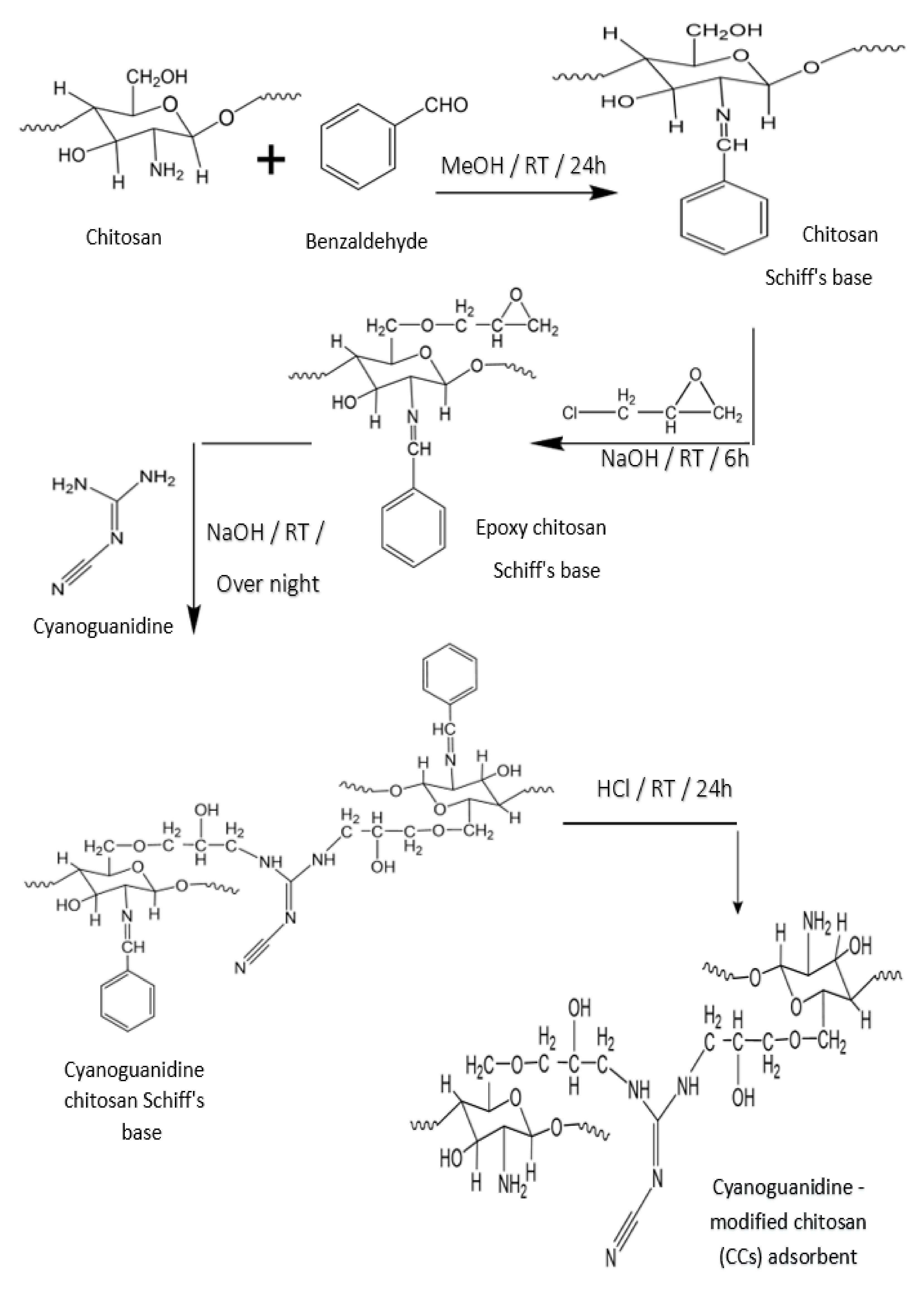
2.2. Preparation of Novel Cyanoguanidine-Modified Chitosan (CCs) Adsorbent
2.3. Measurements
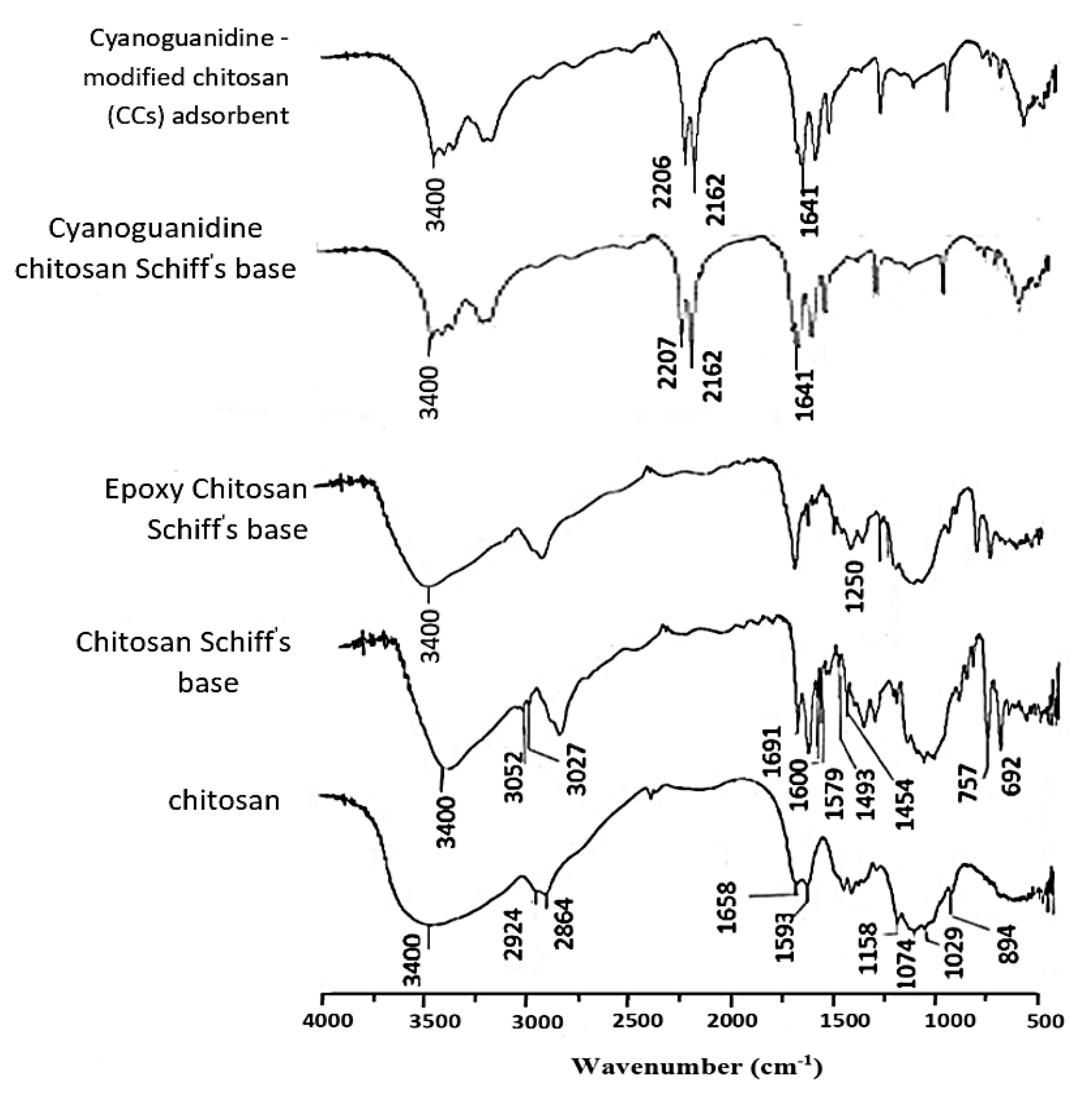
2.3.1. FTIR Spectroscopy
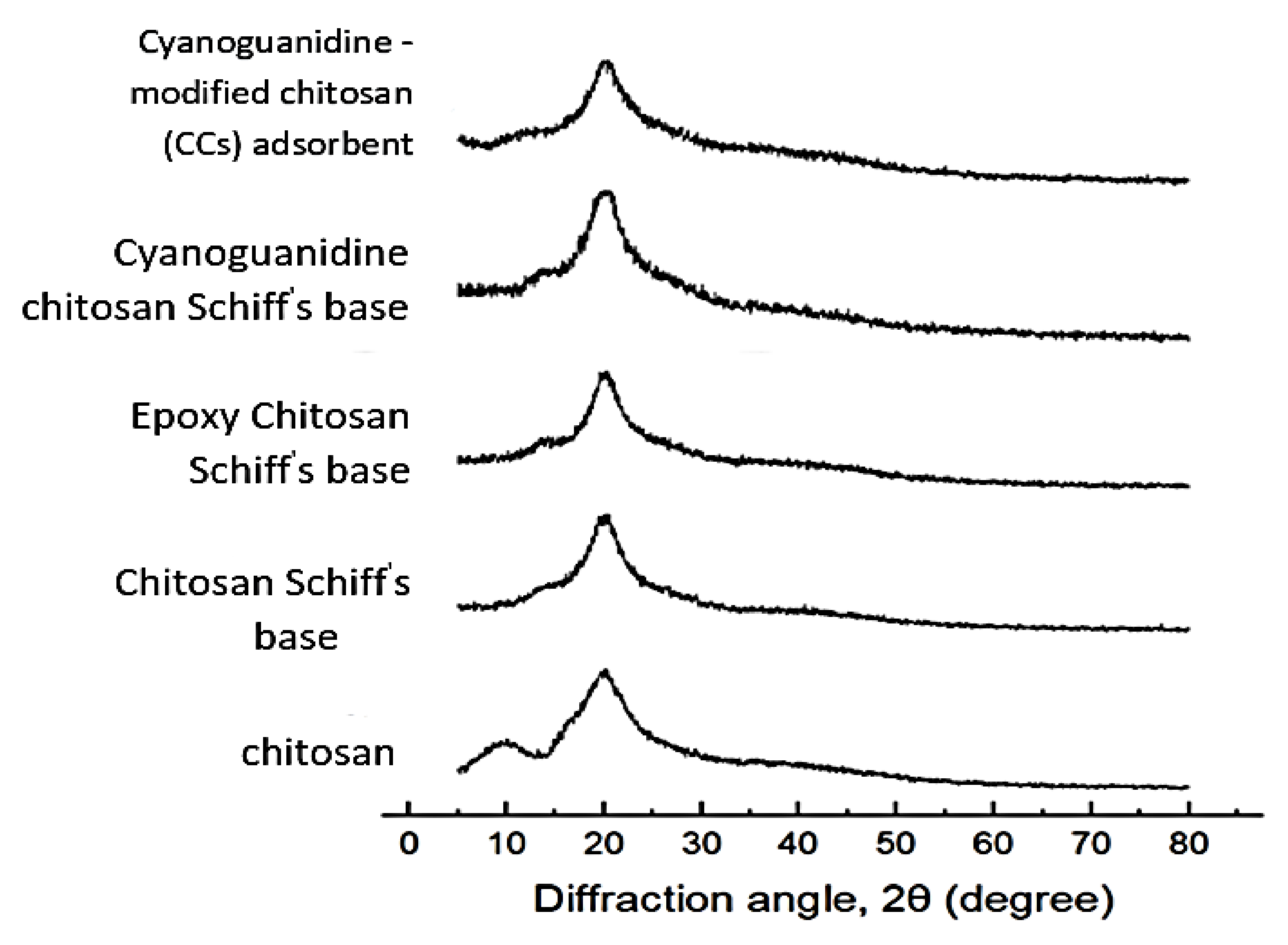
2.3.2. X-ray Diffractometry
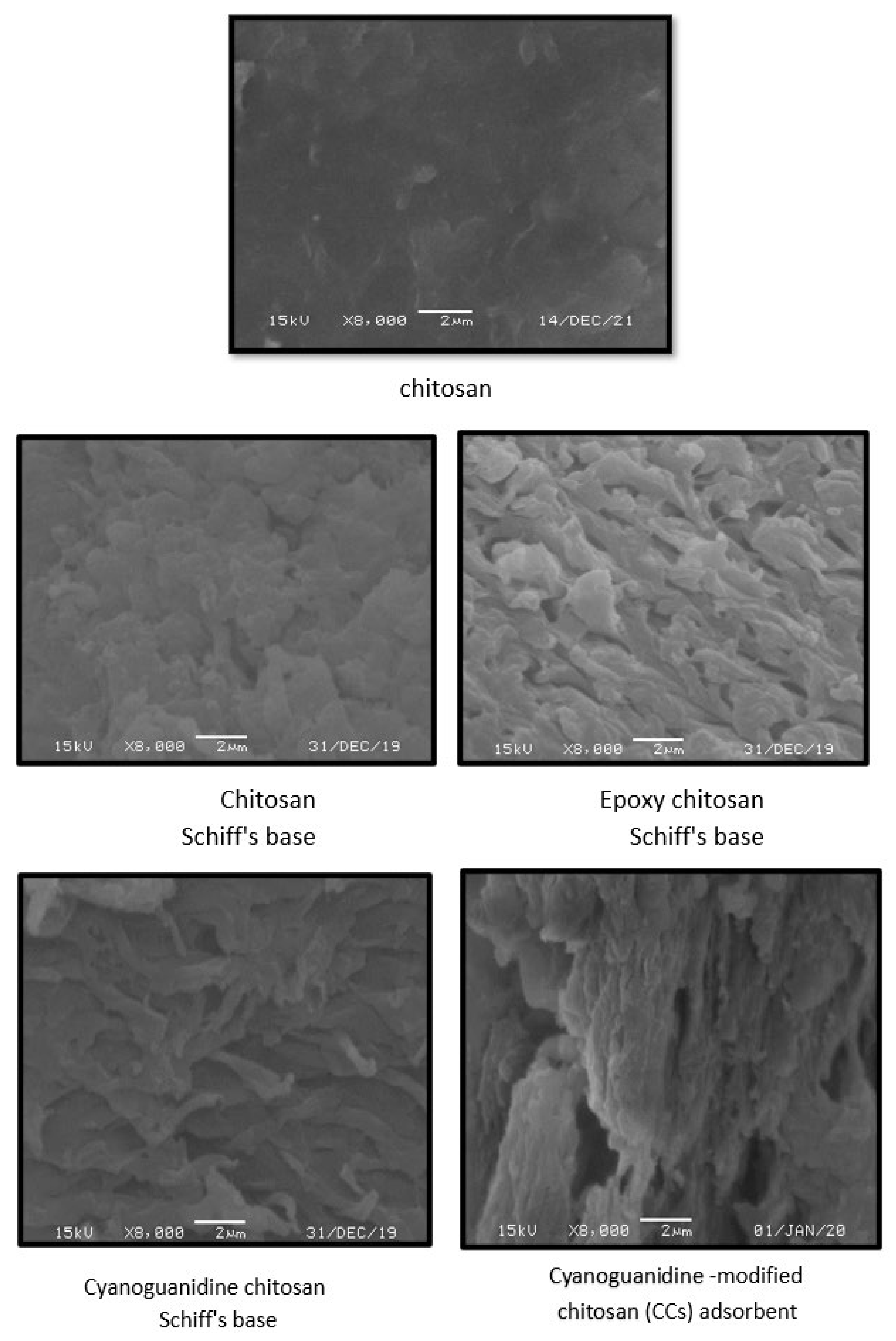
2.3.3. Scanning Electron Microscopy
2.4. Adsorption Studies
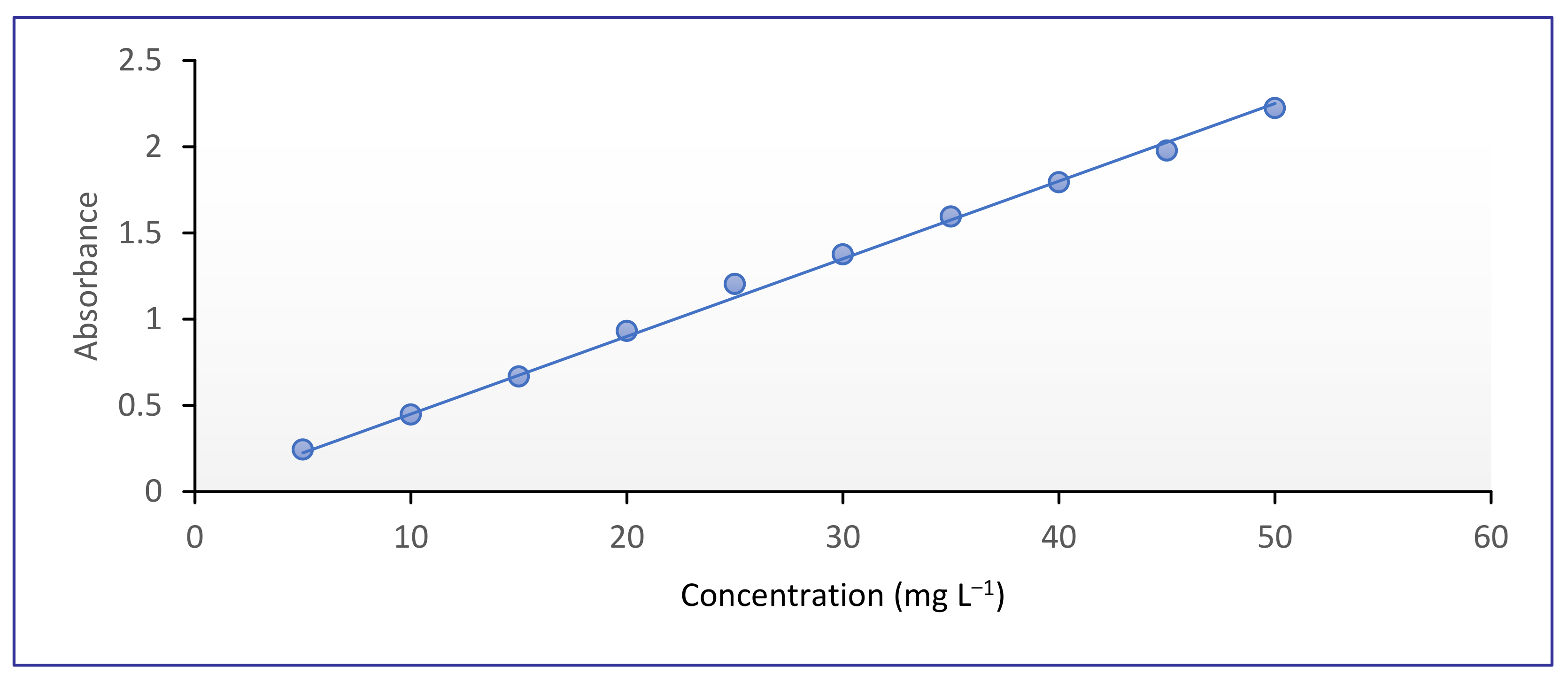
2.4.1. Standard Curve of CR Dye
2.4.2. Adsorption of CR Dye Using CCs Adsorbent
2.4.3. Kinetic Studies
Pseudo-First-Order Model (Lagergren Model)
Pseudo-Second-Order Model (Ho and Mckay Model)
Elovich Model
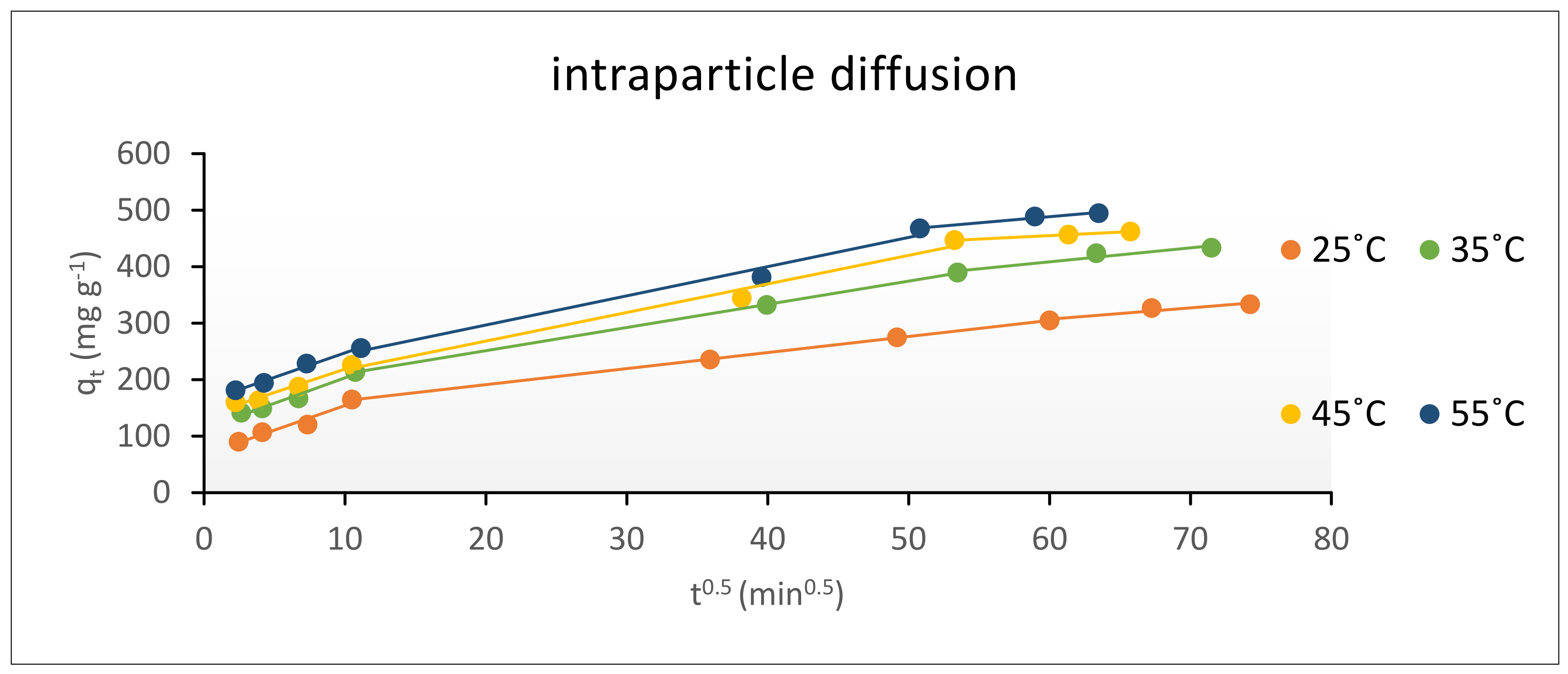
Intraparticle Diffusion Model (Webber and Morris Model)
Kinetic Validation
2.4.4. Adsorption Isotherm for CR Dye
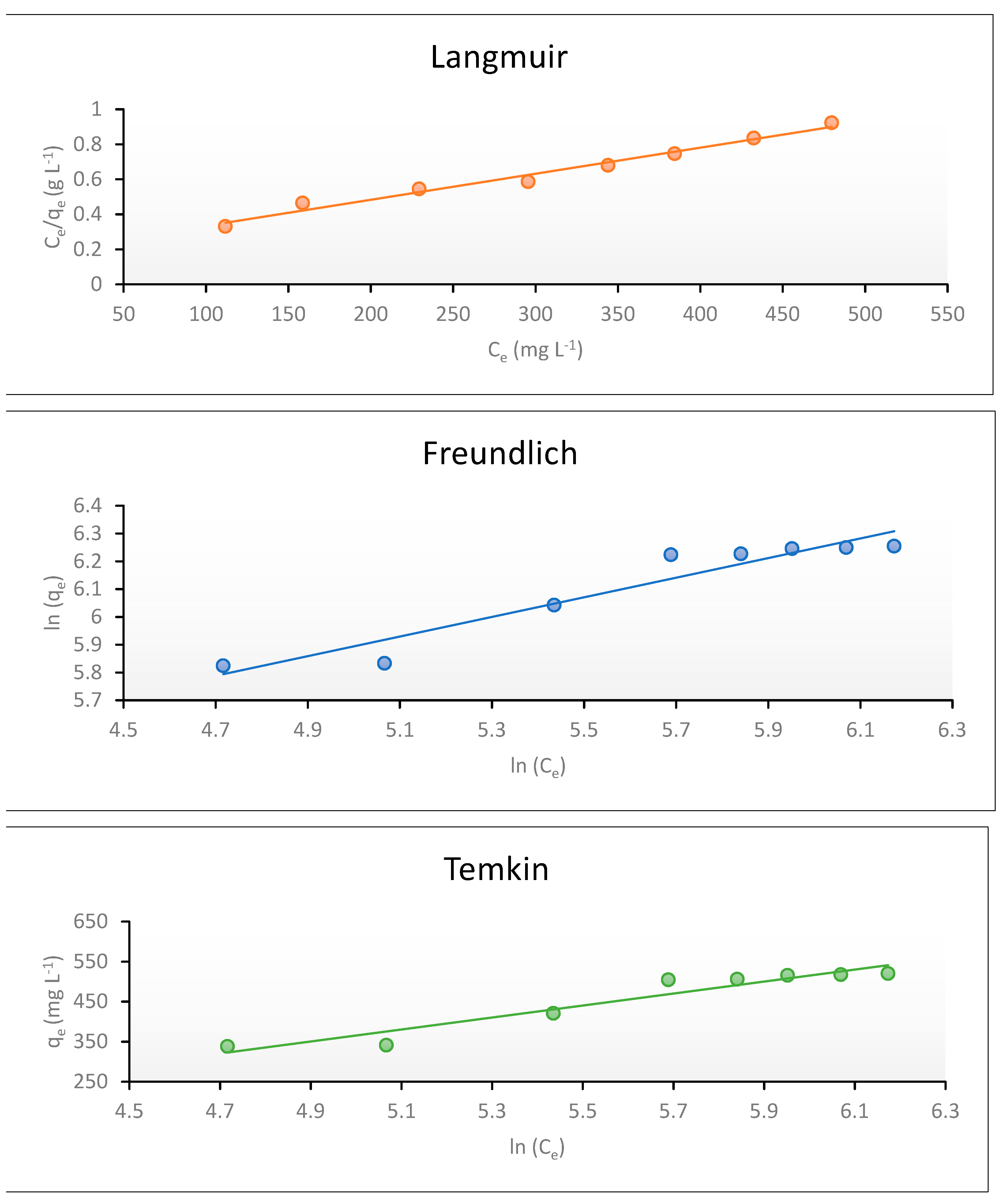
Langmuir Isotherm Model
Freundlich Isotherm Model
Temkin Isotherm Model
Dubinin–Radushkevich (D–R) Isotherm
2.4.5. Thermodynamic Studies
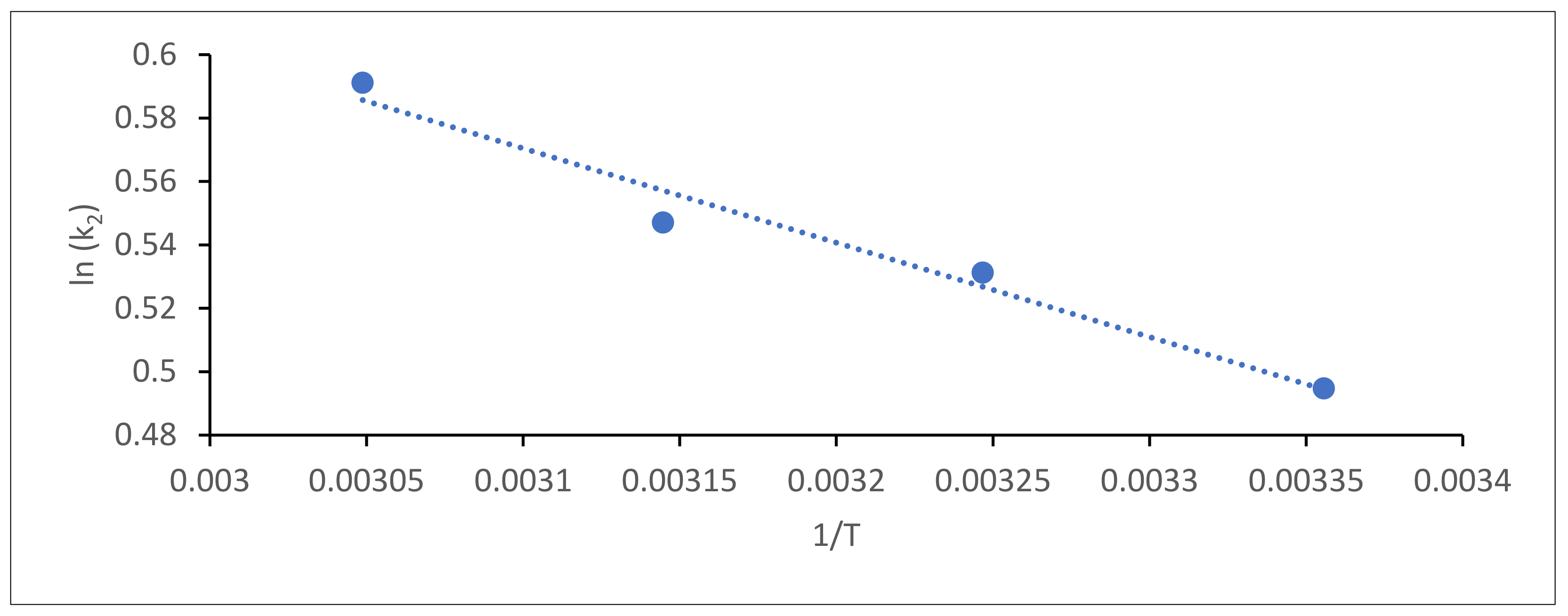
2.4.6. Activation Energy
2.4.7. Desorption Study
3. Results and Discussion
3.1. Synthesis of Novel CCs Adsorbent
3.2. Characterization of Novel CCs Adsorbent
3.2.1. FTIR Spectra of CCs Adsorbent
3.2.2. Powder X-ray Diffraction of CCs Adsorbent
3.2.3. SEM Analysis of CCs Adsorbent
3.3. Adsorption of CR Dye Using CCs Adsorbent
3.3.1. Optimization of the Adsorption Conditions
Effect of pH
Effect of Temperature
Impact of Initial Concentrations of the Dye
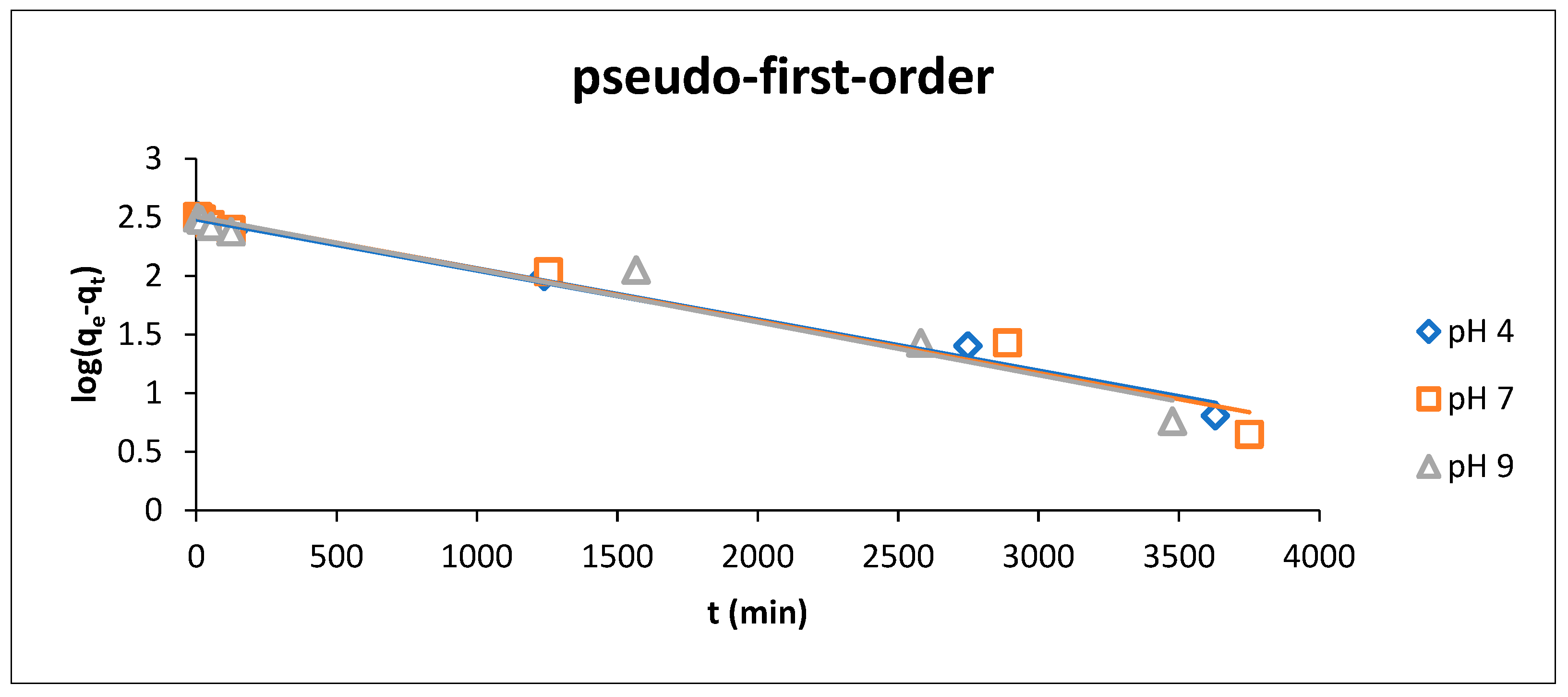
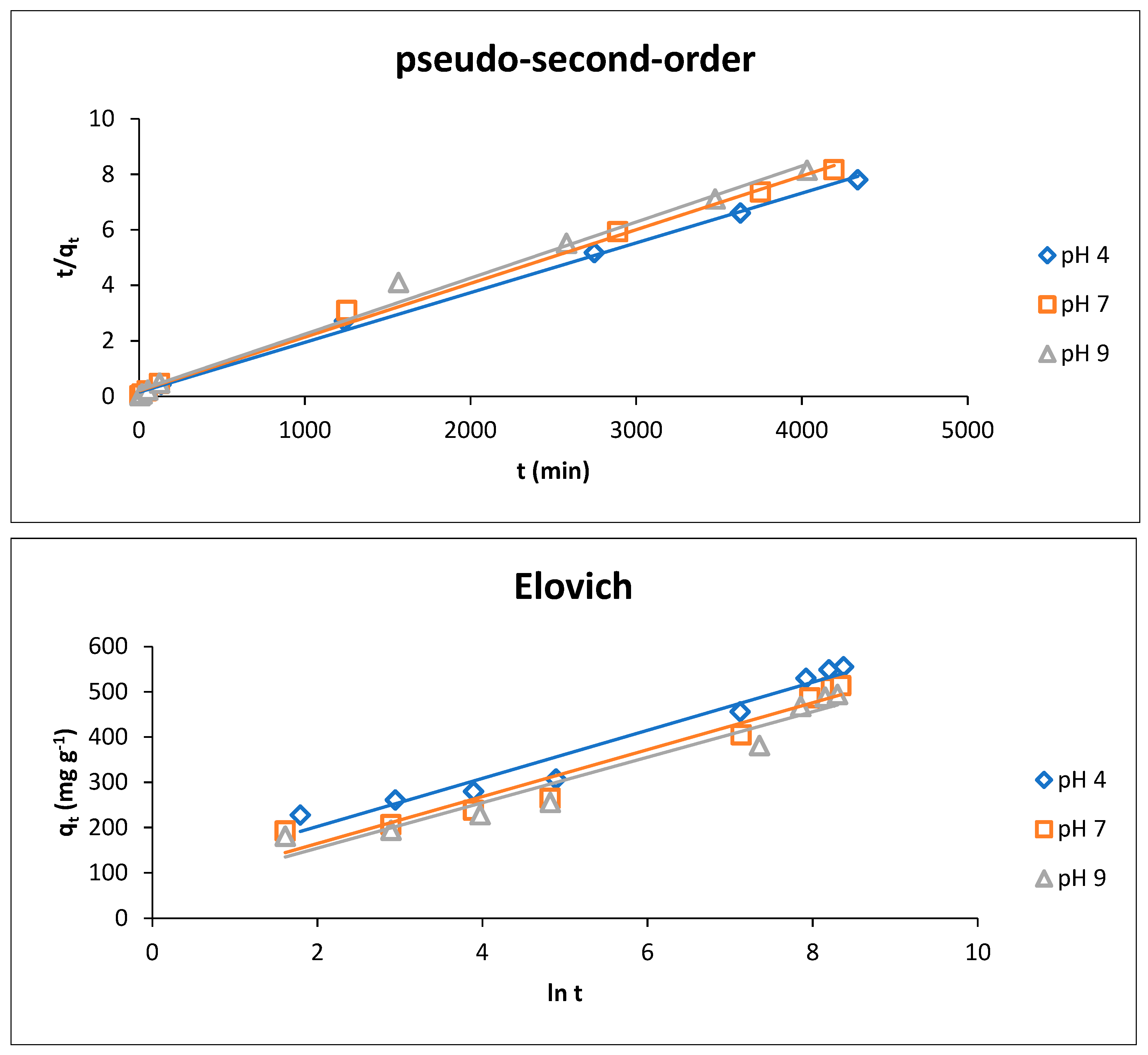
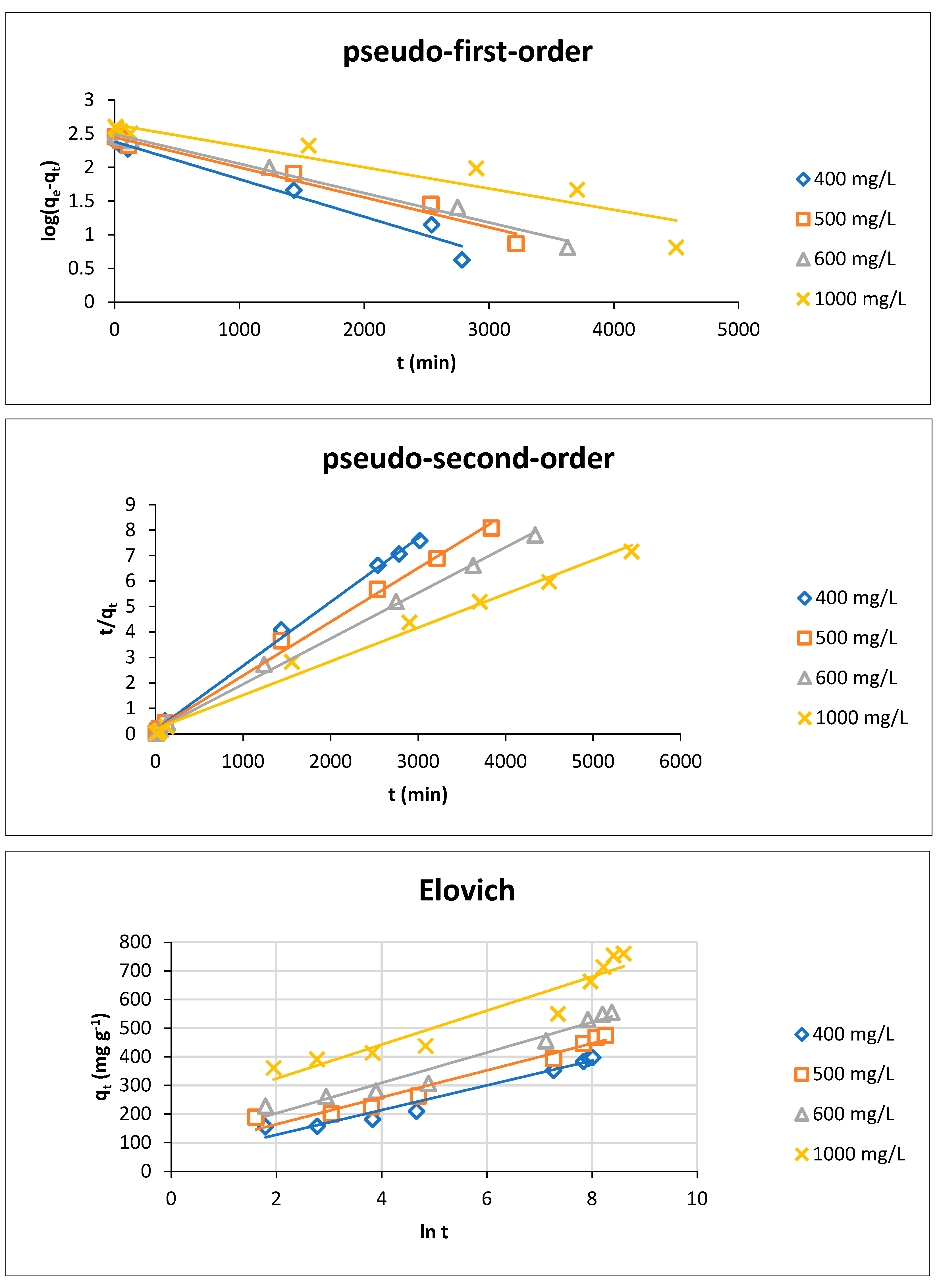
3.3.2. Adsorption Kinetics
At a Different pH
At Different Temperatures
At Different Initial Dye Concentrations
3.3.3. Mechanism of CR Dye Adsorption onto CCs
The Intraparticle Diffusion
3.3.4. Adsorption Isotherm
3.3.5. Adsorption Thermodynamics
3.3.6. Activation Energy
3.3.7. Comparison between CCs and Other Adsorbents for CR Dye Removal
3.3.8. Desorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Al-Harby, N.F.; Almarshed, M.S. Effective removal of Basic Red 12 dye by novel antimicrobial trimellitic anhydride isothiocyanate-cross linked chitosan hydrogels. Polym. Polym. Comp. 2021, 29, S274–S287. [Google Scholar] [CrossRef]
- Buthelezi, S.P.; Olaniran, A.O.; Pillay, B. Textile dye removal from wastewater effluents using bioflocculants produced by indigenous bacterial isolates. Molecules 2012, 17, 14260–14274. [Google Scholar] [CrossRef] [Green Version]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef]
- Annu, S.; Ahmed, S.; Ikram, S. Chitin and Chitosan: History, Composition and Properties, Chitosan; Ahmed, S., Ikram, S., Eds.; Scrivener & Wiley: Hoboken, NJ, USA, 2017; pp. 3–24. [Google Scholar]
- Ahmed, S.; Ikram, S. Chitosan & its derivatives: A review in recent innovations. Int. J. Pharm. Sci. Res. 2015, 6, 14–30. [Google Scholar]
- Elmehbad, N.Y.; Mohamed, N.A. Synthesis, characterization, and antimicrobial activity of novel N-acetyl, N’-chitosanacetohydrazide and its metal complexes. Int. J. Polym. Mater. Polym. Biomater. 2021. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mohamed, N.A.; Mohamed, R.R.; Khalil, N.M.; Abd El Latif, S.M. Synthesis, characterization and antimicrobial activity of poly (N-vinyl imidazole) grafted carboxymethyl chitosan. Carbohydr. Polym. 2010, 79, 998–1005. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mohamed, N.A.; Ali, R.; Abd El Latif, S.M. Chemically induced graft copolymerization of acrylonitrile onto carboxymethyl chitosan and its modification to amidoxime derivative. Polym. Plast. Technol. Eng. 2010, 49, 1055–1064. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mohamed, N.A.; Mohamed, R.R.; Abd El Latif, S.M. Chemically induced graft copolymerization of 4-vinyl pyridine onto carboxymethyl chitosan. Polym. Bull. 2011, 67, 693–707. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abd El-Ghany, N.A. Synthesis, characterization, and antimicrobial activity of carboxymethyl chitosan-graft-poly(N-acryloyl,N’-cyanoacetohydrazide) copolymers. J. Carbohydr. Chem. 2012, 31, 220–240. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Abdallah, H.M.; Mohamed, N.A.; Mohamed, R.R. Synthesis, characterization and application of biodegradable crosslinked carboxymethyl chitosan/poly(vinyl alcohol) clay nanocomposites. Mater. Sci. Eng. C 2015, 56, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Soloman, A.; Rejin, V.O. Preparation of chitosan-polyvinyl alcohol blends and studies on thermal and mechanical properties. Procedia Technol. 2016, 24, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, S.B.; Kordestani, S.S.; Mirzadeh, H.; Mansoori, P. Poly (vinyl alcohol)-chitosan blends: Preparation, mechanical and physical properties. Iran. Polym. J. 2003, 12, 139–146. [Google Scholar]
- Mohamed, N.A.; El-Ghany, N.A.A.; Abdel-Aziz, M.M. Synthesis, characterization, anti-inflammatory and anti-Helicobacter pylori activities of novel benzophenone tetracarboxylimide benzoyl thiourea cross-linked chitosan hydrogels. Int. J. Biol. Macromol. 2021, 181, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Elmehbad, N.Y.; Mohamed, N.A. Terephthalohydrazido cross-linked chitosan hydrogels: Synthesis, characterization and applications. Int. J. Polym. Mater. Polym. Biomater. 2021. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abd El-Ghany, N.A.; Fahmy, M.M. Novel antimicrobial superporous cross-linked chitosan/pyromellitimide benzoyl thiourea hydrogels. Int. J. Biol. Macromol. 2016, 82, 589–598. [Google Scholar] [CrossRef]
- Elsayed, N.H.; Monier, M.; Youssef, I. Fabrication of photo-active trans -3-(4-pyridyl)acrylic acid modified chitosan. Carbohydr. Polym. 2017, 172, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Abd El-Ghany, N.A. Novel aminohydrazide cross-linked chitosan filled with multi-walled carbon nanotubes as antimicrobial agents. Int. J. Biol. Macromol. 2018, 115, 651–662. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Abd El-Ghany, N.A. Synthesis, characterization and antimicrobial activity of novel aminosalicylhydrazide cross linked chitosan modified with multi-walled carbon nanotubes. Cellulose 2019, 26, 1141–1156. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Al-Harby, N.F.; Almarshed, M.S. Enhancement of adsorption of Congo red dye onto novel antimicrobial trimellitic anhydride isothiocyanate-cross-linked chitosan hydrogels. Polym. Bull. 2020, 77, 6135–6160. [Google Scholar] [CrossRef]
- Alharby, N.F.; Almutairi, R.S.; Mohamed, N.A. Adsorption Behavior of Methylene Blue Dye by Novel Crosslinked O-CM-Chitosan Hydrogel in Aqueous Solution: Kinetics, Isotherm and Thermodynamics. Polymers 2021, 13, 3659. [Google Scholar] [CrossRef] [PubMed]
- Surikumaran, H.; Mohamad, S.; Muhamad Sarih, N. Molecular Imprinted Polymer of Methacrylic Functionalised β-Cyclodextrin for Selective Removal of 2,4-Dichlorophenol. Int. J. Mol. Sci. 2014, 15, 6111–6136. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, N.A.; Abd El-Ghany, N.A. Synthesis, characterization, and antimicrobial activity of chitosan hydrazide derivative. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 410–415. [Google Scholar] [CrossRef]
- Elmehbad, N.Y.; Mohamed, N.A. Designing, preparation and evaluation of the antimicrobial activity of biomaterials based on chitosan modified with silver nanoparticles. Int. J. Biol. Macromol. 2020, 151, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.O.; Dotto, G.L.; Pinto, L.A.A. Cyanoguanidine-crosslinked chitosan to adsorption of food dyes in the aqueous binary system. J. Mol. Liq. 2015, 211, 425–430. [Google Scholar] [CrossRef]
- Burkhanova, N.; Yugai, S.; Pulatova, K.P.; Nikononvich, G.; Milusheva, R.Y.; Voropaeva, N.; Rashidova, S.S. Structural investigations of chitin and its deacetylation products. Chem. Nat. Compd. 2000, 36, 352–355. [Google Scholar] [CrossRef]
- Feng, T.; Zhang, F.; Wang, J.; Wang, L. Application of Chitosan-Coated Quartz Sand for Congo Red Adsorption from Aqueous Solution. J. Appl. Polym. Sci. 2013, 125, 1766–1772. [Google Scholar] [CrossRef]
- Salama, H.E.; Saad, G.R.; Sabaa, M.W. Synthesis, characterization, and biological activity of cross-linked chitosan biguanidine loaded with silver nanoparticles. J. Biomater. Sci. Polym. Ed. 2016, 27, 1880–1898. [Google Scholar] [CrossRef]
- El-Harby, N.F.; Ibrahim, S.M.A.; Mohamed, N.A. Adsorption of Congo red dye onto antimicrobial terephthaloyl thiourea cross-linked chitosan hydrogels. Water Sci. Technol. 2017, 76, 2719–2732. [Google Scholar] [CrossRef] [PubMed]
- Guarın, J.R.; Moreno-Pirajan, J.C.; Giraldo, L. Kinetic Study of the Bioadsorption of Methylene Blue on the Surface of the Biomass Obtained from the Algae D. antarctica. J. Chem. 2018, 2018, 2124845. [Google Scholar] [CrossRef] [Green Version]
- Swan, N.B.; Abbas, M.; Zaini, A. Adsrption of Malachite green and Congo red dyes from water: Recent progress and future outlook. Ecol. Chem. Eng. 2019, 26, 119–132. [Google Scholar]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef] [Green Version]
- Ojedokun, A.T.; Bello, O.S. Kinetic modeling of liquid-phase adsorption of Congo red dye using guava leaf-based activated carbon. Appl. Water Sci. 2017, 7, 1965–1977. [Google Scholar] [CrossRef] [Green Version]
- Md Ahsanul Haque, A.N.; Remadevi, R.; Wang, X.; Naebe, M. Adsorption of anionic Acid Blue 25 on chitosan-modified cotton gin trash film. Cellulose 2020, 27, 9437–9456. [Google Scholar] [CrossRef]
- Kumar, R.; Ansari, S.A.; Barakat, M.A.; Aljaafari, A.; Cho, M.H. A polyaniline@MoS2-based organic–inorganic nanohybrid for the removal of Congo red: Adsorption kinetic, thermodynamic and isotherm studies. New J. Chem. 2018, 42, 18802–18809. [Google Scholar] [CrossRef]
- Lin, C.; Li, S.; Chen, M.; Jiang, R. Removal of Congo red dye by gemini surfactant C12-4-C12 ∙ 2Br-modified chitosan hydrogel beads. J. Dispers. Sci. Technol. 2017, 38, 46–57. [Google Scholar] [CrossRef]
- Titi Ojedokun, A.; Solomon Bell, O. Liquid phase adsorption of Congo red dye on functionalized corn cobs. J. Dispers. Sci. Technol. 2017, 38, 1285–1294. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 16530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahira, I.; Aslam, Z.; Abbas, A.; Monim-ul-Mehboob, M.; Ali, S.; Asghar, A. Adsorptive removal of acidic dye onto grafted chitosan: A plausible grafting and adsorption mechanism. Int. J. Biol. Macromol. 2019, 136, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Zahir, A.; Aslam, Z.; Kamal, M.S.; Ahmad, W.; Abbas, A.; Shawabkeh, R.A. Development of novel cross-linked chitosan for the removal of anionic Congo red dye. J. Mol. Liq. 2017, 244, 211–218. [Google Scholar] [CrossRef]
- Bulut, Y.; Karaer, H. Adsorption of Methylene Blue from Aqueous Solution by Crosslinked Chitosan/Bentonite Composite. J. Dispers. Sci. Technol. 2015, 36, 61–67. [Google Scholar] [CrossRef]
- Jabar, J.M.; Odusote, Y.A.; Alabi, K.A.; Ahmed, I.B. Kinetics and mechanisms of congo-red dye removal from aqueous solution using activated Moringa oleifera seed coat as adsorbent. Appl. Water Sci. 2020, 10, 136. [Google Scholar] [CrossRef]
- Dbik, A.; Bentahar, S.; el Khomri, M.; el Messaoudi, N.; Lacherai, A. Adsorption of Congo red dye from aqueous solutions using tunics of the corm of the saffron. Mater. Today 2020, 22, 134–139. [Google Scholar] [CrossRef]
- Cai, L.; Ying, D.; Liang, X.; Zhu, M.; Lin, X.; Xu, Q.; Cai, Z.; Xu, X.; Zhang, L. A novel cationic polyelectrolyte microsphere for ultrafast and ultra-efficient removal of heavy metal ions and dyes. Chem. Eng. J. 2021, 410, 128404. [Google Scholar] [CrossRef]
- Lafi, R.; Montasser, I.; Hafiane, A. Adsorption of Congo red dye from aqueous solutions by prepared activated carbon with oxygen-containing functional groups and its regeneration. Adsorpt. Sci. Technol. 2019, 37, 160–181. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Yu, J.; Liu, C.; Yang, G.; Shi, L.; Zhuang, X. Xanthated chitosan/cellulose sponges for the efficient removal of anionic and cationic dyes. React. Funct. Polym. 2021, 160, 104840. [Google Scholar] [CrossRef]
- Xu, G.; Zhu, Y.; Wang, X.; Wang, S.; Cheng, T.; Ping, R.; Cao, J.; Lv, K. Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), methylene blue and Congo red from aqueous solution. e-Polymers 2019, 19, 244–256. [Google Scholar] [CrossRef]
- Wang, P.; Yan, T.; Wang, L. Removal of Congo Red from Aqueous Solution Using Magnetic Chitosan Composite Microparticles. BioResources 2013, 8, 6026–6043. [Google Scholar] [CrossRef]









| Kinetic Models | Parameters | PH | ||
|---|---|---|---|---|
| 4 | 7 | 9 | ||
| pseudo- first-order | qe.exp (mg g−1) | 555.33 | 513.56 | 494.44 |
| R2 | 0.989 | 0.969 | 0.958 | |
| qe.cal (mg g−1) | 310.38 | 321.07 | 322.18 | |
| K1 (10−4) (min−1) | 9.21 | 9.21 | 11.15 | |
| Δqe (%) | 16.67 | 14.17 | 13.17 | |
| pseudo- second-order | R2 | 0.997 | 0.996 | 0.991 |
| qe,cal (mg g−1) | 555.56 | 526.32 | 500 | |
| K2 (10−5) (g·mg−1·min−1) | 2.12 | 1.86 | 1.81 | |
| Δqe (%) | 0.02 | 0.94 | 0.42 | |
| Elovich | R2 | 0.961 | 0.951 | 0.94 |
| β (g·mg−1) | 0.019 | 0.019 | 0.02 | |
| α (mg g−1 min−1) | 325.64 | 168.8 | 148.17 | |
| Kinetic Models | Parameters | Temperatures | |||
|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | 55 °C | ||
| qe.exp (mg g−1) | 333.11 | 433.33 | 462 | 494.44 | |
| pseudo- first-order | R2 | 0.957 | 0.973 | 0.978 | 0.958 |
| qe.cal (mg g−1) | 230.57 | 288.07 | 313.47 | 322.18 | |
| K1 (10−4) (min−1) | 6.909 | 6.909 | 6.909 | 11.151 | |
| Δqe (%) | 10.88 | 12.67 | 12.15 | 13.17 | |
| pseudo- second-order | R2 | 0.99 | 0.993 | 0.992 | 0.991 |
| qe,cal (mg g−1) | 333.33 | 434.78 | 476.19 | 500 | |
| K2 (10−5) (g·mg−1·min−1) | 1.64 | 1.7 | 1.73 | 1.81 | |
| Δqe (%) | 0.02 | 0.13 | 1.16 | 0.42 | |
| Elovich | R2 | 0.963 | 0.963 | 0.937 | 0.94 |
| β (g·mg−1) | 0.027 | 0.021 | 0.02 | 0.02 | |
| α (mg g−1 min−1) | 36.24 | 64.72 | 96.36 | 148.17 | |
| Kinetic Models | Parameters | Dye Concentrations | |||
|---|---|---|---|---|---|
| 400 mg L−1 | 500 mg L−1 | 600 mg L−1 | 1000 mg L−1 | ||
| qe.exp (mg g−1) | 398 | 474.67 | 555.33 | 760.44 | |
| pseudo- first-order | R2 | 0.972 | 0.975 | 0.989 | 0.879 |
| qe.cal (mg g−1) | 240.99 | 279.58 | 310.38 | 429.64 | |
| K1 (10−4) (min−1) | 13.82 | 9.21 | 9.21 | 6.91 | |
| Δqe (%) | 13.95 | 14.53 | 16.67 | 15.38 | |
| pseudo- second-order | R2 | 0.998 | 0.996 | 0.997 | 0.99 |
| qe,cal (mg g−1) | 400 | 476.19 | 555.56 | 769.23 | |
| K2 (10−5) (g·mg−1·min−1) | 3.97 | 2.58 | 2.12 | 0.88 | |
| Δqe (%) | 0.18 | 0.11 | 0.02 | 0.41 | |
| Elovich | R2 | 0.963 | 0.959 | 0.961 | 0.913 |
| β (g mg−1) | 0.023 | 0.021 | 0.019 | 0.017 | |
| α (mg g−1 min−1) | 111.97 | 207.29 | 325.64 | 1874.6 | |
| Parameters | pH | |||
|---|---|---|---|---|
| 4 | 7 | 9 | ||
| Whole processes | R2 | 0.979 | 0.988 | 0.989 |
| ki | 5.19 | 5.19 | 5.18 | |
| 1st | Ki.1 | 11.26 | 9.72 | 9.54 |
| 2nd | Ki.2 | 5.5 | 5.24 | 5.17 |
| 3rd | Ki.3 | 1.15 | 1.26 | 1.27 |
| Parameters | Temperatures | ||||
|---|---|---|---|---|---|
| 25 °C | 35 °C | 45 °C | 55 °C | ||
| Whole processes | R2 | 0.976 | 0.984 | 0.987 | 0.989 |
| ki | 3.33 | 4.37 | 4.96 | 5.18 | |
| 1st | Ki.1 | 5.9 | 6.25 | 6.43 | 9.54 |
| 2nd | Ki.2 | 2.84 | 4.1 | 5.06 | 5.17 |
| 3rd | Ki.3 | 0.95 | 1.2 | 1.21 | 1.27 |
| Parameters | Dye Concentrations (mg L−1) | ||||
|---|---|---|---|---|---|
| 400 | 500 | 600 | 1000 | ||
| whole processes | R2 | 0.988 | 0.986 | 0.979 | 0.989 |
| ki | 4.7 | 4.88 | 5.19 | 5.55 | |
| 1st | Ki.1 | 6.59 | 7.9 | 11.26 | 11.99 |
| 2nd | Ki.2 | 4.46 | 4.63 | 5.5 | 5.51 |
| 3rd | Ki.3 | 1.91 | 1.41 | 1.15 | 0.96 |
| Isotherm Model | Parameters | |
|---|---|---|
| Langmuir | R2 | 0.98 |
| KL (L mg−1) | 0.008 | |
| qmax (mg g−1) | 666.67 | |
| RL | 0.217–0.111 | |
| Freundlich | R2 | 0.917 |
| Kf (L mg−1) | 62.11 | |
| 1/n | 0.35 | |
| Temkin | R2 | 0.92 |
| KT (L mg−1) | 0.08 | |
| B (J mol−1) | 149.74 | |
| D-R | R2 | 0.807 |
| Xmax (mg g−1) | 520.04 | |
| β (mol2 J−2) | 0.0009 | |
| E (kJ mol−1) | 0.023 |
| Thermodynamic Parameters | Temperature (K) | |
|---|---|---|
| ∆G° (KJ mol−1) | 298 | −0.55 |
| 308 | −2.45 | |
| 318 | −3.19 | |
| 328 | −4.21 | |
| ΔH° (kJ mol−1) | 34.49 | |
| ΔS° (J K−1 mol) | 118.48 | |
| Adsorbent | Qmax (mg g−1) | Temperature °C | Dye Concentration mg L−1 | Adsorbent Dose (g) | pH | Ref. |
|---|---|---|---|---|---|---|
| Tunics of the corm of the saffron | 6.2 | 25 | 50–500 | 0.5 | 10 | [46] |
| Quaternized chitosan/chitosan cationic polyelectrolyte microsphere | 1500 | 25 | 0–1000 | 0.075 | 5 | [47] |
| Activated carbon coffee waste | 90.90 | 25 | 50 mg | 0.1 | 3 | [48] |
| xanthated chitosan/cellulose sponges | 289.855 | 30 | 6.25–200 | 0.05 | 6 | [49] |
| Chitosan and Laponite based nanocomposite | 390.3 | 30 | 500 | 1 | 6 | [50] |
| CCs | 760.44 | 55 | 1000 | 0.05 | 4 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Kinetics, Isotherm and Thermodynamic Studies for Efficient Adsorption of Congo Red Dye from Aqueous Solution onto Novel Cyanoguanidine-Modified Chitosan Adsorbent. Polymers 2021, 13, 4446. https://doi.org/10.3390/polym13244446
Al-Harby NF, Albahly EF, Mohamed NA. Kinetics, Isotherm and Thermodynamic Studies for Efficient Adsorption of Congo Red Dye from Aqueous Solution onto Novel Cyanoguanidine-Modified Chitosan Adsorbent. Polymers. 2021; 13(24):4446. https://doi.org/10.3390/polym13244446
Chicago/Turabian StyleAl-Harby, Nouf F., Ebtehal F. Albahly, and Nadia A. Mohamed. 2021. "Kinetics, Isotherm and Thermodynamic Studies for Efficient Adsorption of Congo Red Dye from Aqueous Solution onto Novel Cyanoguanidine-Modified Chitosan Adsorbent" Polymers 13, no. 24: 4446. https://doi.org/10.3390/polym13244446
APA StyleAl-Harby, N. F., Albahly, E. F., & Mohamed, N. A. (2021). Kinetics, Isotherm and Thermodynamic Studies for Efficient Adsorption of Congo Red Dye from Aqueous Solution onto Novel Cyanoguanidine-Modified Chitosan Adsorbent. Polymers, 13(24), 4446. https://doi.org/10.3390/polym13244446







