Composite Materials Based on Iron Oxide Nanoparticles and Polyurethane for Improving the Quality of MRI
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Methods
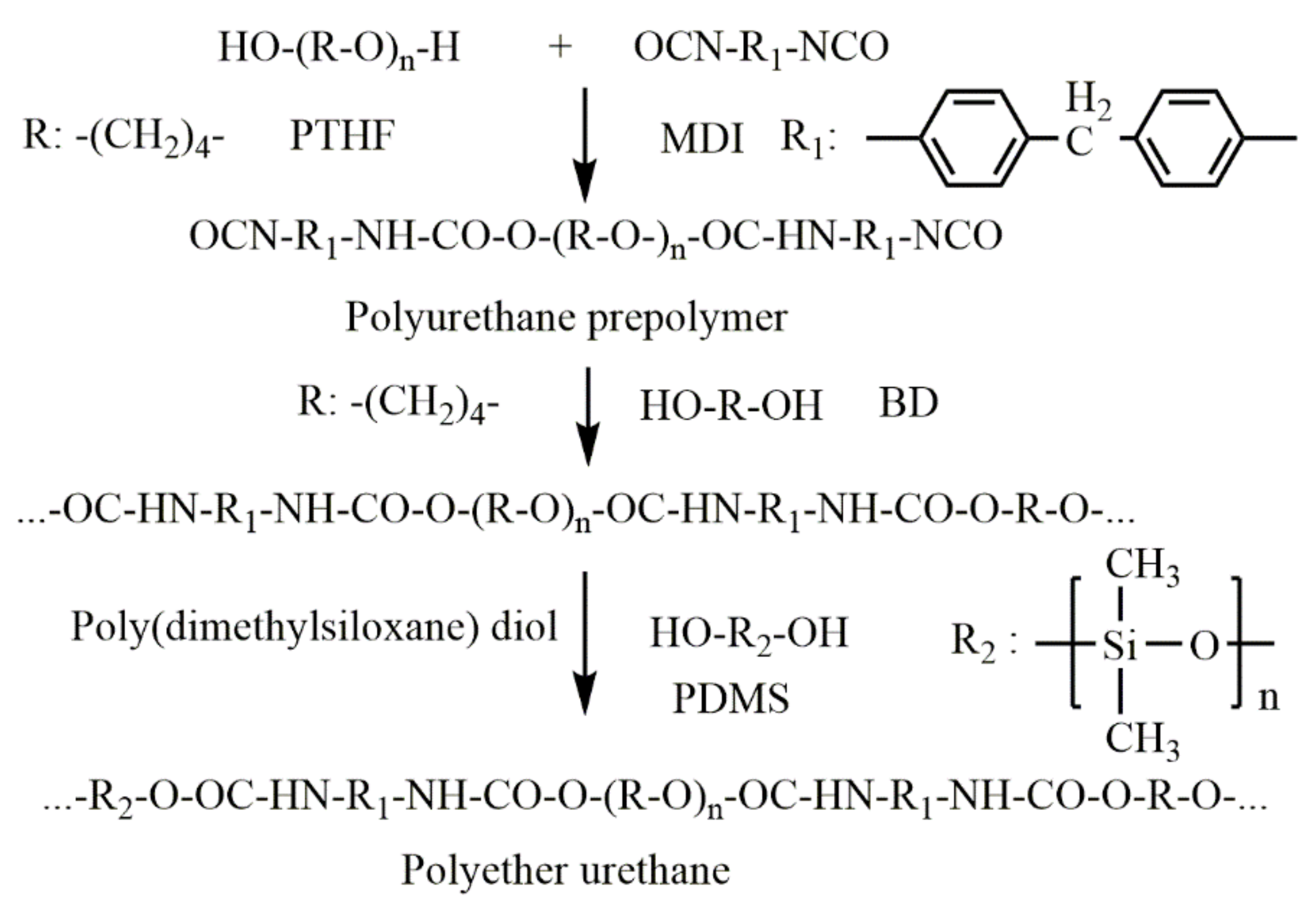
2.2.1. Synthesis of Pristine PU
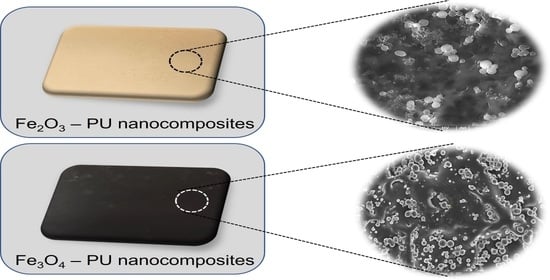
2.2.2. Preparation of Fe2O3 and Fe3O4 PU Nanocomposites
2.3. Characterization Technique
2.3.1. Fourier Transform Infrared–Attenuated Total Reflectance (FTIR-ATR) Spectroscopy
2.3.2. Mechanical Analysis
2.3.3. Scanning Electron Microscopy (SEM) Analysis
2.3.4. Surface Topography
2.3.5. Contact Angle Determination and Surface Wettability Study
2.3.6. Determination of Dynamic Vapours’ Sorption and Diffusion Coefficients
2.3.7. Dielectric Analysis
2.3.8. Magnetic Determination
2.4. In Vitro Evaluation of Biocompatibility—MTT Assay
2.5. Statistical Analysis
3. Results and Discussion
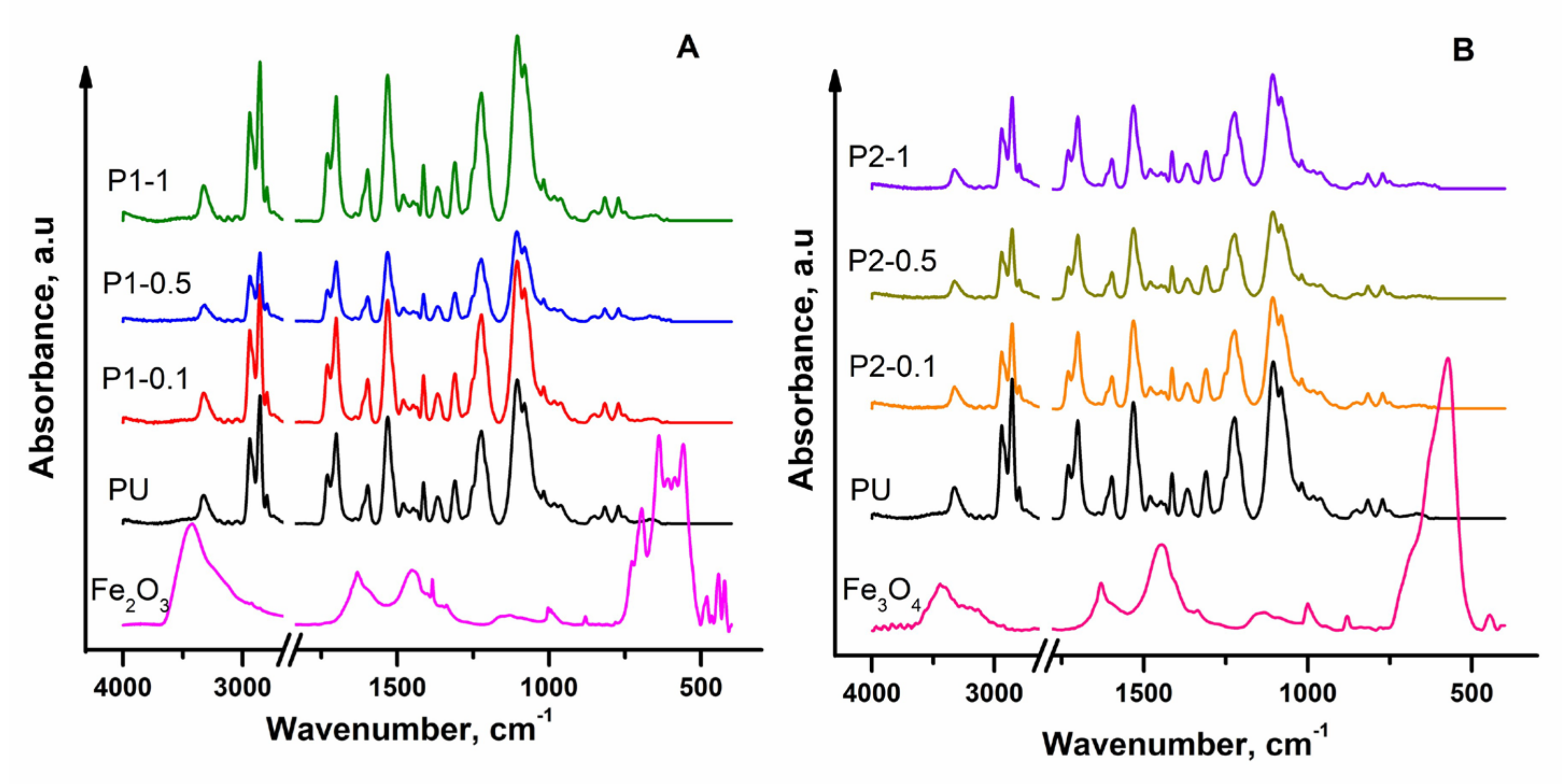
3.1. FTIR-ATR Analysis
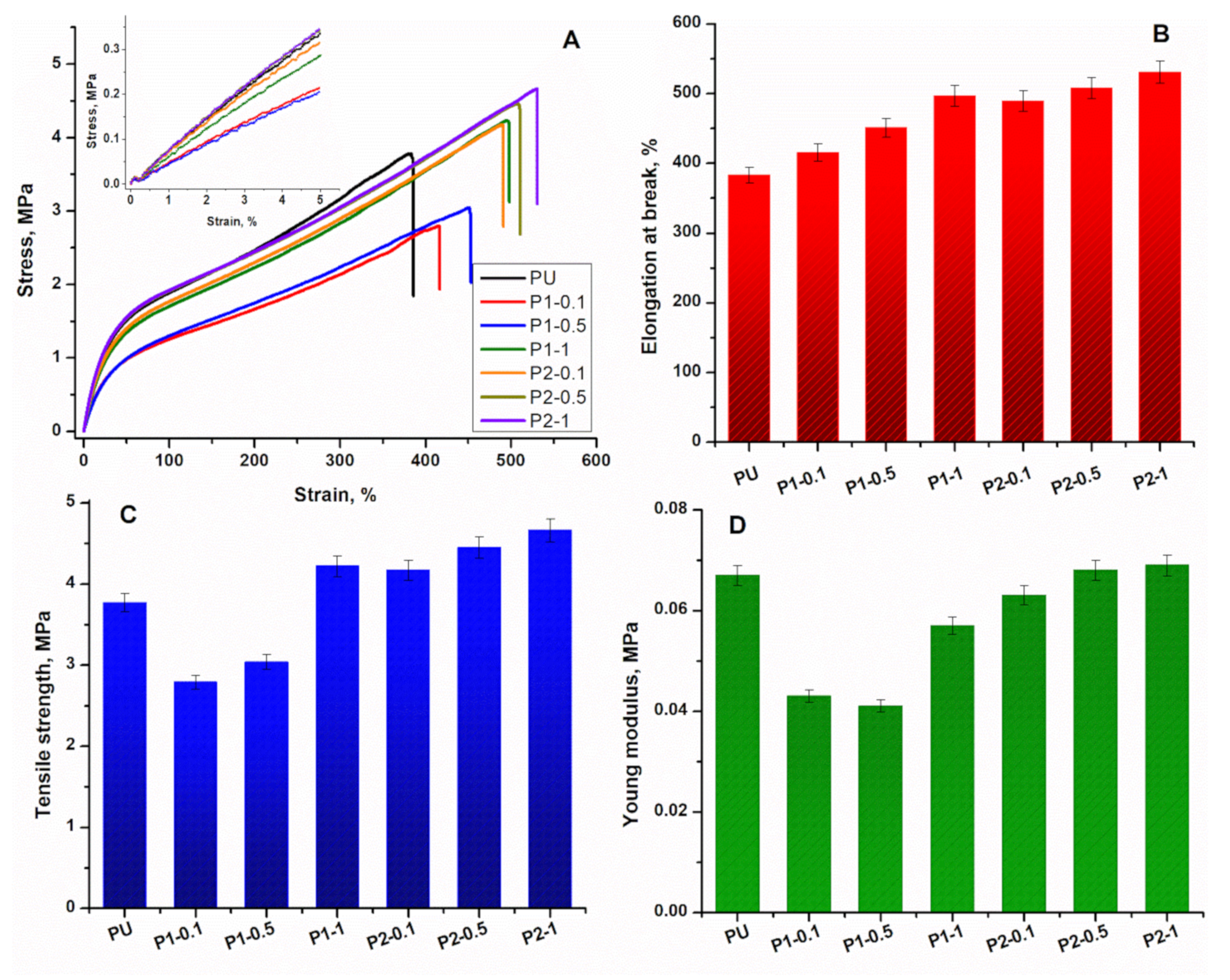
3.2. Mechanical Analysis
3.3. Surface Morphology of the Samples
3.4. Profilometry Analysis
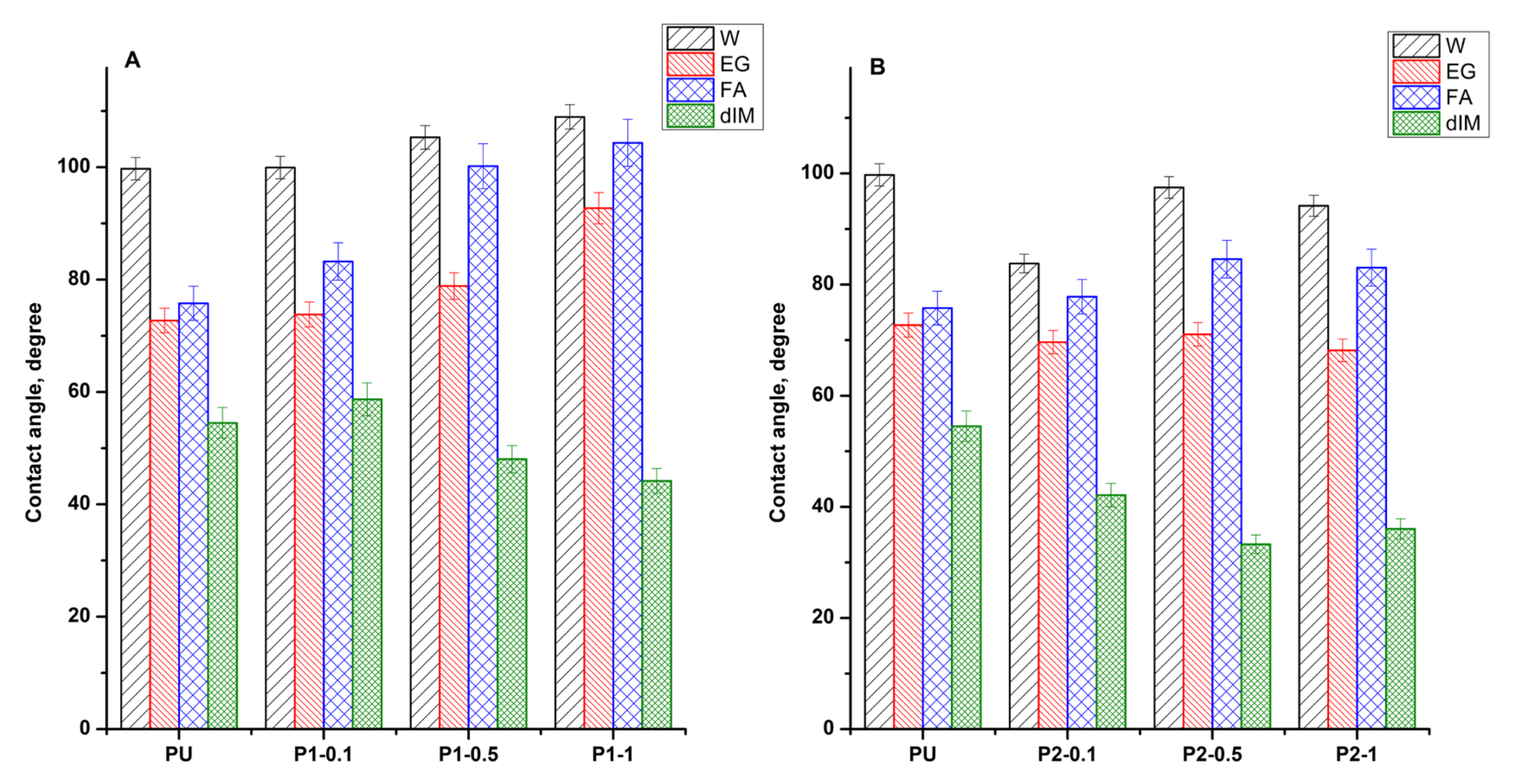
3.5. Wettability and Surface Energy Parameters Study
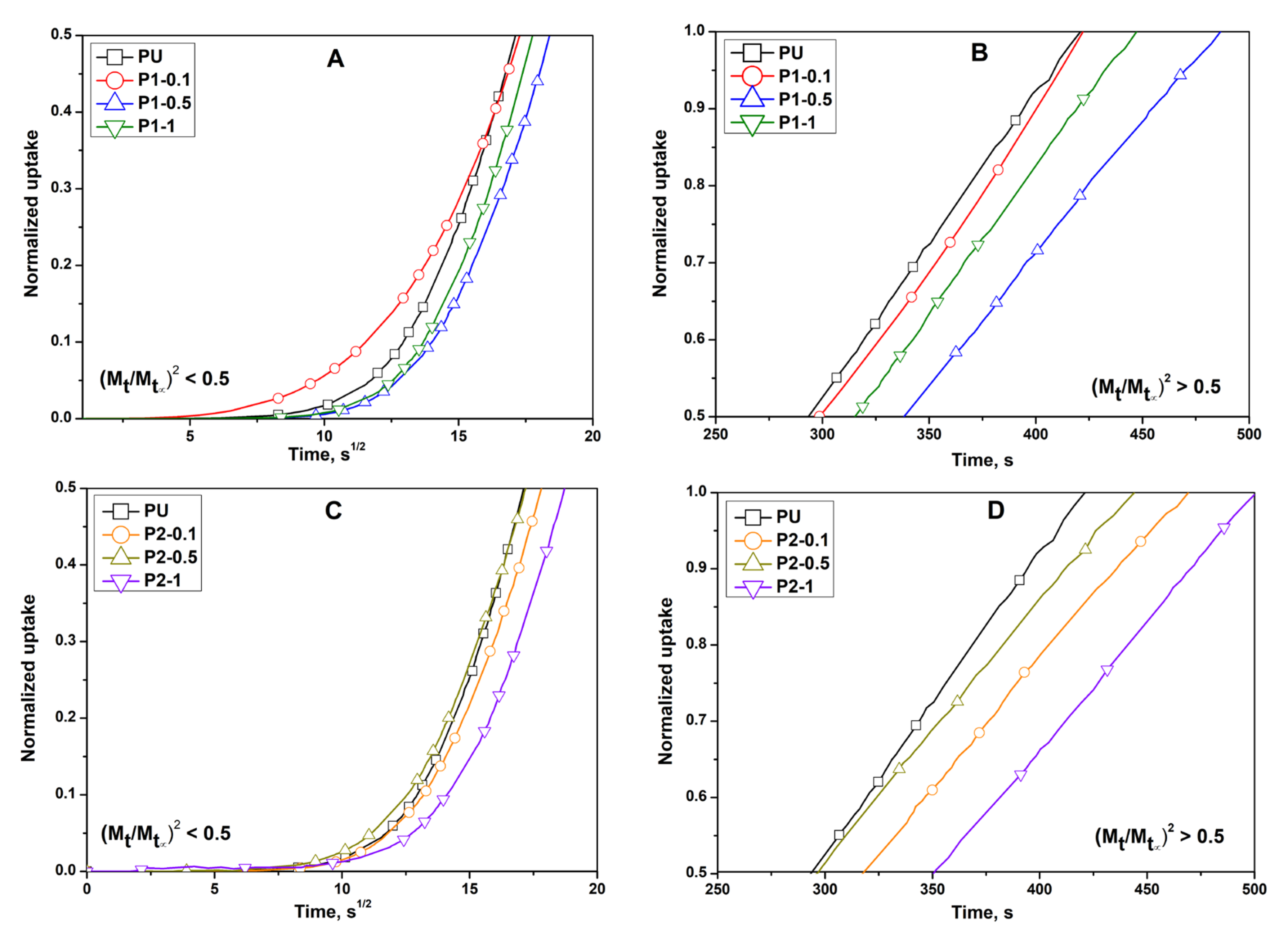
3.6. Determination of Dynamic Vapours’ Sorption and Diffusion Coefficients
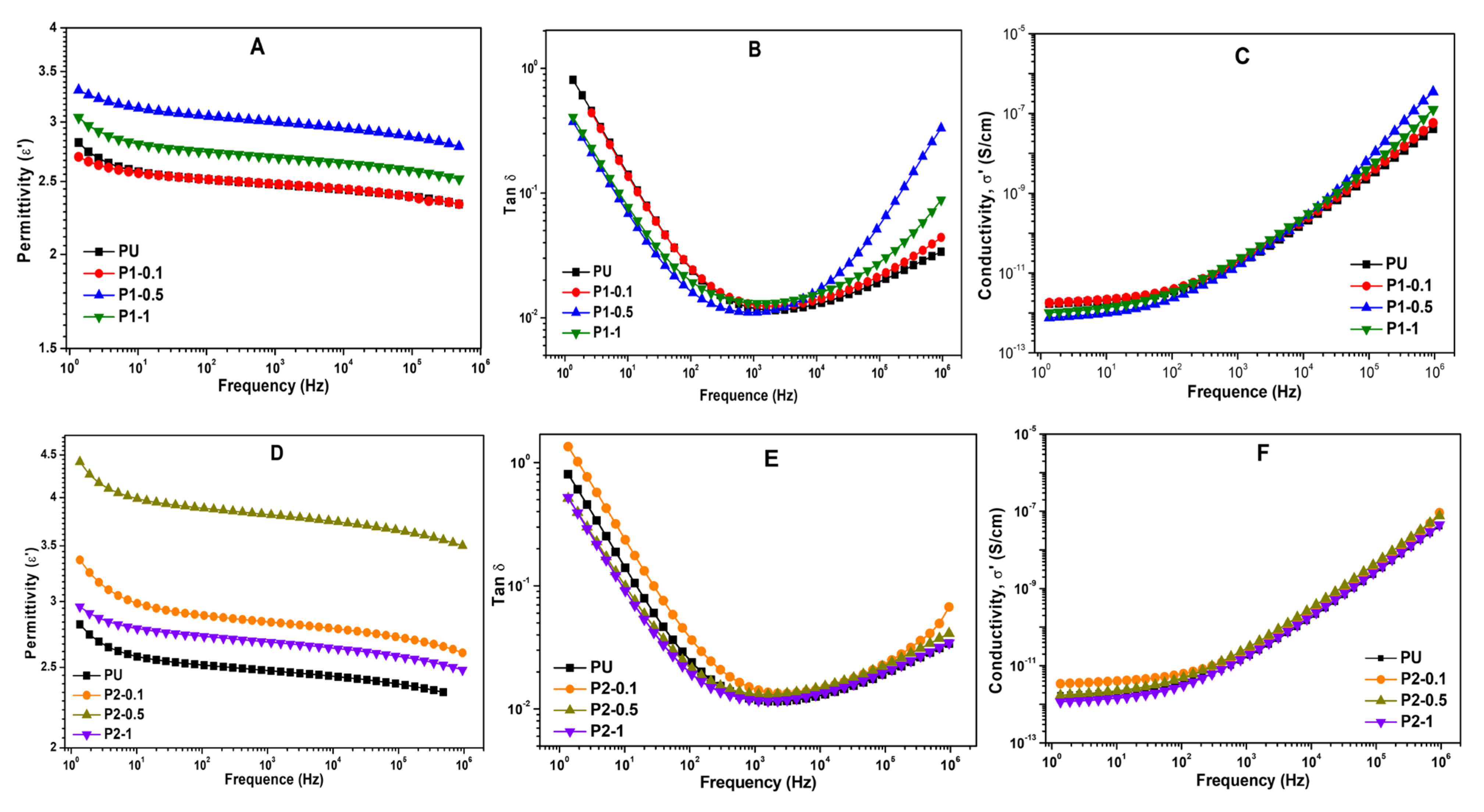
3.7. Dielectric Properties
3.8. Magnetic Properties
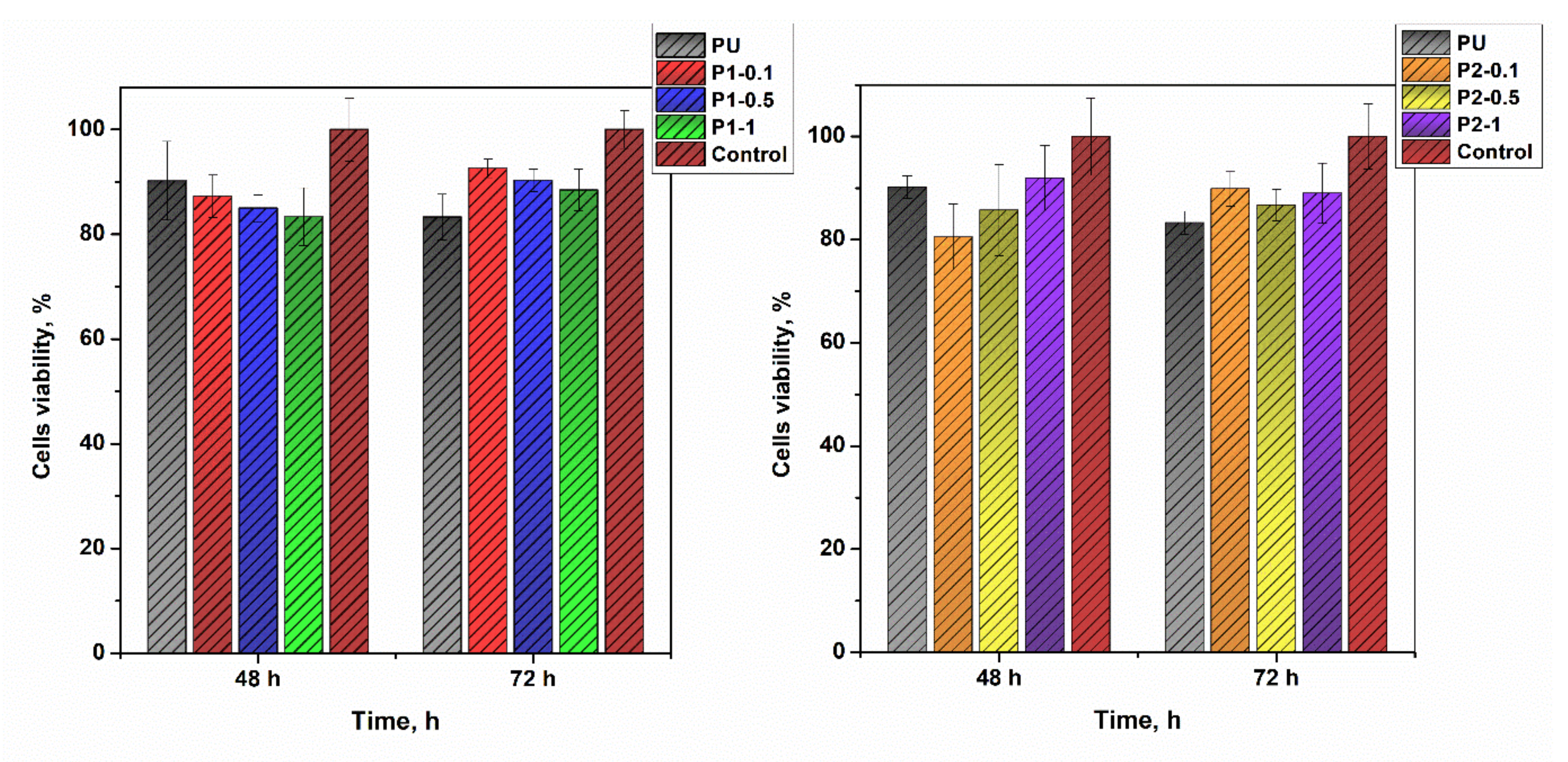
3.9. Cytotoxicity Screenings and In Vitro Evaluation of Biocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, M.; Shu, J. Multimodal molecular imaging: Current status and future directions. Contrast Media Mol. Imaging 2018, 2018, 7890241. [Google Scholar] [CrossRef]
- Ahmad Saad, F.F. Molecular imaging. In Selected Topics on Computed Tomography; InTech: Oxford, UK, 2013; Volume 30, pp. 326–332. [Google Scholar]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef]
- Hu, H. Recent advances of bioresponsive nano-sized contrast agents for ultra-high-field magnetic resonance imaging. Front. Chem. 2020, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.B.; Nayak, K.S. MRI artifacts and correction strategies. Imaging Med. 2010, 2, 445–457. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Gradinaru, L.M.; Mandru, M.; Ciobanu, C.; Aflori, M. Opportunities and challenges in polymer-noble metal nanocomposites. In Intelligent Polymers for Nanomedicine and Biotechnologies; Taylor Francis Group: Abingdon, UK, 2017; ISBN 9781351977449. [Google Scholar]
- Vlad, S.; Gradinaru, L.M.; Gradinaru, R.V.; Ciobanu, C. Recent developments in ZnO-polyurethane nanomaterials. In Intelligent Polymers for Nanomedicine and Biotechnologies; Taylor Francis Group: Abingdon, UK, 2017; ISBN 9781351977449. [Google Scholar]
- Moghaddam, S.T.; Naimi-Jamal, M.R. Preparation of novel magnetic polyurethane flexible foam nanocomposites. Macromol. Symp. 2017, 375, 1600151. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M. Antibacterial nanocomposites based on thermosetting polymers derived from vegetable oils andmetal oxide nanoparticles. Polymers 2019, 11, 1790. [Google Scholar] [CrossRef]
- Suhasini, A.; Kumar, K.P.V.; Maiyalagan, T. Synthesis, thermal and magnetic behavior of iron oxide-polymer nanocomposites. Sci. Eng. Compos. Mater. 2018, 25, 189–195. [Google Scholar] [CrossRef]
- Kirchberg, S.; Abdin, Y.; Ziegmann, G. Influence of particle shape and size on the wetting behavior of soft magnetic micropowders. Powder Technol. 2011, 207, 311–317. [Google Scholar] [CrossRef]
- Achary, P.G.R.; Deo, S.S.; Munisha, B.; Choudhary, R.N.P.; Parida, S.K. Effect of temperature on electrical properties of PU/Fe (30%) nanocomposite. J. Polym. Res. 2020, 27, 244. [Google Scholar] [CrossRef]
- Vlad, S.; Filip, D.; Macocinschi, D.; Spiridon, I.; Nistor, A.; Gradinaru, L.M.; Musteata, V.E. New polyetherurethanes based on cellulose derivative for biomedical applications. Optoelectron. Adv. Mater. Rapid Commun. 2010, 4, 407–414. [Google Scholar]
- Gradinaru, L.M.; Ciobanu, C.; Vlad, S.; Drobota, M.; Butnaru, M.; Saint-Pierre, G. Thermal behavior, surface energy analysis, and hemocompatibility of some polycarbonate urethanes for cardiac engineering. High Perform. Polym. 2015, 27, 637–645. [Google Scholar] [CrossRef]
- Gradinaru, L.M.; Ciobanu, C.; Drobota, M.; Vlad, S. Poly(alkylene sebacate ether)urethane hydrogels for indomethacin delivery formulations. J. Polym. Res. 2017, 24, 99. [Google Scholar] [CrossRef]
- Vlad, S.; Butnaru, M.; Filip, D.; Macocinschi, D.; Nistor, A.; Gradinaru, L.M.; Ciobanu, C. Polyetherurethane membranes modified with renewable resource as a potential candidate for biomedical applications. Dig. J. Nanomater. Biostruct. 2010, 5, 1089–1100. [Google Scholar]
- Shahrousvand, M.; Hoseinian, M.S.; Ghollasi, M.; Karbalaeimahdi, A.; Salimi, A.; Tabar, F.A. Flexible magnetic polyurethane/Fe2O3 nanoparticles as organic-inorganic nanocomposites for biomedical applications: Properties and cell behavior. Mater. Sci. Eng. C 2017, 74, 556–567. [Google Scholar] [CrossRef]
- Salimi, S.; Babra, T.S.; Dines, G.S.; Baskerville, S.W.; Hayes, W.; Greenland, B.W. Composite polyurethane adhesives that debond-on-demand by hysteresis heating in an oscillating magnetic field. Eur. Polym. J. 2019, 121, 109264. [Google Scholar] [CrossRef]
- Yuwawech, K.; Wootthikanokkhan, J.; Tanpichai, S. Preparation and characterization of iron oxide decorated graphene nanoplatelets for use as barrier enhancing fillers in polyurethane based solar cell encapsulant. Mater. Today Proc. 2020, 23, 703–711. [Google Scholar] [CrossRef]
- Morsi, S.M.M.; Mohamed, H.A.; Ghany, N.A.A. Development of advanced-functional polyurethane/red iron oxide composites as protective one coating systems for steel. Prog. Org. Coat. 2019, 136, 105236. [Google Scholar] [CrossRef]
- Okoli, C.P.; Ofomaja, A.E. Development of sustainable magnetic polyurethane polymer nanocomposite for abatement of tetracycline antibiotics aqueous pollution: Response surface methodology and adsorption dynamics. J. Clean. Prod. 2019, 217, 42–55. [Google Scholar] [CrossRef]
- Yu, T.; Halouane, F.; Mathias, D.; Barras, A.; Wang, Z.; Lv, A.; Lu, S.; Xu, W.; Meziane, D.; Tiercelin, N.; et al. Preparation of magnetic, superhydrophobic/superoleophilic polyurethane sponge: Separation of oil/water mixture and demulsification. Chem. Eng. J. 2020, 384, 123339. [Google Scholar] [CrossRef]
- Hussein, F.B.; Abu-Zahra, N.H. Adsorption kinetics and evaluation study of iron oxide nanoparticles impregnated in polyurethane matrix for water filtration application. J. Miner. Mater. Charact. Eng. 2017, 5, 298–310. [Google Scholar] [CrossRef][Green Version]
- Dai, M.; Zhai, Y.; Wu, L.; Zhang, Y. Magnetic aligned Fe3O4-reduced graphene oxide/waterborne polyurethane composites with controllable structure for high microwaves absorption capacity. Carbon N. Y. 2019, 152, 661–670. [Google Scholar] [CrossRef]
- Cheng, K.W.; Hsu, S.H. A facile method to prepare superparamagnetic iron oxide and hydrophobic drug-encapsulated biodegradable polyurethane nanoparticles. Int. J. Nanomed. 2017, 12, 1775–1789. [Google Scholar] [CrossRef]
- Das, B.; Mandal, M.; Upadhyay, A.; Chattopadhyay, P.; Karak, N. Bio-based hyperbranched polyurethane/Fe3O4 nanocomposites: Smart antibacterial biomaterials for biomedical devices and implants. Biomed. Mater. 2013, 8, 035003. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.; Wang, Z.; Wang, J.; Sun, Z. Biodegradable shape memory nanocomposites with thermal and magnetic field responsiveness. J. Biomater. Sci. Polym. Ed. 2013, 24, 1057–1070. [Google Scholar] [CrossRef]
- Urban, M.; Strankowski, M. Shape memory polyurethane materials containing ferromagnetic iron oxide and graphene nanoplatelets. Materials 2017, 10, 1083. [Google Scholar] [CrossRef]
- Stanciu, A.; Bulacovschi, V.; Lungu, M.; Vlad, S.; Balint, S.; Oprea, S. Mechanical behaviour of crosslinked poly(ester-siloxane) urethanes. Eur. Polym. J. 1999, 35, 2039–2044. [Google Scholar] [CrossRef]
- Stanciu, A.; Bulacovschi, V.; Oprea, S.; Vlad, S. Thermal and mechanical behaviour of some new crosslinked poly(ester-siloxane)urethanes. Polym. Degrad. Stab. 2001, 72, 551–558. [Google Scholar] [CrossRef]
- Kozbial, A.; Li, Z.; Conaway, C.; McGinley, R.; Dhingra, S.; Vahdat, V.; Zhou, F.; Durso, B.; Liu, H.; Li, L. Study on the surface energy of graphene by contact angle measurements. Langmuir 2014, 30, 8598–8606. [Google Scholar] [CrossRef]
- Erbil, Y. (Ed.) Surface Chemistry of Solid and Liquid Interfaces; Blackwell Publishing: Oxford, UK, 2006; ISBN 1-4051-1968-3. [Google Scholar]
- Spiridon, I.; Anghel, N.; Dinu, M.V.; Vlad, S.; Bele, A.; Ciubotaru, B.I.; Verestiuc, L.; Pamfil, D. Development and performance of bioactive compounds-loaded cellulose/collagen/polyurethane materials. Polymers 2020, 12, 1191. [Google Scholar] [CrossRef]
- Gradinaru, L.M.; Barbalata-Mandru, M.; Drobota, M.; Aflori, M.; Spiridon, M.; Pircalabioru, G.G.; Bleotu, C.; Butnaru, M.; Vlad, S. Preparation and evaluation of nanofibrous hydroxypropyl cellulose and β-cyclodextrin polyurethane composite mats. Nanomaterials 2020, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Ji, Y.; Ou, J.; Wang, F.; Li, C.; Lei, S.; Li, W. Surface wettability and strong adhesion of medical polyurethane elastomer porous films by microphase separation. AIP Adv. 2019, 9, 075309. [Google Scholar] [CrossRef]
- Blanco, D.; Rivera, N.; Oulego, P.; Díaz, M.; González, R.; Battez, A.H. Novel fatty acid anion-based ionic liquids: Contact angle, surface tension, polarity fraction and spreading parameter. J. Mol. Liq. 2019, 288, 110995. [Google Scholar] [CrossRef]
- Crank, J. The mathematics of diffusion. J. Am. Chem. Soc. 1975, 79, 1267–1268. [Google Scholar]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef]
- Pooladian, B.; Alavi Nikje, M.M. Synthesis and characterization of poly(urethane-imide) nanocomposite films filled with iron oxide–silica and clay–silane–iron oxide nanoparticles. J. Plast. Film Sheeting 2018, 34, 196–218. [Google Scholar] [CrossRef]
- Song, C.; Wang, X.-X.; Zhang, J.; Nie, G.-D.; Luo, W.-L.; Fu, J.; Ramakrishna, S.; Long, Y.-Z. Electric field-assisted in situ precise deposition of electrospun γ-Fe2O3/polyurethane nanofibers for magnetic hyperthermia. Nanoscale Res. Lett. 2018, 13, 273. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, G.; Xu, S.; Ma, T.; Nie, J. Biodegradable magnetic-sensitive shape memory poly(ɛ-caprolactone)/Fe3O4 nanocomposites. J. Appl. Polym. Sci. 2018, 135, 45652. [Google Scholar] [CrossRef]
- Ghosh, T.; Voit, B.; Karak, N. Polystyrene/thermoplastic polyurethane interpenetrating network-based nanocomposite with high-speed, thermo-responsive shape memory behavior. Polymer 2020, 200, 122575. [Google Scholar] [CrossRef]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate-polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Zhao, X.; Courtney, J.M.; Qian, H. Blood interface biomaterials. In Biomaterials and Devices for the Circulatory System; Woodhead Publishing: Sawston, UK, 2010; pp. 24–53. [Google Scholar]
- Erbulut, D.U.; Lazoglu, I. Biomaterials for improving the blood and tissue compatibility of total artificial hearts (TAH) and ventricular assist devices (VAD). In Biomaterials for Artificial Organs; Woodhead Publishing: Sawston, UK, 2011; pp. 207–235. ISBN 9781845696535. [Google Scholar]
- Balaji, A.; Jaganathan, S.K.; Ismail, A.F.; Rajasekar, R. Fabrication and hemocompatibility assessment of novel polyurethane-based bio-nanofibrous dressing loaded with honey and Carica papaya extract for the management of burn injuries. Int. J. Nanomed. 2016, 11, 4339–4355. [Google Scholar] [CrossRef]
- Polini, A.; Yang, F. Physicochemical characterization of nanofiber composites. In Nanofiber Composites for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 97–115. ISBN 9780081002087. [Google Scholar]
- Tran, V.H.T.; Lee, B.K. Novel fabrication of a robust superhydrophobic PU@ZnO@Fe3O4@SA sponge and its application in oil-water separations. Sci. Rep. 2017, 7, 17520. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I. Modification of the wettability of polymer surfaces using nanoparticles. Prog. Org. Coatings 2014, 77, 331–338. [Google Scholar] [CrossRef]
- Selvakumar, N.; Barshilia, H.C.; Rajam, K.S. Effect of substrate roughness on the apparent surface free energy of sputter deposited superhydrophobic polytetrafluoroethylene coatings: A comparison of experimental data with different theoretical models. J. Appl. Phys. 2010, 108, 732–741. [Google Scholar] [CrossRef]
- Menzies, K.L.; Jones, L. The impact of contact angle on the biocompatibility of biomaterials. Optom. Vis. Sci. 2010, 87, 387–399. [Google Scholar] [CrossRef]
- Balik, C.M. On the extraction of diffusion coefficients from gravimetric data for sorption of small molecules by polymer thin films. Macromolecules 1996, 29, 3025–3029. [Google Scholar] [CrossRef]
- Ramesan, M.T. Effects of magnetite nanoparticles on morphology, processability, diffusion and transport behavior of ethylene vinyl acetate nanocomposites. Int. J. Plast. Technol. 2015, 19, 368–380. [Google Scholar] [CrossRef]
- MacOcinschi, D.; Filip, D.; Vlad, S.; Cristea, M.; Musteata, V.; Ibanescu, S. Thermal, dynamic mechanical, and dielectric analyses of some polyurethane biocomposites. J. Biomater. Appl. 2012, 27, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Smaranda, I.; Nila, A.; Ganea, P.; Daescu, M.; Zgura, I.; Ciobanu, R.C.; Trandabat, A.; Baibarac, M. The influence of the ceramic nanoparticles on the thermoplastic polymers matrix: Their structural, optical, and conductive properties. Polymers 2021, 13, 2273. [Google Scholar] [CrossRef]
- Iacob, M.; Tugui, C.; Tiron, V.; Bele, A.; Vlad, S.; Vasiliu, T.; Cazacu, M.; Vasiliu, A.L.; Racles, C. Iron oxide nanoparticles as dielectric and piezoelectric enhancers for silicone elastomers. Smart Mater. Struct. 2017, 26, 105046. [Google Scholar] [CrossRef]
- Bele, A.; Cazacu, M.; Stiubianu, G.; Vlad, S. Silicone-barium titanate composites with increased electromechanical sensitivity. the effects of the filler morphology. RSC Adv. 2014, 4, 58522–58529. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 23501. [Google Scholar] [CrossRef]
- Kalita, H.; Karak, N. Hyperbranched polyurethane/Fe3O4 thermosetting nanocomposites as shape memory materials. Polym. Bull. 2013, 70, 2953–2965. [Google Scholar] [CrossRef]
- Salahuddin, N.; Rehab, A.; Abd-Elghany, S. In vitro thermo-triggered drug release from magnetic polyurethane-urea nanocomposite. J. Drug Deliv. Sci. Technol. 2020, 56, 101564. [Google Scholar] [CrossRef]
- Li, L.; Zheng, S. Poly(ε-caprolactone)-grafted Fe3O4 nanoparticles: Preparation and superparamagnetic nanocomposites with epoxy thermosets. Ind. Eng. Chem. Res. 2015, 54, 171–180. [Google Scholar] [CrossRef]
- Barrioni, B.R.; De Carvalho, S.M.; Oréfice, R.L.; De Oliveira, A.A.R.; Pereira, M.D.M. Synthesis and characterization of biodegradable polyurethane films based on HDI with hydrolyzable crosslinked bonds and a homogeneous structure for biomedical applications. Mater. Sci. Eng. C 2015, 52, 22–30. [Google Scholar] [CrossRef]
- Amiri, B.; Ghollasi, M.; Shahrousvand, M.; Kamali, M.; Salimi, A. Osteoblast differentiation of mesenchymal stem cells on modified PES-PEG electrospun fibrous composites loaded with Zn2SiO4 bioceramic nanoparticles. Differentiation 2016, 92, 148–158. [Google Scholar] [CrossRef]


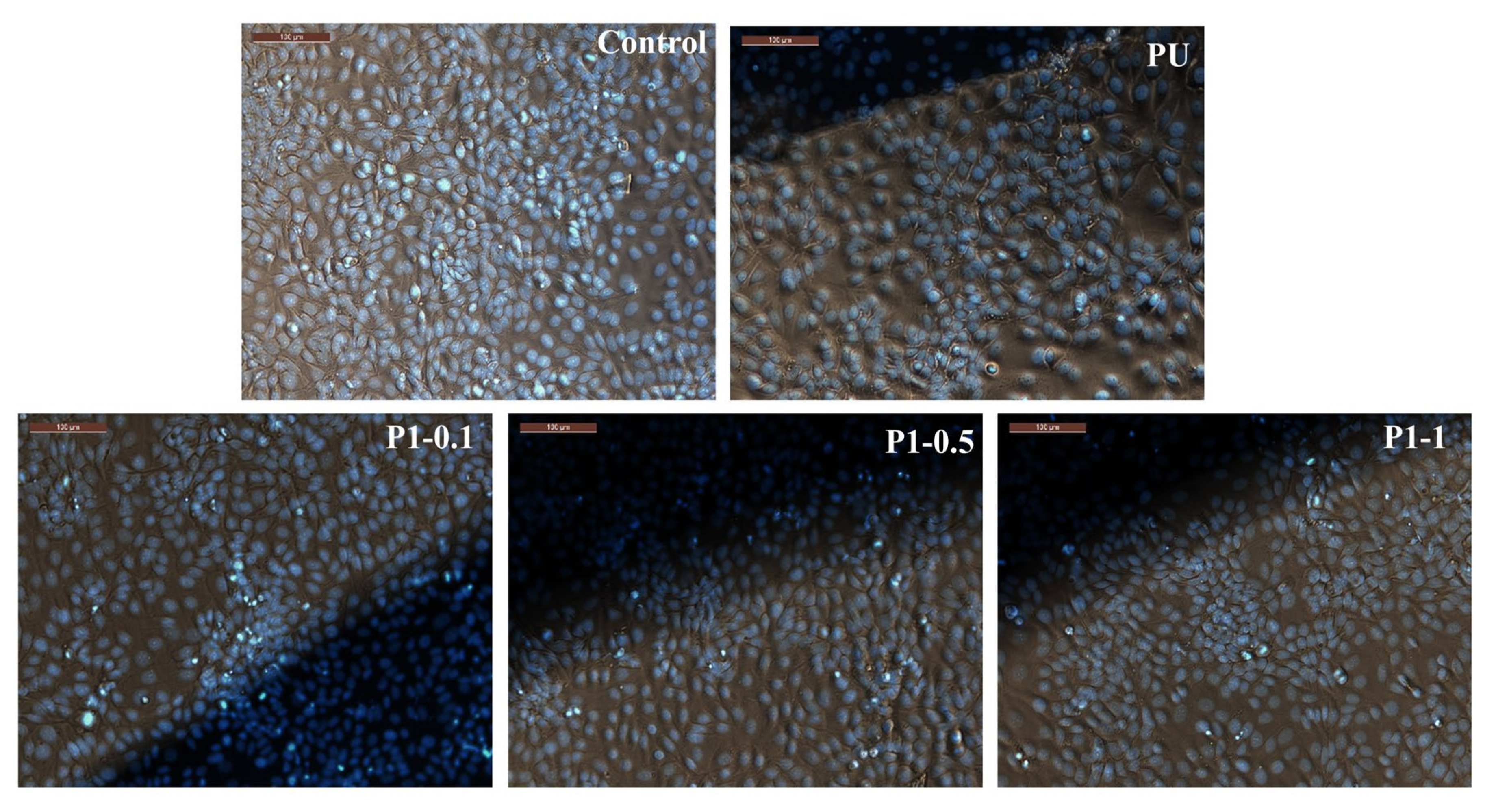
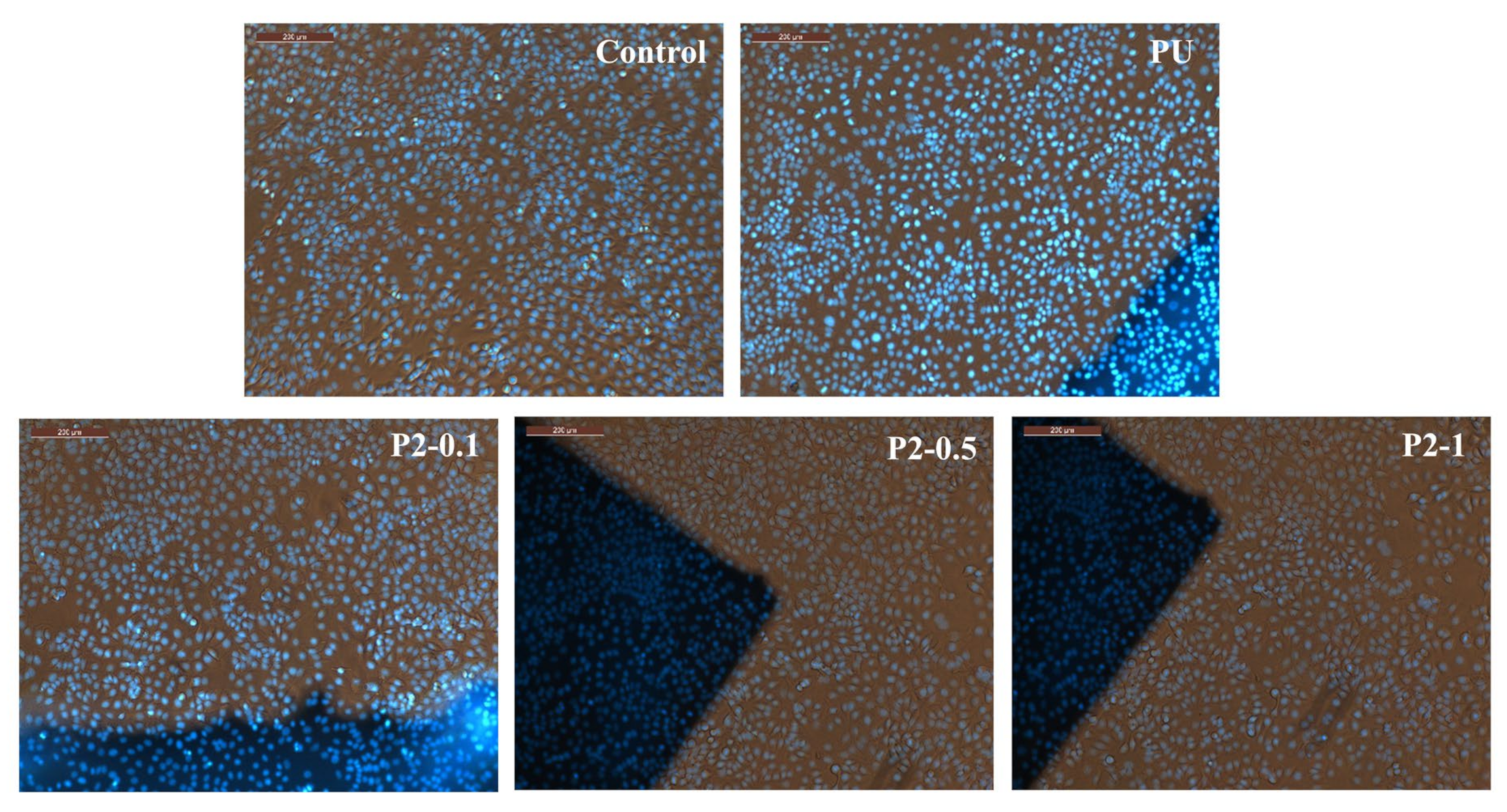
| Sample Code | Molar Ratio * (PTHF:MDI:BG/PDMS) | Nanoparticle Type | Nanoparticle Content (wt%) |
|---|---|---|---|
| PU | 1:3:2 | None | 0 |
| P1-0.1 | 1:3:2 | Fe2O3 | 0.1 |
| P1-0.5 | 1:3:2 | Fe2O3 | 0.5 |
| P1-1 | 1:3:2 | Fe2O3 | 1 |
| P2-0.1 | 1:3:2 | Fe3O4 | 0.1 |
| P2-0.5 | 1:3:2 | Fe3O4 | 0.5 |
| P2-1 | 1:3:2 | Fe3O4 | 1 |
| Sample Code | Wa (mN/m) | S (mN/m) | ||||||
|---|---|---|---|---|---|---|---|---|
| W | EG | FA | dIM | W | EG | FA | dIM | |
| PU | 60.47 | 62.26 | 72.49 | 80.29 | −85.12 | −33.73 | −43.90 | −21.30 |
| P1-0.1 | 60.22 | 61.38 | 65.06 | 77.18 | −85.37 | −34.61 | −51.33 | −24.41 |
| P1-0.5 | 53.57 | 57.27 | 47.90 | 84.76 | −92.02 | −38.72 | −68.49 | −16.83 |
| P1-1 | 49.14 | 45.72 | 43.77 | 87.23 | −96.45 | −50.27 | −72.62 | −14.36 |
| P2-0.1 | 80.65 | 64.68 | 70.48 | 88.47 | −64.94 | −31.31 | −45.91 | −13.12 |
| P2-0.5 | 63.33 | 63.58 | 63.69 | 93.26 | −82.26 | −32.41 | −52.70 | −8.33 |
| P2-1 | 67.47 | 65.84 | 65.22 | 91.87 | −78.12 | −30.15 | −51.17 | −9.72 |
| Sample Code | γds (mN/m) | γps (mN/m) | γs (mN/m) | γsl (mN/m) | γc (mN/m) |
|---|---|---|---|---|---|
| PU | 30.25 | 0.30 | 30.55 | 38.24 | 26.43 |
| P1-0.1 | 26.58 | 0.49 | 27.08 | 37.24 | 22.58 |
| P1-0.5 | 27.50 | 0.02 | 27.52 | 43.03 | 25.61 |
| P1-1 | 26.66 | 0.25 | 26.91 | 35.27 | 19.22 |
| P2-0.1 | 29.09 | 2.60 | 31.69 | 14.78 | 19.85 |
| P2-0.5 | 35.06 | 0.04 | 35.11 | 35.13 | 32.15 |
| P2-1 | 33.87 | 0.32 | 34.19 | 31.35 | 31.49 |
| Sample Code | D1 × 10−7 (cm2/s) | D2 × 10−7 (cm2/s) | K1 × 10−3 (s−1) | K2 × 10−3 (s−1) | l (cm) |
|---|---|---|---|---|---|
| PU | 0.90 | 7.14 | 1.15 | −17.62 | 0.02 |
| P1-0.1 | 1.04 | 5.05 | 1.48 | −12.46 | 0.02 |
| P1-0.5 | 1.16 | 5.81 | 1.33 | −14.33 | 0.02 |
| P1-1 | 0.76 | 6.42 | 0.97 | −15.84 | 0.02 |
| P2-0.1 | 1.05 | 6.22 | 1.34 | −15.33 | 0.02 |
| P2-0.5 | 1.13 | 5.59 | 1.44 | −13.78 | 0.02 |
| P2-1 | 0.77 | 5.87 | 0.98 | −14.49 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradinaru, L.M.; Barbalata Mandru, M.; Drobota, M.; Aflori, M.; Butnaru, M.; Spiridon, M.; Doroftei, F.; Aradoaei, M.; Ciobanu, R.C.; Vlad, S. Composite Materials Based on Iron Oxide Nanoparticles and Polyurethane for Improving the Quality of MRI. Polymers 2021, 13, 4316. https://doi.org/10.3390/polym13244316
Gradinaru LM, Barbalata Mandru M, Drobota M, Aflori M, Butnaru M, Spiridon M, Doroftei F, Aradoaei M, Ciobanu RC, Vlad S. Composite Materials Based on Iron Oxide Nanoparticles and Polyurethane for Improving the Quality of MRI. Polymers. 2021; 13(24):4316. https://doi.org/10.3390/polym13244316
Chicago/Turabian StyleGradinaru, Luiza Madalina, Mihaela Barbalata Mandru, Mioara Drobota, Magdalena Aflori, Maria Butnaru, Maria Spiridon, Florica Doroftei, Mihaela Aradoaei, Romeo Cristian Ciobanu, and Stelian Vlad. 2021. "Composite Materials Based on Iron Oxide Nanoparticles and Polyurethane for Improving the Quality of MRI" Polymers 13, no. 24: 4316. https://doi.org/10.3390/polym13244316
APA StyleGradinaru, L. M., Barbalata Mandru, M., Drobota, M., Aflori, M., Butnaru, M., Spiridon, M., Doroftei, F., Aradoaei, M., Ciobanu, R. C., & Vlad, S. (2021). Composite Materials Based on Iron Oxide Nanoparticles and Polyurethane for Improving the Quality of MRI. Polymers, 13(24), 4316. https://doi.org/10.3390/polym13244316