Fluorescence Modulation of Conjugated Polymer Nanoparticles Embedded in Poly(N-Isopropylacrylamide) Hydrogel
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Instrumentation
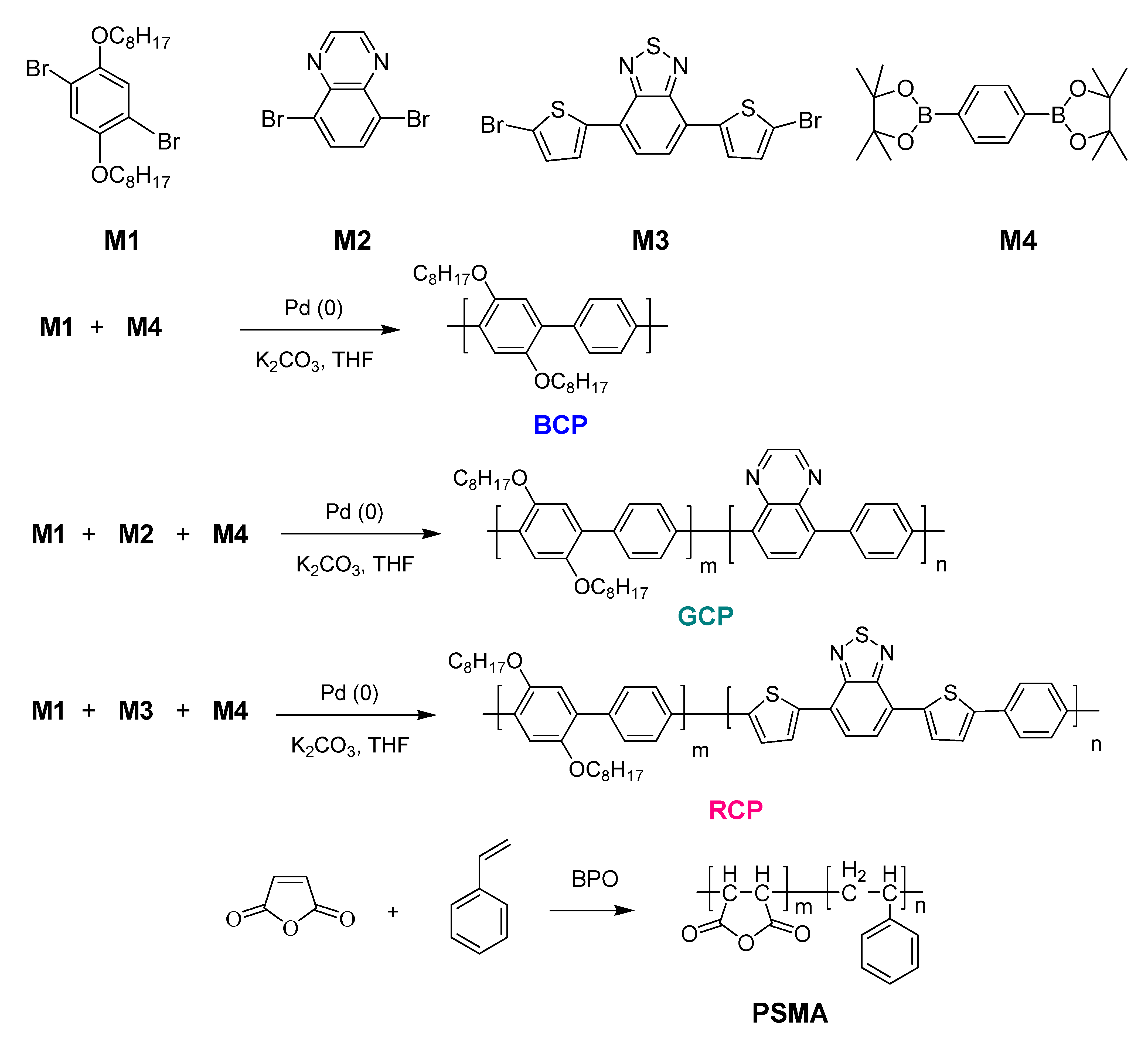
2.2. Synthesis of a Blue-Emitting CP (BCP)
2.3. Synthesis of a Green-Emitting CP (GCP)
2.4. Synthesis of a Red-Emitting CP (RCP)
2.5. Synthesis of Poly(Styrene-Co-Maleic Anhydride) (PSMA)
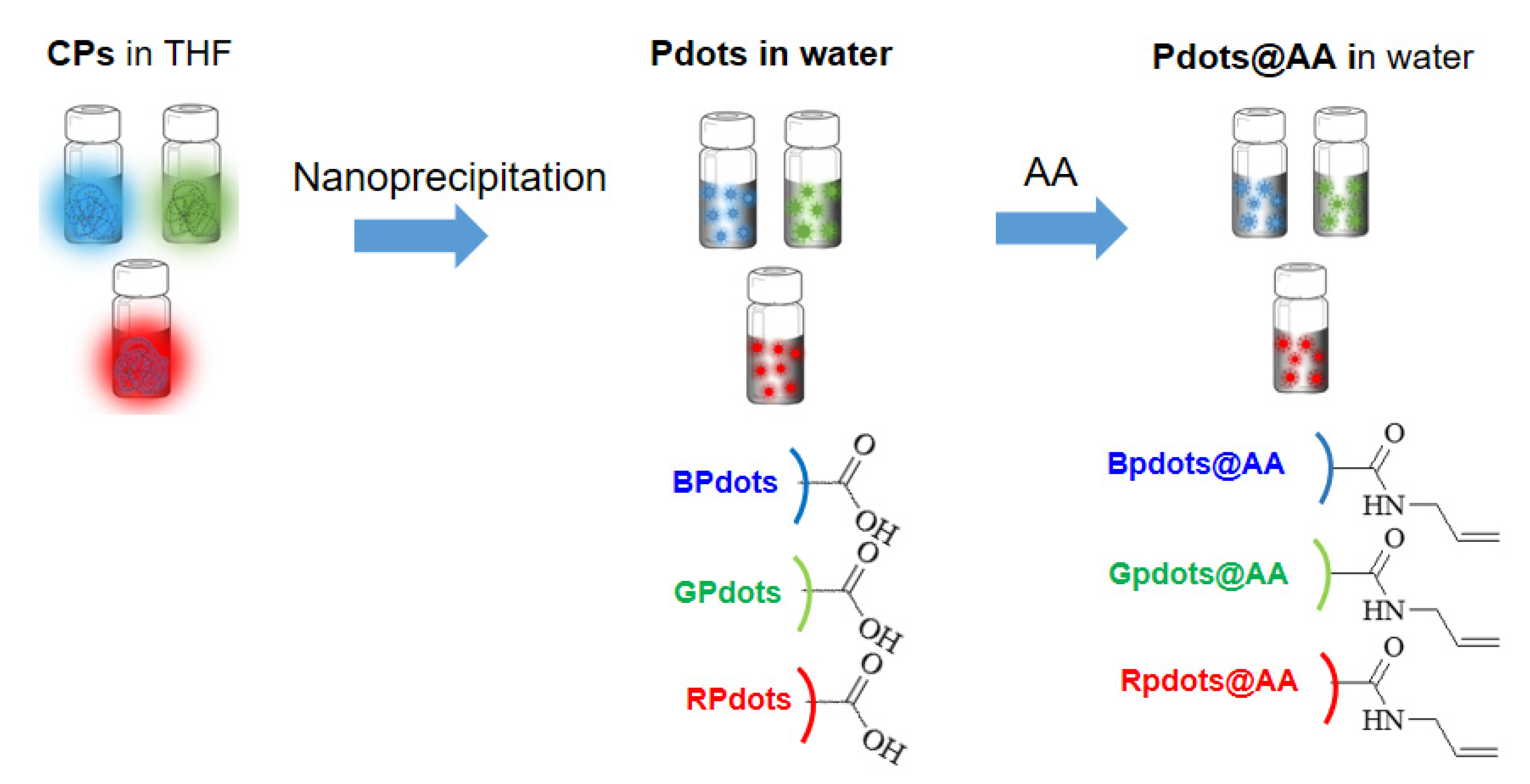
2.6. Fabrication of Polymer Nanoparticles (NPs)
2.7. Pdots Coated with Allyl Amine (AA)
2.8. Pdot-Embedded Hydrogel
3. Results and Discussion
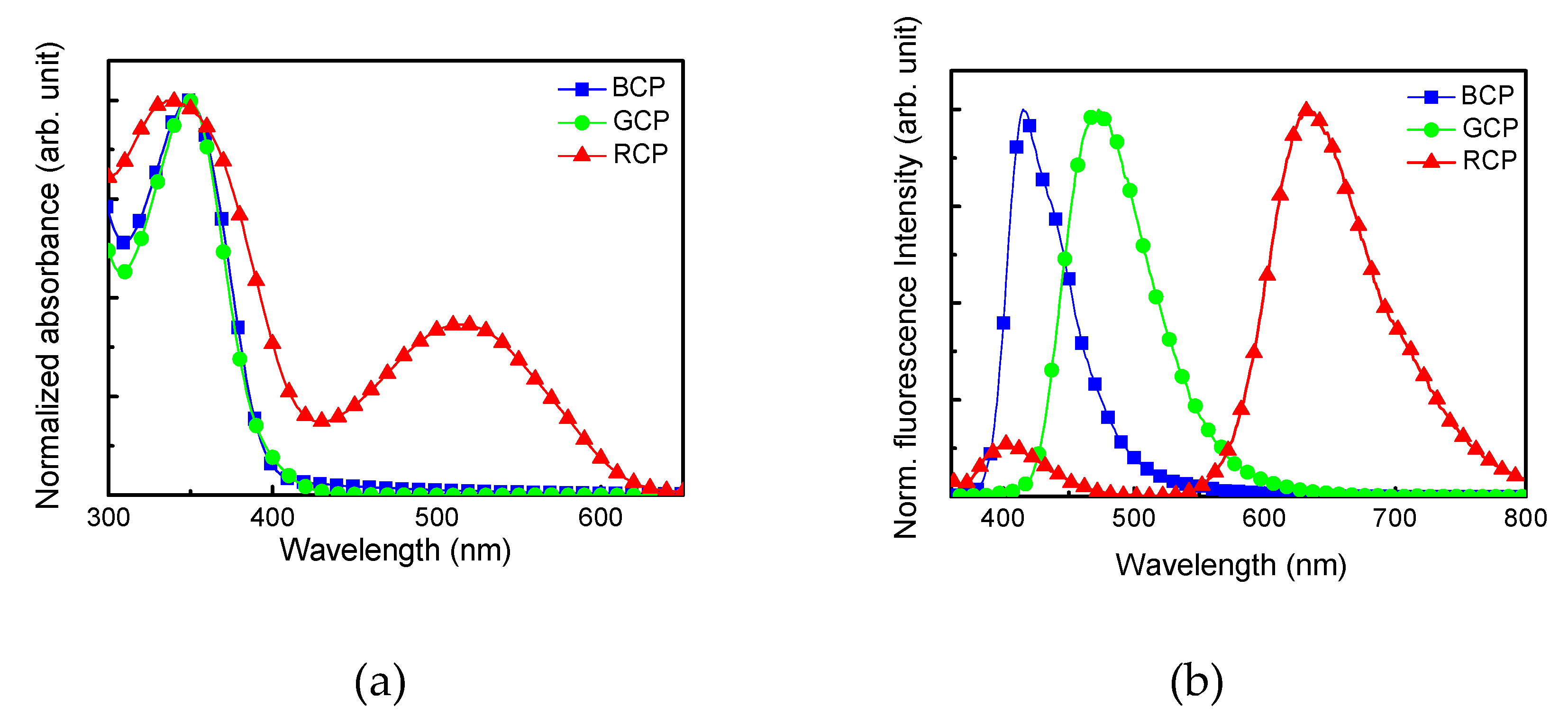
3.1. Polymer Synthesis
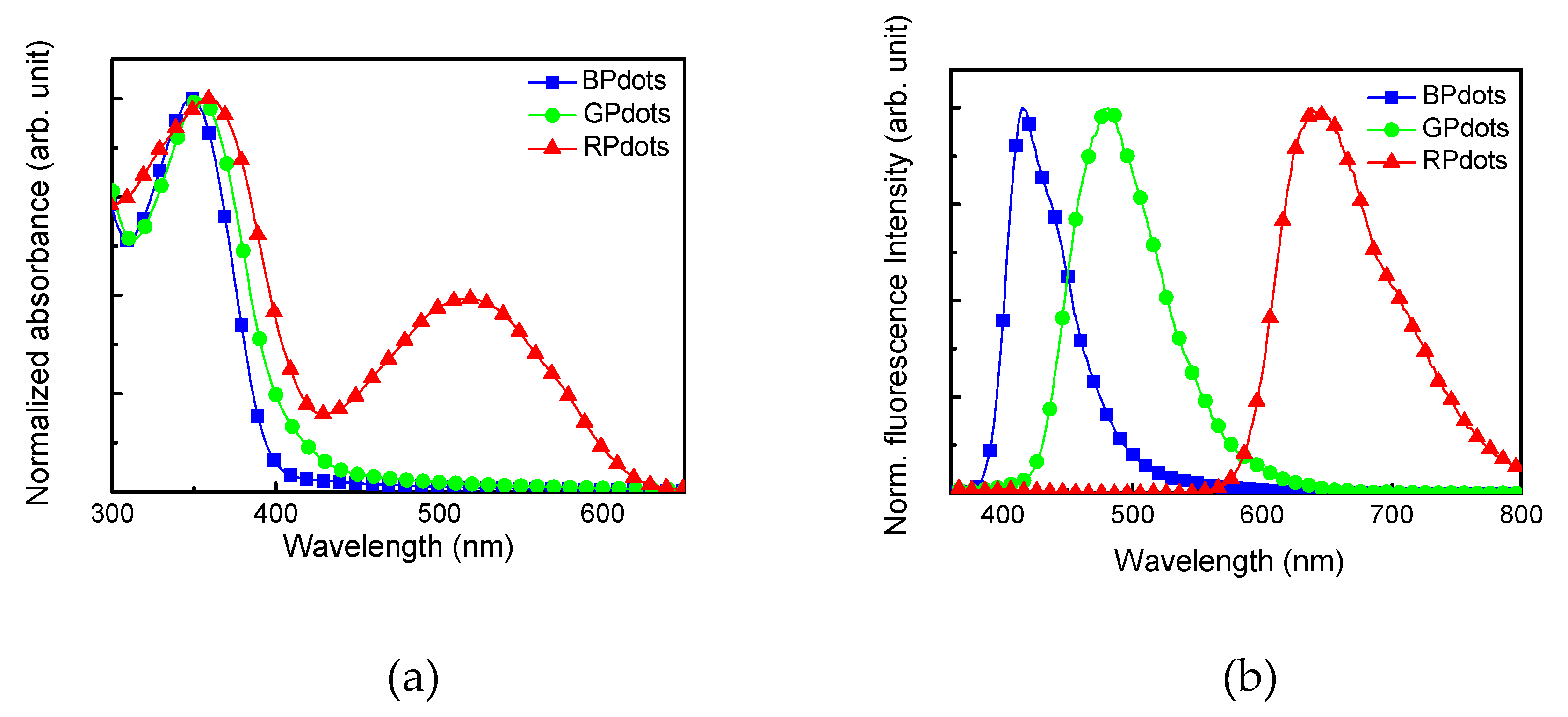
3.2. Preparation of Polymer Nanoparticle Coated with Allyl Amine
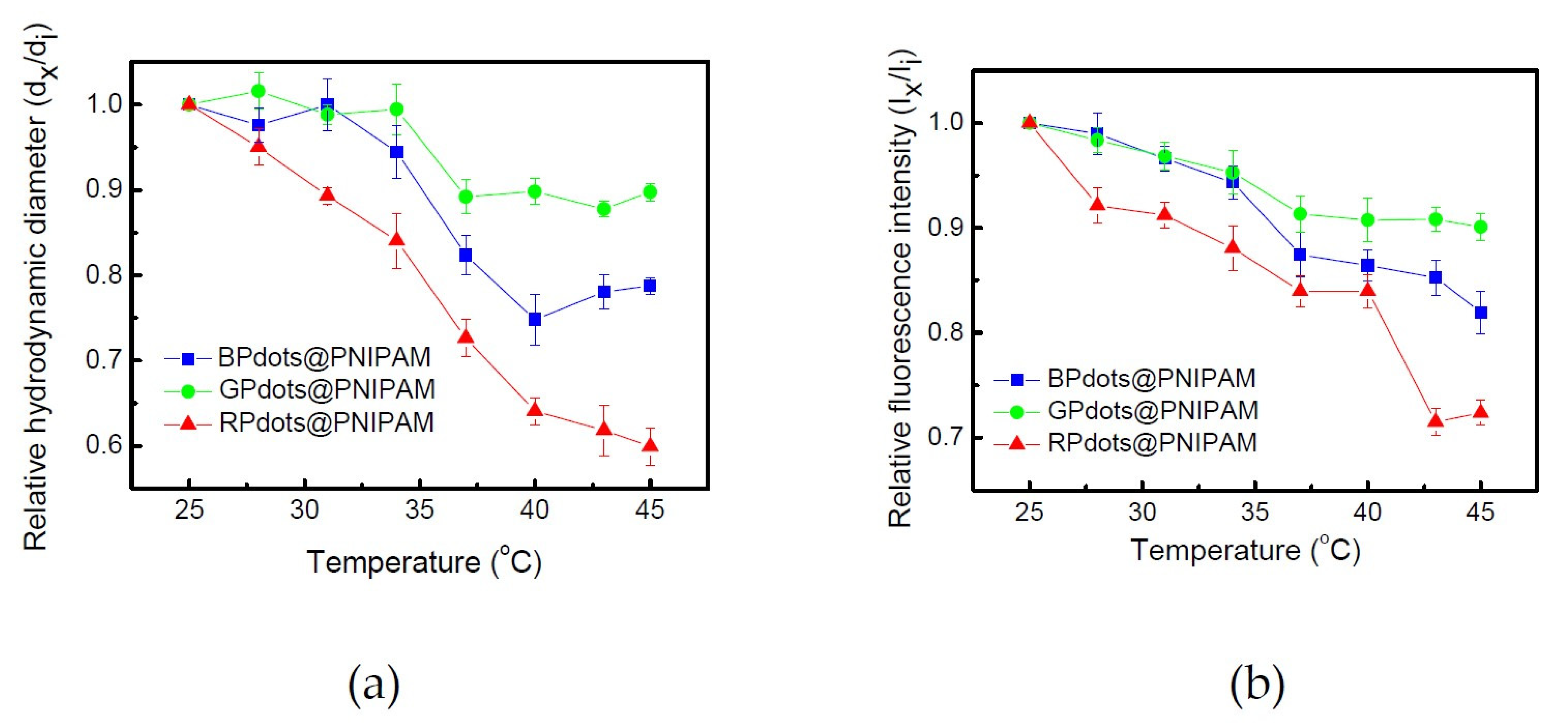
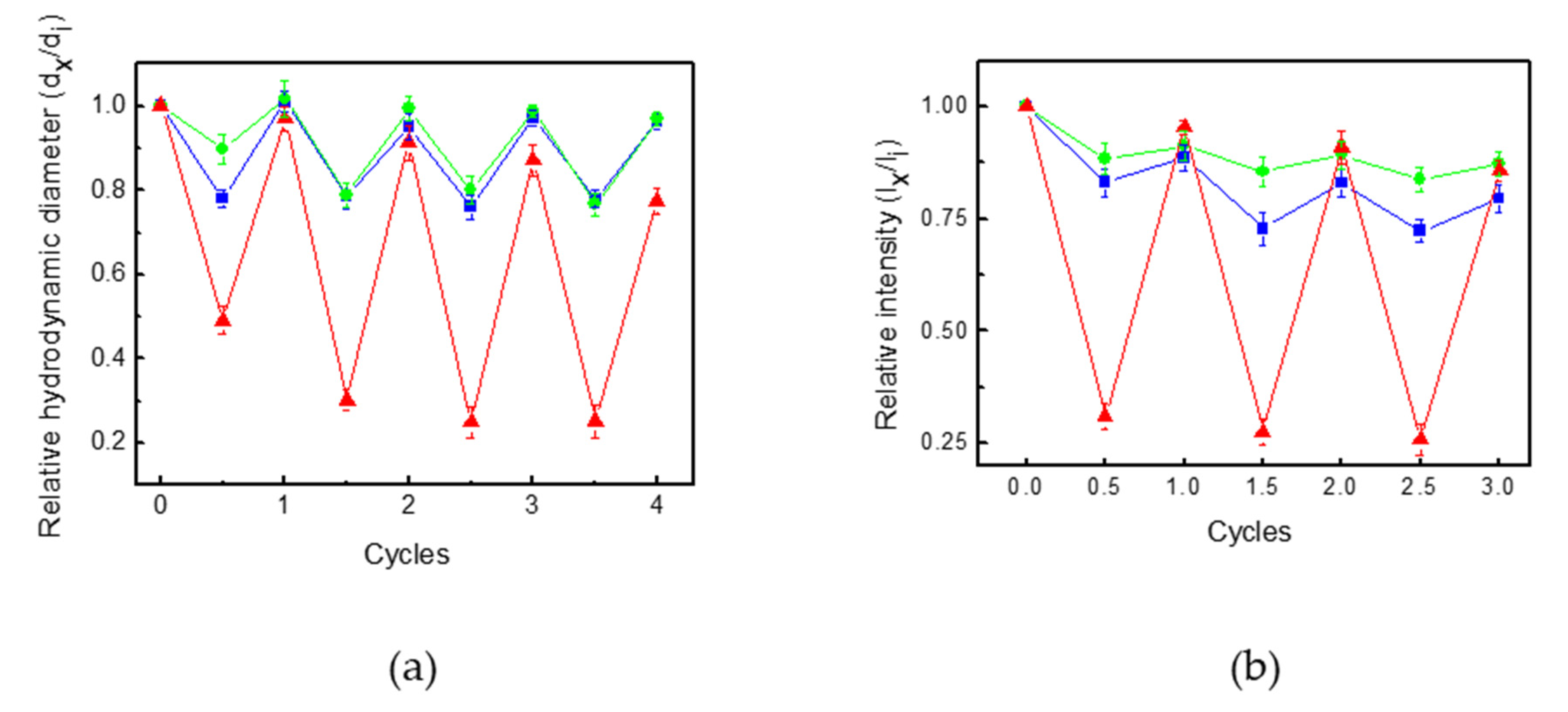
3.3. Pdot-Embedded Hydrogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dreiss, C.A. Hydrogel design strategies for drug delivery. Curr. Opin. Colloid Interface Sci. 2020, 48, 1–17. [Google Scholar] [CrossRef]
- Gao, Y.; Ahiabu, A.; Serpe, M.J. Controlled drug release from the aggregation–disaggregation behavior of pH-responsive microgels. ACS Appl. Mater. Interfaces 2014, 6, 13749–13756. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, T.; Berliner, A.; Banerjee, P.; Zhou, S. Smart core−shell hybrid nanogels with Ag nanoparticle core for cancer cell imaging and gel shell for pH-regulated drug delivery. Chem. Mater. 2010, 22, 1966–1976. [Google Scholar] [CrossRef]
- Nayak, S.; Bhattacharjee, S.; Chaudhary, Y.S. In situ encapsulation and release kinetics of pH and temperature responsive nanogels. J. Phys. Chem. C 2012, 116, 30–36. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V.; Barrera, E.V. Covalently functionalized carbon nano-onions integrated gelatin methacryloyl nanocomposite hydrogel containing γ-cyclodextrin as drug carrier for high-performance pH-triggered drug release. Pharmaceuticals 2021, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Castrejón, J.V.; González-Oritz, A. Rational design and engineering of carbon nano-onions reforced natural protein nanocomposite hydroges for biomedical applications. J. Mech. Behav. Biomed. Mater. 2021, 104, 103696. [Google Scholar] [CrossRef] [PubMed]
- Ngyuen, Q.K.; Hoang, T.H.; Bui, X.T.; Ngyuen, H.T.A. Synthesis and application of polycation-stabilized gold nanoparticles as a highly sensitive sensor for molecular cysteine determination. Microchem. J. 2021, 168, 106481. [Google Scholar] [CrossRef]
- Kim, Y.; Namgung, H.; Lee, T.S. Synthesis of a glucose oxidase-conjugated, polyacrylamide-based, fluorescent hydrogel as a reusable, ratiometric glucose sensor. Polym. Chem. 2016, 7, 6655–6661. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, I.; Lee, T.S.; Lee, E.; Lee, K.J. Porous hydrogel containing Prussian blue nanoparticles for effective cesium ion adsorption in aqueous media. J. Ind. Eng. Chem. 2018, 60, 465–474. [Google Scholar] [CrossRef]
- Cho, E.; Jang, G.; Kim, D.; Lee, T.S. Fabrication of hollow-centered sodium-alginate-based hydrogels embedded with various particles. Mol. Cryst. Liq. Cryst. 2017, 659, 71–76. [Google Scholar] [CrossRef]
- Hu, J.; Dai, L.; Liu, S. SAnalyte-reactive amphiphilic thermoresponsive diblock copolymer micelles-based multifunctional ratiometric fluorescent chemosensors. Macromolecules 2011, 44, 4699–4710. [Google Scholar] [CrossRef]
- Yin, J.; Hu, H.; Wu, Y.; Liu, S. Thermo-and light-regulated fluorescence resonance energy transfer processes within dually responsive microgels. Polym. Chem. 2011, 2, 363–371. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, T.S. Thermoresponsive, and reversibly-emissive, core-shell nanogel composed of PNIPAM and carbon nanodots. Polym. Bull. 2016, 73, 2615–2625. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive polymers and their biomedical application in tissue engineering—A review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and copolymers: A review on recent progresses in biomedical applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Jin, Y.; Wu, C.; Chiu, D.T. Copper (II) and iron (II) ion sensing with semiconducting polymer dots. Chem. Commun. 2011, 47, 2820–2822. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chiu, D.T. Highly fluorescent semiconducting polymer dots for biology and medicine. Angew. Chem. Int. Ed. 2013, 52, 3086–3109. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, C. A PNIPAM-based fluorescent nanothermometer with ratiometric readout. Chem. Commun. 2011, 47, 994–996. [Google Scholar] [CrossRef]
- Wu, C.; Schneider, T.; Zeigler, M.; Yu, J.; Schiro, P.G.; Burnham, D.R.; McNeill, J.D.; Chiu, D.T. Bioconjugation of ultrabright semiconducting polymer dots for specific cellular targeting. J. Am. Chem. Soc. 2010, 132, 15410–15417. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lee, T.S. Emission tuning with size-controllable polymer dots from a single conjugated polymer. Small 2018, 14, 1702758. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.-C.; Yu, J.; Ye, F.; Rong, Y.; Gallina, M.E.; Fujimoto, B.S.; Zhang, Y.; Chan, Y.-H.; Sun, W.; Zhou, X.-H.; et al. Squaraine-based polymer dots with narrow, bright near-infrared fluorescence for biological applications. J. Am. Chem. Soc. 2015, 137, 173–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Lee, Y.-D.; Jo, S.; Kim, S.; Lee, T.S. Detection and imaging of cathepsin L in cancer cells using the aggregation of conjugated polymer dots and magnetic nanoparticles. Sens. Actuat. B Chem. 2020, 307, 127641. [Google Scholar] [CrossRef]
- Wu, C.; Hansen, S.J.; Hou, Q.; Yu, J.; Zeigler, M.; Jin, Y.; Burnham, D.R.; McNeill, J.D.; Olson, J.M.; Chiu, D.T. Design of highly emissive polymer dot bioconjugates for in vivo tumor targeting. Angew. Chem. Int. Ed. 2011, 50, 3430–3434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Chen, C.; Wu, P.; Kuo, S.; Chan, Y. Coumarin dye-embedded semiconducting polymer dots for ratiometric sensing of fluoride ions in aqueous solution and bio-imaging in cells. J. Mater. Chem. B 2014, 2, 6188–6191. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Bull, B.; Szymanski, C.; Christensen, K.; McNeill, J. Multicolor conjugated polymer dots for biological fluorescence imaging. ACS Nano 2008, 2, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, P.; Kuo, S.; Chen, C.; Chang, E.; Wu, C.; Chan, Y. Quinoxaline-based polymer dots with ultrabright red to near-infrared fluorescence for in vivo biological imaging. J. Am. Chem. Soc. 2015, 137, 10420–10429. [Google Scholar] [CrossRef]
- Yu, J.; Wu, C.; Zhang, X.; Ye, F.; Gallina, M.E.; Rong, Y.; Wu, I.; Sun, W.; Chan, Y.; Chiu, D.T. Stable functionalization of small semiconducting polymer dots via covalent cross-linking and their application for specific cellular Imaging. Adv. Mater. 2012, 24, 3498–3504. [Google Scholar] [CrossRef] [Green Version]
- Kuo, S.; Li, H.; Wu, P.; Chen, C.; Huang, Y.; Chan, Y. Dual colorimetric and fluorescent sensor based on semiconducting polymer dots for ratiometric detection of lead ions in living cells. Anal. Chem. 2015, 87, 4765–4771. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Chen, J.; Chen, C.; Chan, Y. Photoactivated ratiometric copper (II) ion sensing with semiconducting polymer dots. Chem. Commun. 2013, 49, 898–900. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jung, Y.; Lee, D.; Jang, W.-D. Thermoresponsive polymer and fluorescent dye hybrids for tunable multicolor emission. Adv. Mater. 2016, 28, 3499–3503. [Google Scholar] [CrossRef]
- Lee, J.; Yang, H.; Park, C.H.; Cho, H.-H.; Yun, H.; Kim, B.J. Colorimetric thermometer from graphene oxide platform integrated with red, green, and blue emitting, responsive block copolymers. Chem. Mater. 2016, 28, 3446–3453. [Google Scholar] [CrossRef]
- Jo, S.; Kim, D.; Son, S.-H.; Kim, Y.; Lee, T.S. Conjugated poly(fluorene-quinoxaline) for fluorescence imaging and chemical detection of nerve agents with its paper-based strip. ACS Appl. Mater. Interfaces 2014, 6, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Bijileveld, J.C.; Shahid, M.; Gilot, J.; Wienk, M.M.; Janssen, R.A.J. Copolymers of cyclopentadithiophene and electron-deficient aromatic units designed for photovoltaic applications. Adv. Funct. Mater. 2009, 19, 3262–3270. [Google Scholar] [CrossRef]
- Son, S.; Kim, Y.; Heo, M.B.; Lim, Y.T.; Lee, T.S. A fluorescence turn-on probe for the detection of thiol-containing amino acids in aqueous solution and bioimaging in cells. Tetrahedron 2014, 70, 2034–2039. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Lee, T.S. Dual-signal detection of trypsin using controlled aggregation of conjugated polymer dots and magnetic nanoparticles. Sens Actuat. B Chem. 2018, 264, 45–51. [Google Scholar] [CrossRef]
- Jo, S.; Kim, H.; Lee, T.S. Decoration of conjugated polyquinoxaline dots on mesoporous TiO2 nanofibers for visible-light-driven photocatalysis. Polymer 2021, 228, 123892. [Google Scholar] [CrossRef]
- Mamidi, N.; Delgadillo, R.M.V. Design, fabrication and drug release potential of dual stimuli-responsive composite hydrogel nanoparticle interfaces. Colloids Interfaces B Biointerfaces 2021, 204, 111819. [Google Scholar] [CrossRef] [PubMed]
- Jalili, N.A.; Jaiswal, M.K.; Peak, C.W.; Cross, L.M.; Gaharwar, A.K. Injectable nanoengineered stimuli-responsive hydrogels for on-demand and localized therapeutic delivery. Nanoscale 2017, 9, 15379–15389. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, N. Gelatin/carboxymethyl cellulose based stimuli-responsive hydrogels for controlled delivery of 5-fluorouracil, development, in vitro characterization, in vivo safety and bioavailability evaluation. Carbohydr. Polym. 2021, 257, 117617. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Chen, B. Elastomeric and pH-responsive hydrogels based on direct crosslinking of the poly(glycerol sebacate) pre-polymer and gelatin. Polym. Chem. 2018, 9, 3727–3740. [Google Scholar] [CrossRef] [Green Version]
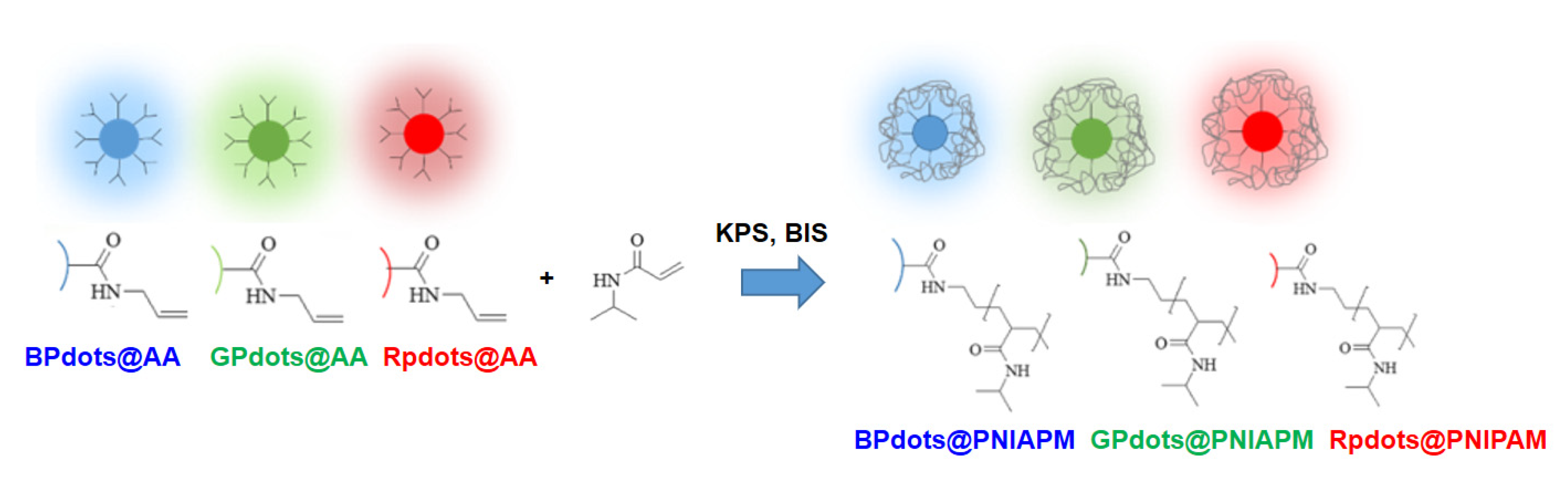

| m | n | Mn | Mw | Polydispersity | |
|---|---|---|---|---|---|
| BCP | 11,100 | 22,100 | 1.99 | ||
| GCP | 0.89 | 0.11 | 10,200 | 21,100 | 2.07 |
| RCP | 0.63 | 0.37 | 8200 | 10,660 | 1.30 |
| PMAS | 0.47 | 0.53 | 12,100 | 45,300 | 3.74 |
| Source | Hydrodynamic Diameter (nm) | Zeta Potential (mV) | ||
|---|---|---|---|---|
| Pdots | Pdots@AA | Pdots | Pdots@AA | |
| BCP | 89.0 ± 0.03 | 78.9 ± 0.03 | −34.0 ± 0.3 | −19.0 ± 0.5 |
| GCP | 130 ± 0.01 | 125.0 ± 0.7 | −27.0 ± 0.8 | −13.0 ± 0.5 |
| RCP | 122.0 ± 0.8 | 126.0 ± 0.7 | −42.0 ± 0.7 | −11.0 ± 0.6 |
| Size (nm) | Zeta Potential (mV) | |
|---|---|---|
| BPdots@PNIPAM | 104 ± 0.7 | −7.5 ± 0.03 |
| GPdost@PNIPAM | 126 ± 0.4 | −6.0 ± 0.06 |
| RPdots@PNIPAM | 175 ± 0.1 | −1.38 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namgung, H.; Jo, S.; Lee, T.S. Fluorescence Modulation of Conjugated Polymer Nanoparticles Embedded in Poly(N-Isopropylacrylamide) Hydrogel. Polymers 2021, 13, 4315. https://doi.org/10.3390/polym13244315
Namgung H, Jo S, Lee TS. Fluorescence Modulation of Conjugated Polymer Nanoparticles Embedded in Poly(N-Isopropylacrylamide) Hydrogel. Polymers. 2021; 13(24):4315. https://doi.org/10.3390/polym13244315
Chicago/Turabian StyleNamgung, Ho, Seonyoung Jo, and Taek Seung Lee. 2021. "Fluorescence Modulation of Conjugated Polymer Nanoparticles Embedded in Poly(N-Isopropylacrylamide) Hydrogel" Polymers 13, no. 24: 4315. https://doi.org/10.3390/polym13244315
APA StyleNamgung, H., Jo, S., & Lee, T. S. (2021). Fluorescence Modulation of Conjugated Polymer Nanoparticles Embedded in Poly(N-Isopropylacrylamide) Hydrogel. Polymers, 13(24), 4315. https://doi.org/10.3390/polym13244315






