Composites Based on Natural Polymers and Microbial Biomass for Biosorption of Brilliant Red HE-3B Reactive Dye from Aqueous Solutions
Abstract
:1. Introduction
- Bacteria: Pseudomonas aeruginosa immobilised in sodium alginate have been used for the retention of Reactive Green 6 from wastewaters with a maximum adsorption capacity of 21.2 mg/g; Bacillus sp., immobilized in 1% sodium alginate, allows the obtaining of a maximum adsorption capacity of 588.235 mg/g for Brilliant Red HE-3B; Bacillus cereus immobilised in 3% sodium alginate yields a maximum retention of 83% for Malachite Green [10,11,12].
- Fungi: Penicillium sp. immobilised in 2% sodium alginate has been used for the removal of C.I. Reactive Red with a maximum adsorption capacity of 120.48 mg/g; Penicillium crustosum immobilised in 2% agar retains Congo red with an efficiency of 81.86%; Rhizophus orizae immobilised in carboxymethyl cellulose has been used for the biosorption of Reactive Blue with an efficiency of 97.44%. Saccharomyces cerevisiae immobilized in sodium alginate and present in the form of gel beads has also been used for the retention of Brilliant Red HE-3B dye, and leads to a sorption capacity of 104.67 mg/g [13,14,15,16].
2. Materials and Methods
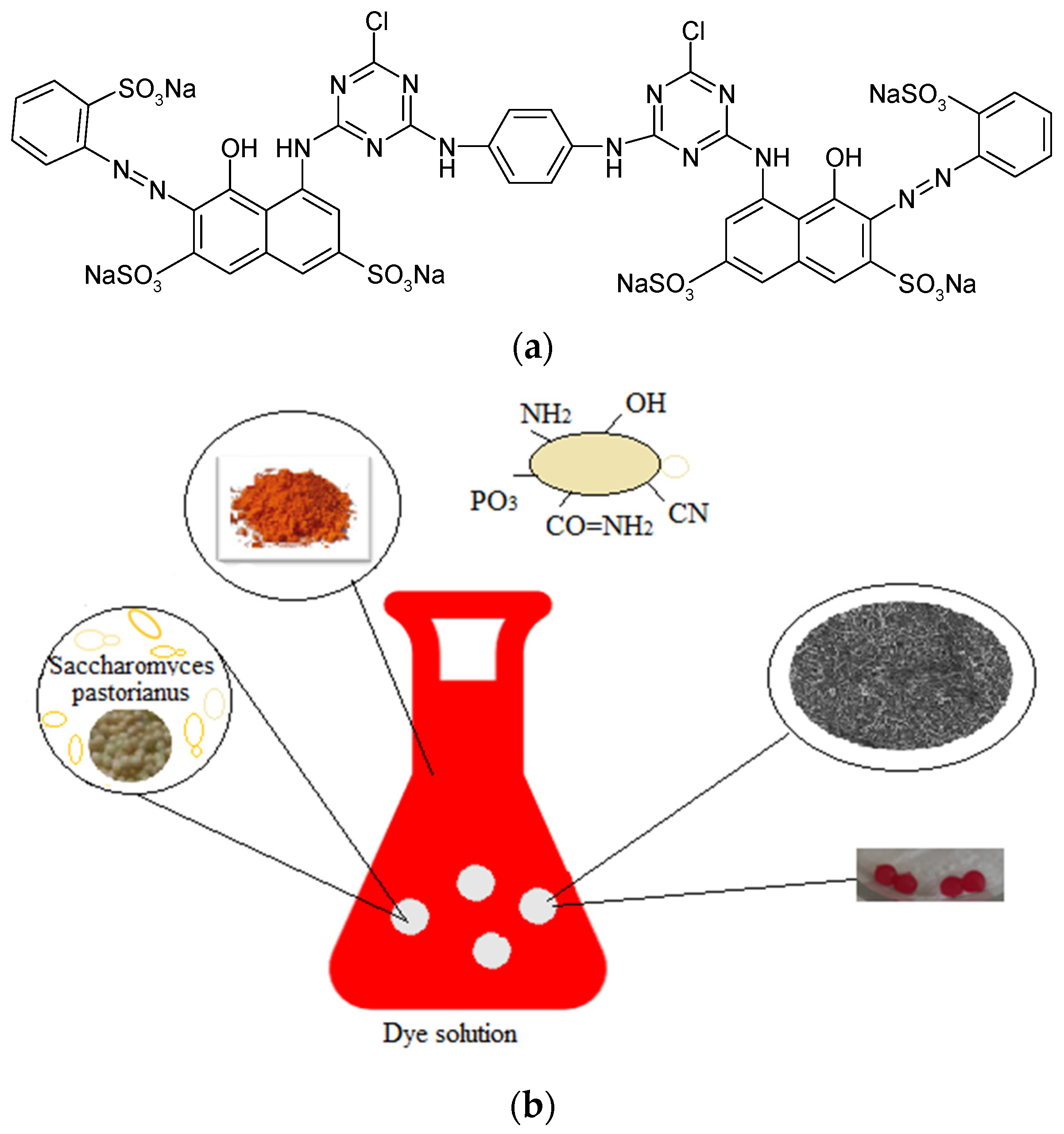
2.1. Materials
2.2. Methods
2.2.1. Batch Biosorption Methodology
2.2.2. Physicochemical Characterization of Composite Biosorbent
2.2.3. Modelling the Biosorption Experimental Data
2.3. Thermodynamic Parameters of the Biosorption Process
3. Results and Discussion
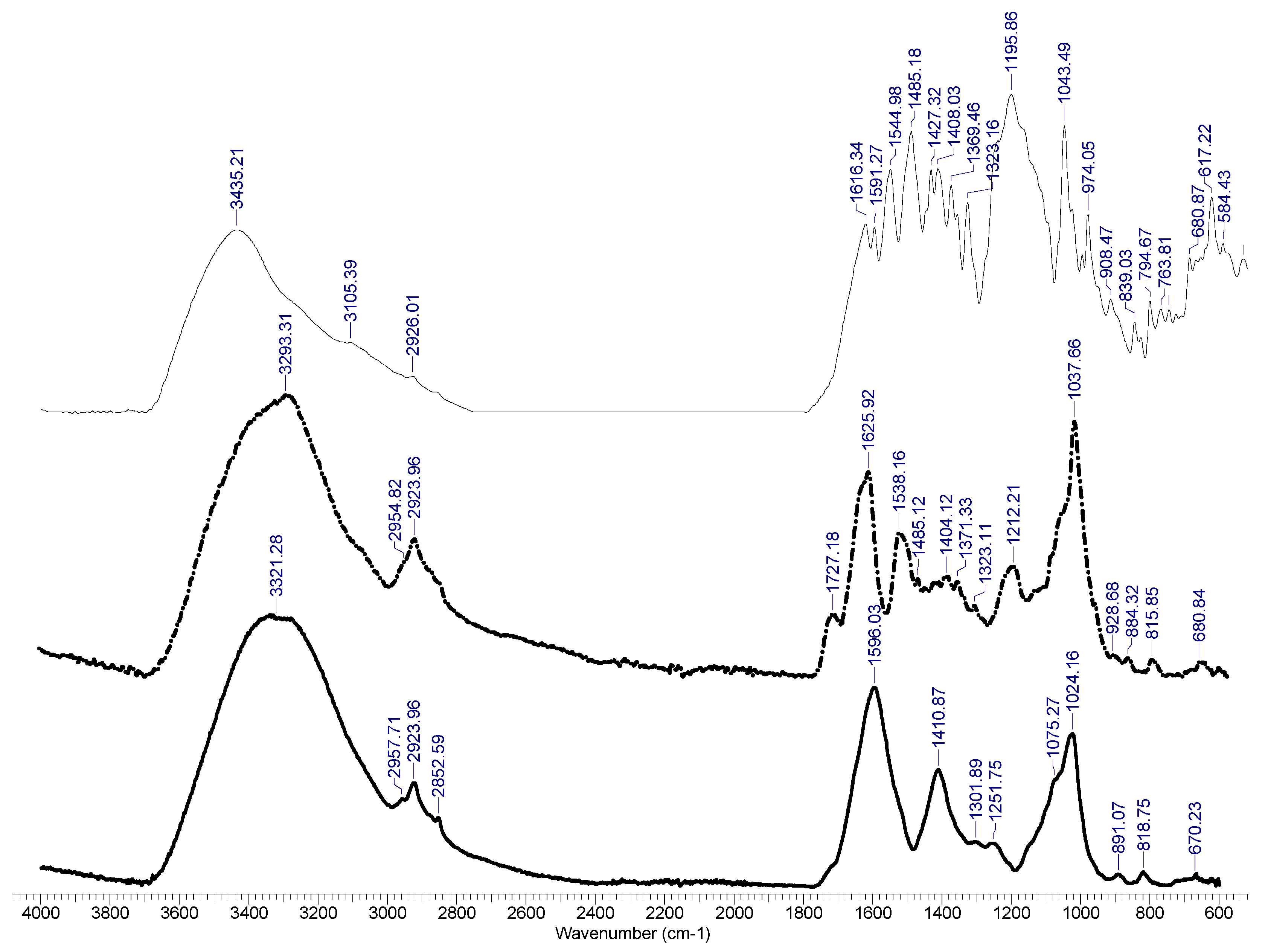
3.1. Analysis of the Biosorbent Based on Residual Biomass Using SEM, EDAX and FT-IR Spectra
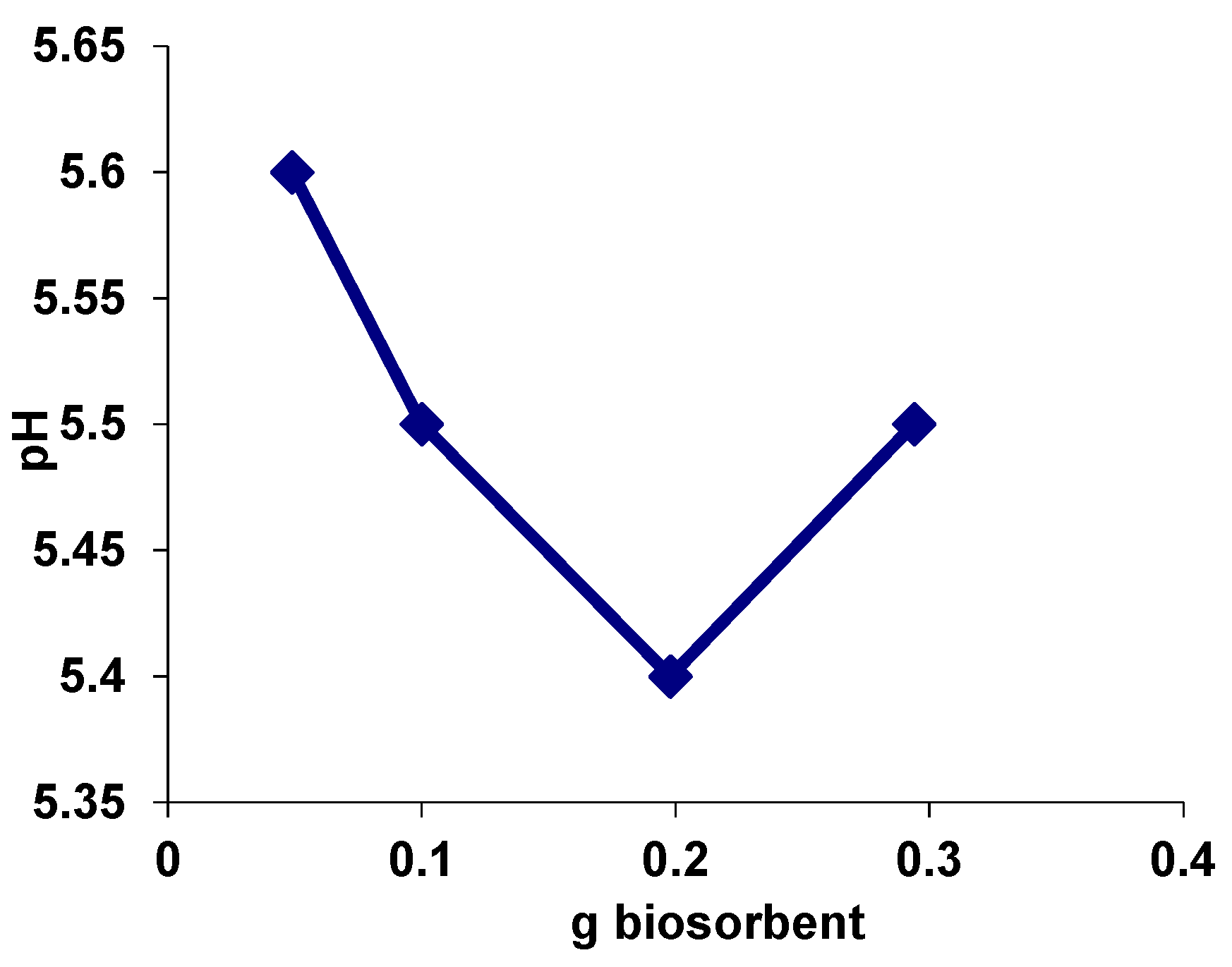
3.2. Evaluation of the Value of the Point of Zero Charge (pHPZC) for Biosorbent
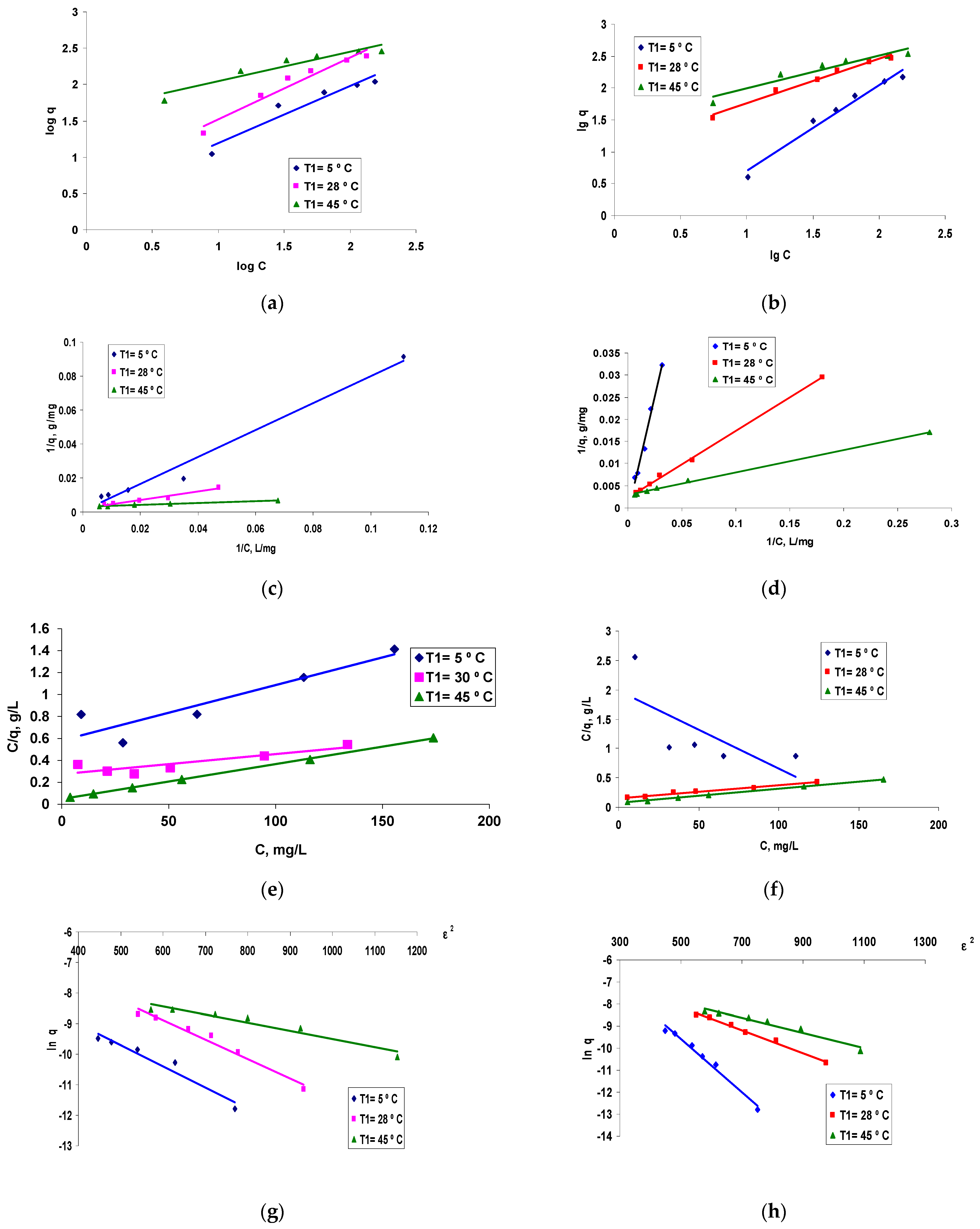
3.3. Modelling the Biosorption Equilibrium Process
- -
- Assessment of the efficiency of polymeric composites based on residual biomass as bioadsorbent material (q),
- -
- Subsequent analyses to evaluate the effect of temperature on the development of the biosorption process and its feasibility from a thermodynamic point of view (q, KL).
- -
- Additionally, a series of preliminary conclusions can be drawn regarding the mechanism of the biosorption process, both from the information provided by the value of the biosorption energy (E) determined from the DR model, and from the subsequent data obtained by the thermodynamic study based on the Langmuir model: biosorption capacity, q and Langmuir constant, KL.
3.4. Analysis of the Proposed Thermodynamic Parameters
3.5. Recovery of Biosorbent Loaded with Dye
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Suteu, D.; Zaharia, C.; Blaga, A.C. Biosorption-current bioprocess for wastewater treatment (chapter 10). In Current Topics, Concepts and Research Priorities in Environmental Chemistry; Zaharia, C., Ed.; Universitatii ‘Al.I. Cuza’: Iasi, Romania, 2012; Volume I, pp. 221–244. [Google Scholar]
- Berillo, D.; Al-Jwaid, A.; Caplin, J. Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water. Polymers 2021, 13, 1073. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.R. Recent Advances in Chitosan Based Biosorbent for Environmental Clean-Up. J. Bioremed. Biodeg. 2016, 7, e173. [Google Scholar] [CrossRef]
- Blaga, A.C.; Zaharia, C.; Suteu, D. Polysaccharides as Support for Microbial Biomass-Based Adsorbents with Applications in Removal of Heavy Metals and Dyes. Polymers 2021, 13, 2893. [Google Scholar] [CrossRef] [PubMed]
- Rusu, L.; Grigoras, C.G.; Suceveanu, E.M.; Simion, A.I.; Dediu Botezatu, A.V.; Istrate, B.; Doroftei, I. Eco-Friendly Biosorbents Based on Microbial Biomass and Natural Polymers: Synthesis, Characterization and Application for the Removal of Drugs and Dyes from Aqueous Solutions. Materials 2021, 14, 4810. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, A. Chemically Modified Biosorbents and Their Role in the Removal of Emerging Pharmaceutical Waste in the Water System. Water 2020, 12, 1551. [Google Scholar] [CrossRef]
- Pires, C.; Marques, A.P.; Guerreiro, A.; Magan, N.; Castro, P.M. Removal of heavy metals using different polymer matrixes as support for bacterial immobilisation. J. Hazard Mater. 2011, 191, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Saha, P.; Bhaskara Rao, K.V. Immobilization as a powerful bioremediation tool for abatement of dye pollution: A review. Environ. Rev. 2020, 29, 277–299. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Gargh, S. Recent developments in composite biosorbents and their applications for wastewater treatment. Res. J. Chem. Environ. 2015, 19, 42–58. [Google Scholar]
- Saravanan, N.; Kannadasan, T.; Basha, C.; Manivasagan, V. Biosorption of Textile Dye Using Immobilized Bacterial (Pseudomonas aeruginosa) and Fungal (Phanerochatechrysosporium) Cells. Am. J. Environ. Sci. 2013, 9, 377–387. [Google Scholar] [CrossRef]
- Horciu, L.I.; Zaharia, C.; Blaga, A.C.; Rusu, L.; Suteu, D. Brilliant Red HE-3B dye biosorption by immobilized residual consortium Bacillus sp. biomass: Fixed–Bed-Column Studies. Appl. Sci. 2021, 11, 4498. [Google Scholar] [CrossRef]
- Nath, J.; Das, A.; Ray, L. Biosorption of Malachite Green from Aqueous Solution Using Resting and Immobilised Biomass of Bacillus cereus M116 (MTCC 5521). Indian Chem. Eng. 2015, 57, 82–100. [Google Scholar] [CrossRef]
- Zhang, L.F.; Wang, M. Biosorption of C. I. Reactive Red 2 by Immobilized Fungal Biomass. Adv. Mater. Res. 2011, 213, 432–436. [Google Scholar] [CrossRef]
- Sharma, B.; Tiwari, S.; Bisht, N.; Tewari, L. Eco-friendly bioprocess using agar plug immobilized Penicilliumcrustosum PWWS-6 biomass for treatment of wastewater contaminated with toxic Congo red dye for use in agriculture. Ind. Crop. Prod. 2021, 170, 113755. [Google Scholar] [CrossRef]
- Bagchi, M.; Bera, D.; Adhikari, S. Biosorption of an azo dye Reactive Blue 4 from aqueous solution using dead and CMC immobilized biomass of Rhizopus oryzae (MTCC 262). Bioremediation J. 2021, 25, 326–346. [Google Scholar] [CrossRef]
- Suteu, D.; Blaga, A.C.; Diaconu, M.; Malutan, T. Biosorption of reactive dye from aqueous media using Saccharomyces cerevisiae biomass. Equilibrium and kinetic study. Cent. Eur. J. Chem. 2013, 11, 2048–2057. [Google Scholar] [CrossRef] [Green Version]
- Khashei, S.; Etemadifar, Z.; Rahmani, H.R. Immobilization of Pseudomonas putida PT in resistant matrices to environmental stresses: A strategy for continuous removal of heavy metals under extreme conditions. Ann. Microbiol. 2018, 68, 931–942. [Google Scholar] [CrossRef]
- Stewart, G.G. Saccharomyces species in the Production of Beer. Beverages 2016, 2, 34. [Google Scholar] [CrossRef]
- Bastos, R.; Coelho, E.; Coimbra, M.A. Modifications of Saccharomyces pastorianus cell wall polysaccharides with brewing process. Carbohydr. Polym. 2015, 124, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Dias Monte Blanco, S.P.; Scheufele, F.B.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Marin, P.; Kroumov, A.D.; Borba, C.E. Kinetic, equilibrium and thermodynamic phenomenological modeling of reactive dye adsorption onto polymeric adsorbent. Chem. Eng. J. 2017, 307, 466–475. [Google Scholar] [CrossRef]
- Bouras, H.D.; Yeddou, A.R.; Bouras, N.; Hellel, D.; Michael, H.; Sabaou, D.N.; Chergui, A.; Nadjemi, B. Biosorption of Congo red dye by Aspergillus carbonarius M333 and Penicillium glabrum Pg1: Kinetics, equilibrium and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2017, 80, 915–923. [Google Scholar] [CrossRef]
- Nouri, S.; Haghseresht, F. Adsorption of p-nitrophenol in untreated and treated activated carbon. Adsorption 2004, 10, 79–86. [Google Scholar] [CrossRef]
- Suteu, D.; Blaga, A.C.; Zaharia, C.; Horciu, I.L. Residual biomass of Lactobacillus immobilized in alginate for Orange 16 dye retention from aqueous medium. Bull. Polytech. Inst. Iasi 2021, in press. [Google Scholar]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.; Smith, D.J. Studies in adsorption. Part X: A system of classification of solution. Adsorption isotherms and its use in diagnosis of adsorption mechanism and in measurement of specific surface areas of solids. J. Chem. Soc. Lond. 1960, 2, 3973. [Google Scholar] [CrossRef]
- Akar, T.; Kurşunlu, G.; Celik, S.; Akar, S.T. Immobilized Mucor plumbeus on sepiolite support: A potential decolorization agent suitable for batch and continuous mode water treatment. J. Clean. Prod. 2021, 294, 126283. [Google Scholar] [CrossRef]
- Celik, S.; Duman, N.; Sayin, F.; Akar, S.T.; Akar, T. Microbial cells immobilized on natural biomatrix as a new potential ecofriendly biosorbent for the biotreatment of reactive dye contamination. J. Water Process. Eng. 2021, 39, 101731. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Cengiz Ozalp, V.; Yakup Arıca, M. Removal of Disperse Red 60 dye from aqueous solution using free and composite fungal biomass of Lentinus concinnus. Water Sci. Technol. 2017, 72, 366–377. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Yilmaz, M. Azo Dye Removal Using Free and Immobilized Fungal Biomasses: Isotherms, Kinetics and Thermodynamic Studies. Fibers Polym. 2018, 19, 877–886. [Google Scholar] [CrossRef]
- Horciu, I.L.; Blaga, A.C.; Rusu, L.; Zaharia, C.; Suteu, D. Biosorption of reactive dyes from aqueous media using the Bacillus sp. residual biomass. Des. Water Treat. 2020, 195, 353–360. [Google Scholar] [CrossRef]
- Suteu, D.; Zaharia, C.; Blaga, A.C.; Peptu, A.C. Biosorbents based on residual biomass of Lactobacillus sp. bacteria consortium immobilized in sodium alginate for Orange 16 dye retention from aqueous solutions. Des. Water Treat. in press.
- Kumar, M.R.; Girma, E. Aklilu, Equilibrium and thermodynamic studies for dye removal using biosorption. Int. J. Sci. Res. 2016, 5, 905–908. [Google Scholar] [CrossRef]
- Gedam, V.V.; Raut, P.; Chahande, A.; Pathak, P. Kinetic, thermodynamics and equilibrium studies on the removal of Congo red dye using activated teak leaf powder. Appl. Water Sci. 2019, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Tataru-Farmus, R.E.; Zaharia, C.; Suteu, D.; Blaga, A.C. Biomass-based soil in ecological agriculture: CHARACTERISTICS and wheat seeds development trends. J. Appl. Life Sci. Environ. under review.
- Chojnackaa, K.; Moustakasb, K.; Witek-Krowiakca, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Tehnol. 2020, 295, 122223. [Google Scholar] [CrossRef] [PubMed]




| Isotherm | Φ1 = 1500 μm | Φ2 = 900 μm | ||||
|---|---|---|---|---|---|---|
| 278 K | 303 | 318 | 278 K | 303 | 318 | |
| Freundlich | ||||||
| KF ((mg/g) (L/mg)1/n) | 2.5275 | 4.6709 | 43.944 | 0.222 | 11.416 | 30.4369 |
| n | 1.2711 | 1.1737 | 2.4845 | 0.7402 | 14.431 | 1.9558 |
| R2 | 0.9242 | 0.9525 | 0.9025 | 0.971 | 0.9838 | 0.9321 |
| Langmuir I (1/q = f (1/C)) | ||||||
| q0 (mg/g) | 2500 | 555.55 | 312.5 | 454.545 | 344.827 | |
| KL (L/g) | 0.000504 | 0.00712 | 0.0667 | 0.01453 | 0.0571 | |
| R2 | 0.9799 | 0.9761 | 0.9987 | 0.9881 | 0.9985 | 0.9969 |
| Langmuir II (C/q = f (C)) | ||||||
| q0 (mg/g) | 200 | 555.55 | 312.5 | 476.19 | 416.667 | |
| KL (L/g) | 0.00859 | 0.00656 | 0.06639 | 0.01345 | 0.03162 | |
| R2 | 0.8363 | 0.7778 | 0.9994 | 0.4894 | 0.9838 | 0.9991 |
| Dubinin-Radushkevich (DR) | ||||||
| q0 (mg/g) | 3013.02 | 8814.576 | 1572.725 | 42,327.8 | 5348.6 | 2923.39 |
| β (mol2/KJ2) | 0.007 | 0.0063 | 0.0027 | 0.0121 | 0.0051 | 0.0034 |
| E (KJ/mol) | 8.4515 | 8.909 | 13.608 | 6.428 | 9.901 | 12.127 |
| R2 | 0.9426 | 0.9724 | 0.9317 | 0.9827 | 0.9917 | 0.954 |
| T (K) | KL (L/g) | ΔG0 (KJ/mol) | ΔH0 (KJ/mol) | ΔS0 (J/mol K) |
|---|---|---|---|---|
| 278 | 0.0000504 | −0.704 | −87.795 | 312.232 |
| 303 | 0.00712 | −5.903 | ||
| 318 | 0.0667 | −12.111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suteu, D.; Blaga, A.C.; Cimpoesu, R.; Puiţel, A.C.; Tataru-Farmus, R.-E. Composites Based on Natural Polymers and Microbial Biomass for Biosorption of Brilliant Red HE-3B Reactive Dye from Aqueous Solutions. Polymers 2021, 13, 4314. https://doi.org/10.3390/polym13244314
Suteu D, Blaga AC, Cimpoesu R, Puiţel AC, Tataru-Farmus R-E. Composites Based on Natural Polymers and Microbial Biomass for Biosorption of Brilliant Red HE-3B Reactive Dye from Aqueous Solutions. Polymers. 2021; 13(24):4314. https://doi.org/10.3390/polym13244314
Chicago/Turabian StyleSuteu, Daniela, Alexandra Cristina Blaga, Ramona Cimpoesu, Adrian Cătălin Puiţel, and Ramona-Elena Tataru-Farmus. 2021. "Composites Based on Natural Polymers and Microbial Biomass for Biosorption of Brilliant Red HE-3B Reactive Dye from Aqueous Solutions" Polymers 13, no. 24: 4314. https://doi.org/10.3390/polym13244314
APA StyleSuteu, D., Blaga, A. C., Cimpoesu, R., Puiţel, A. C., & Tataru-Farmus, R.-E. (2021). Composites Based on Natural Polymers and Microbial Biomass for Biosorption of Brilliant Red HE-3B Reactive Dye from Aqueous Solutions. Polymers, 13(24), 4314. https://doi.org/10.3390/polym13244314











