Influence of Solvent and Substrate on Hydrophobicity of PLA Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Section
2.2. Substrates Preparation
2.3. Films Preparation
2.4. Contact Angle Measurements and Surface Tension Calculations
2.5. X-ray Diffraction
2.6. AFM
2.7. ToF-SIMS
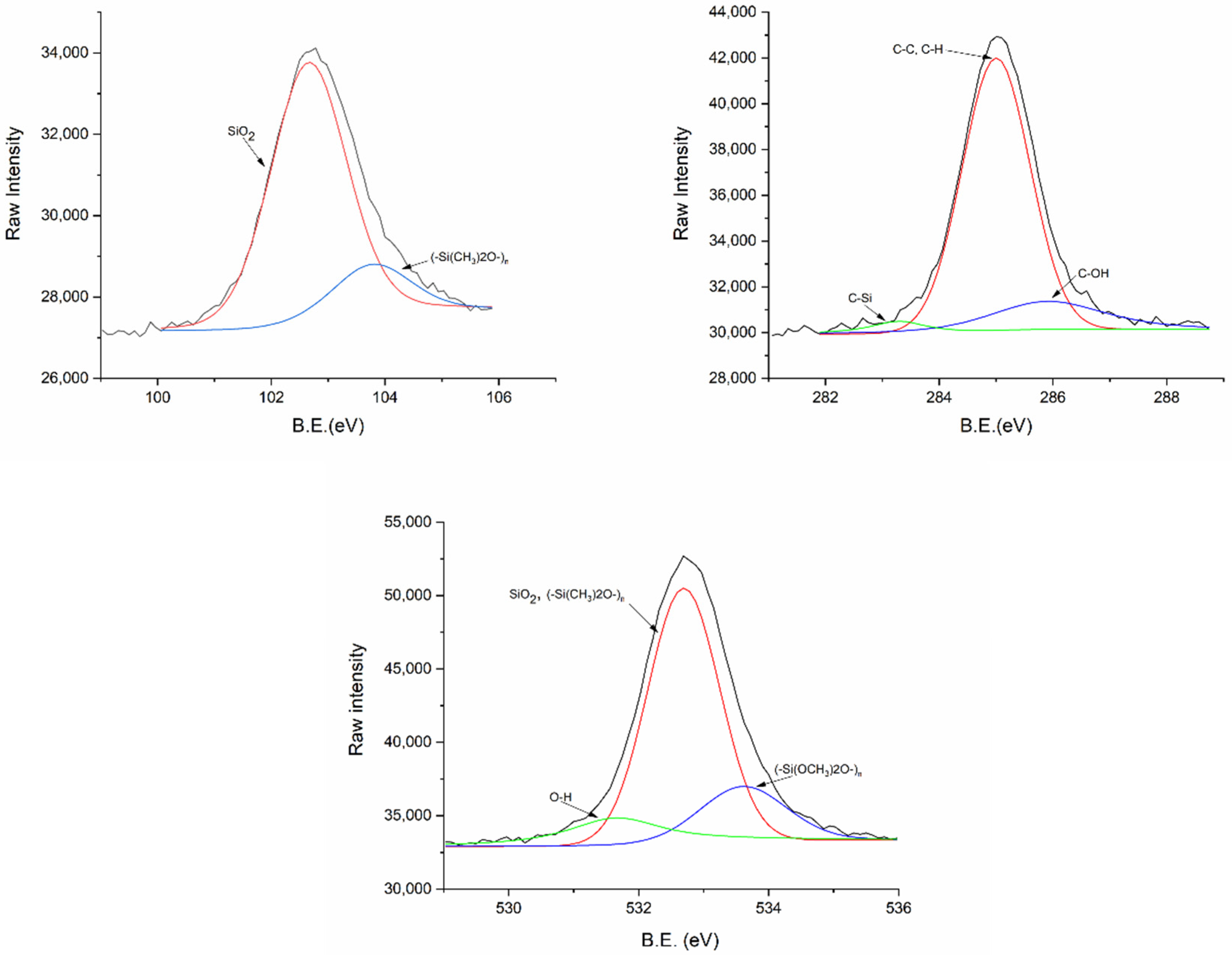
2.8. XPS
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhu, B.; Wang, Y.; Liu, C.; Shen, C. Effect of different sterilization methods on the properties of commercial biodegradable polyesters for single-use, disposable medical devices. Mater. Sci. Eng. C 2019, 105, 110041. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Dwivedi, A.K. Recent advancement in wound healing dressing material. Int. J. Res. Pharm. Sci. 2019, 10, 2572–2577. [Google Scholar] [CrossRef]
- Han, J.; Ma, B.; Liu, H.; Wang, T.; Wang, F.; Xie, C.; Li, M.; Liu, H.; Ge, S. Hydroxyapatite nanowires modified polylactic acid membrane plays barrier/osteoinduction dual roles and promotes bone regeneration in a rat mandible defect model. J. Biomed. Mater. Res.-Part A 2018, 106, 3099–3110. [Google Scholar] [CrossRef] [PubMed]
- Fairag, R.; Rosenzweig, D.H.; Ramirez-Garcialuna, J.L.; Weber, M.H.; Haglund, L. Three-Dimensional Printed Polylactic Acid Scaffolds Promote Bone-like Matrix Deposition in Vitro. ACS Appl. Mater. Interfaces 2019, 11, 15306–15315. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Roosta Azad, R. Preparation and characterization of poly-lactic acid based films containing propolis ethanolic extract to be used in dry meat sausage packaging. J. Food Sci. Technol. 2020, 57, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Lin, T.Y.; Zhu, T.T.; Xun, Y.; Tao, Y.S.; Yang, Y.Q.; Xie, J.L.; Zhang, X.M.; Chen, S.X.; Ding, B.J.; Chen, W.D. A novel drug delivery system of mixed micelles based on poly(ethylene glycol)-poly(lactide) and poly(ethylene glycol)-poly(ɛ-caprolactone) for gambogenic acid. Kaohsiung J. Med. Sci. 2019, 35, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Q.; Gong, Y.C.; Li, Z.L.; Li, Y.P.; Xiong, X.Y. Folate-conjugated pluronic/polylactic acid polymersomes for oral delivery of paclitaxel. Int. J. Biol. Macromol. 2019, 139, 377–386. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, M.F.C.; Nonato, R.C.; Bottini, R.; Morales, A.R. Quality evaluation of solvent-cast 3D printing of poly(lactic acid) films. Bull. Mater. Sci. 2020, 43, 1–6. [Google Scholar] [CrossRef]
- Tang, T.O.; Holmes, S.; Dean, K.; Simon, G.P. Design and fabrication of transdermal drug delivery patch with milliprojections using material extrusion 3D printing. J. Appl. Polym. Sci. 2019, 48777, 1–17. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Ayob, N.A.I.; Blanford, C.F.; Rawi, N.F.M.; Szekely, G. Nonwoven Membrane Supports from Renewable Resources: Bamboo Fiber Reinforced Poly(Lactic Acid) Composites. ACS Sustain. Chem. Eng. 2019, 7, 11885–11893Sd. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Ong, X.Y.; Koh, J.J.; Kong, J.; Zhang, X.; Thitsartarn, W.; Li, Z.; He, C. Dual-Phase Poly(lactic acid)/Poly(hydroxybutyrate)-Rubber Copolymer as High-Performance Shape Memory Materials. ACS Appl. Polym. Mater. 2021, 3, 389–399. [Google Scholar] [CrossRef]
- Rosenberg, M.; Kjelleberg, S. Hydrophobic Interactions: Role in Bacterial Adhesion. Adv. Microb. Ecol. 1986, 9, 353–393. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef] [Green Version]
- Luque-Agudo, V.; Romero-Guzmán, D.; Fernández-Grajera, M.; González-Martín, M.L.; Gallardo-Moreno, A.M. Aging of Solvent-Casting PLA-Mg Hydrophobic Films: Impact on Bacterial Adhesion and Viability. Coatings 2019, 9, 814. [Google Scholar] [CrossRef] [Green Version]
- Cai, K.; Yao, K.; Lin, S.; Yang, Z.; Li, X.; Xie, H.; Qing, T.; Gao, L. Poly(D,L-lactic acid) surfaces modified by silk fibroin: Effects on the culture of osteoblast in vitro. Biomaterials 2002, 23, 1153–1160. [Google Scholar] [CrossRef]
- Yang, J.; Bei, J.; Wang, S. Enhanced cell affinity of poly (D,L-lactide) by combining plasma treatment with collagen anchorage. Biomaterials 2002, 23, 2607–2614. [Google Scholar] [CrossRef]
- Chen, Y.; Geever, L.M.; Higginbotham, C.L.; Devine, D.M. Analysis of the mechanical properties of solvent cast blends of PLA/PCL. Appl. Mech. Mater. 2014, 679, 50–56. [Google Scholar] [CrossRef]
- Fernández-Calderón, M.C.; Romero-Guzmán, D.; Ferrández-Montero, A.; Pérez-Giraldo, C.; González-Carrasco, J.L.; Lieblich, M.; Benavente, R.; Ferrari, B.; González-Martín, M.L.; Gallardo-Moreno, A.M. Impact of PLA/Mg films degradation on surface physical properties and biofilm survival. Colloids Surf. B Biointerfaces 2020, 185, 110617. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Sutera, F.; Botta, L. Biopolymeric bilayer films produced by co-extrusion film blowing. Polym. Test. 2018, 65, 35–43. [Google Scholar] [CrossRef]
- Onder, O.C.; Nazeer, M.A.; Yilgör, E.; Yilgör, I. Spontaneous formation of microporous poly(lactic acid) coatings. Prog. Org. Coat. 2018, 125, 249–256. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, X.; Ye, L.; Coates, P.; Caton-Rose, F.; Martyn, M. Fibrillation of chain branched poly (lactic acid) with improved blood compatibility and bionic structure. Chem. Eng. J. 2015, 279, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ye, L.; Zhao, X.; Coates, P.; Caton-Rose, F.; Martyn, M. Structure and biocompatibility of highly oriented poly(lactic acid) film produced by biaxial solid hot stretching. J. Ind. Eng. Chem. 2017, 52, 338–348. [Google Scholar] [CrossRef]
- Paragkumar N, T.; Edith, D.; Six, J.L. Surface characteristics of PLA and PLGA films. Appl. Surf. Sci. 2006, 253, 2758–2764. [Google Scholar] [CrossRef]
- Fukuoka, T.; Morita, T.; Saika, A.; Habe, H. Application of glycolipid biosurfactants as surface modifiers in bioplastics. J. Oleo Sci. 2018, 67, 1609–1616. [Google Scholar] [CrossRef] [Green Version]
- Narladkar, A.; Balnois, E.; Vignaud, G.; Grohens, Y.; Bardeau, J.-F. Morphology and Glass Transition of Thin Polylactic Acid Films. Polym. Eng. Sci. 2008, 48, 1655–1660. [Google Scholar] [CrossRef]
- Winterton, N. The green solvent: A critical perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef] [PubMed]
- Cseri, L.; Razali, M.; Pogany, P.; Szekely, G. Organic Solvents in Sustainable Synthesis and Engineering. In Green Chemistry; Török, B., Dransfield, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 513–553. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Ju, L.; Chaudhury, M.K.; Good, R.J. Estimation of the polar parameters of the surface tension of liquids by contact angle measurements on gels. J. Colloid Interface Sci. 1989, 128, 313–319. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Monopolar surfaces. Adv. Colloid Interface Sci. 1987, 28, 35–64. [Google Scholar] [CrossRef]
- Ringard-Lefebvre, C.; Baszkin, A. Behavior of Poly(d,l-lactic acid) Monolayers at the Air—Water Interface. Effect of Spreading Solvents. Langmuir 1994, 10, 2376–2381. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, X.; Chan, C.M.; Mi, Y. Manipulating the surface properties of polyacrylamide with nitrogen plasma. Eur. Polym. J. 2006, 42, 2914–2920. [Google Scholar] [CrossRef]
- Brizzolara, D.; Cantow, H.J.; Diederichs, K.; Keller, E.; Domb, A.J. Mechanism of the stereocomplex formation between enantiomeric poly(lactide)s. Macromolecules 1996, 29, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, Z.; Ye, L.; Zhao, X.; Coates, P.; Caton-Rose, F. Structure and biocompatibility improvement mechanism of highly oriented poly(lactic acid) produced by solid die drawing. Eur. Polym. J. 2017, 97, 68–76. [Google Scholar] [CrossRef]
- Aliotta, L.; Cinelli, P.; Coltelli, M.B.; Righetti, M.C.; Gazzano, M.; Lazzeri, A. Effect of nucleating agents on crystallinity and properties of poly (lactic acid) (PLA). Eur. Polym. J. 2017, 93, 822–832. [Google Scholar] [CrossRef]
- Taylor, J.A.; Lancaster, G.M.; Rabalais, J.W. Surface alteration of graphite, graphite monofluoride and teflon by interaction with Ar+ and Xe+ beams. Appl. Surf. Sci. 1978, 1, 503–514. [Google Scholar] [CrossRef]
- Miura, Y.; Kusano, H.; Nanba, T.; Matsumoto, S. X-ray photoelectron spectroscopy of sodium borosilicate glasses. J. Non. Cryst. Solids 2001, 290, 1–14. [Google Scholar] [CrossRef]
- Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. XPS Analysis of Ti6Al4V Oxidation Under UHV Conditions. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2014, 45, 6285–6290. [Google Scholar] [CrossRef]
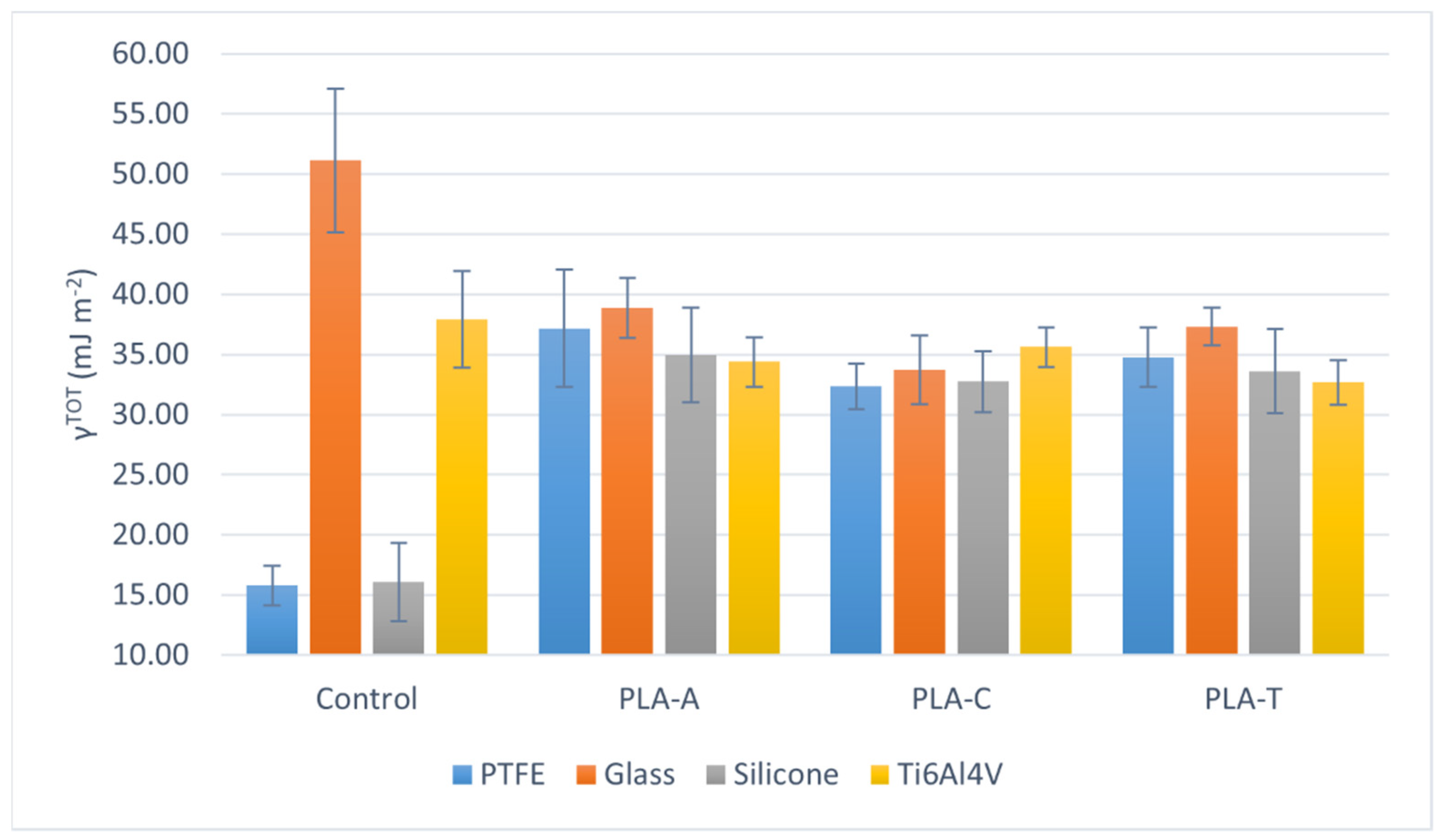

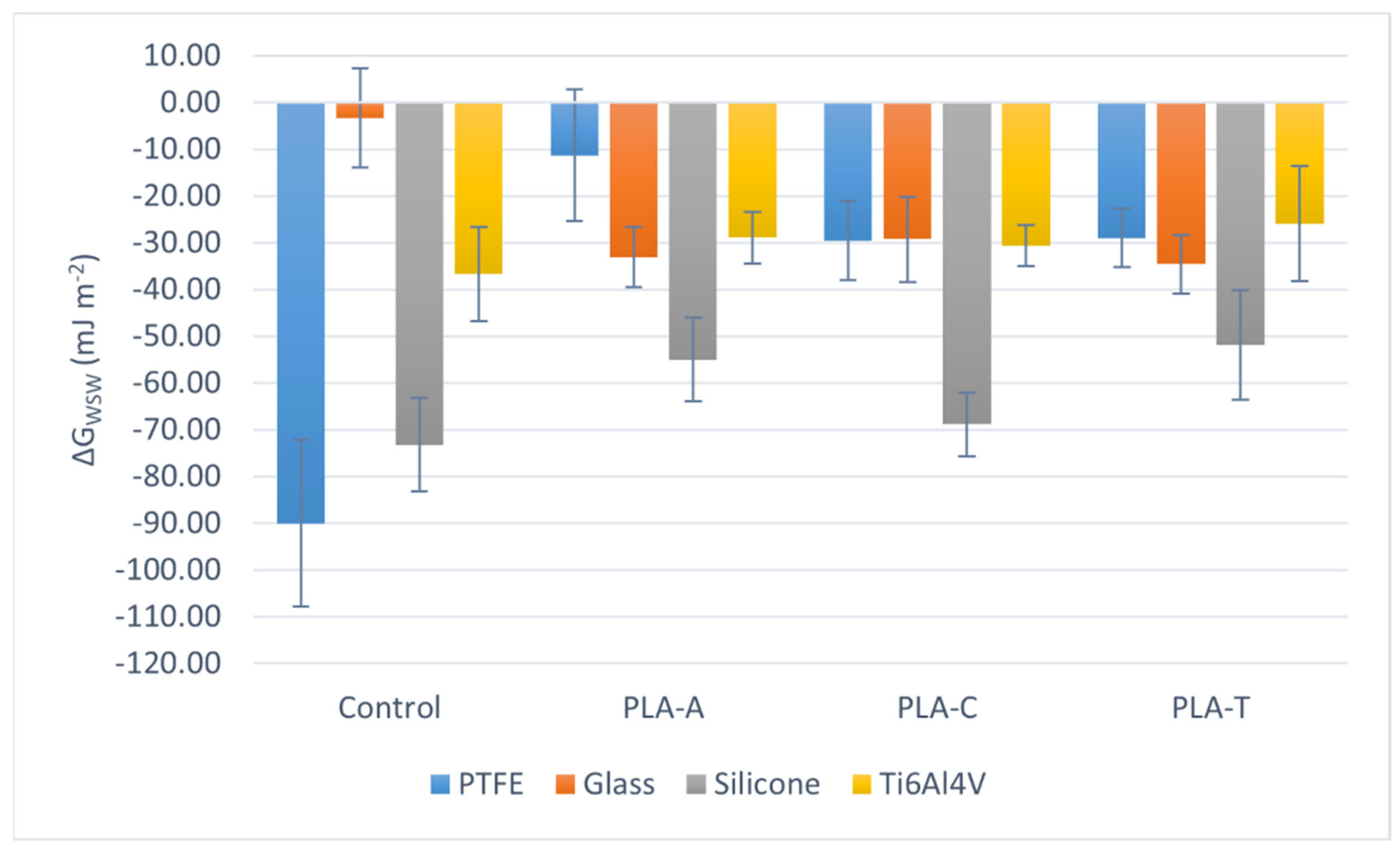

| θW ± sW [°] | θF ± sF [°] | θD ± sD [°] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | PLA-C | PLA-A | PLA-T | Control | PLA-C | PLA-A | PLA-T | Control | PLA-C | PLA-A | PLA-T | |
| PTFE | 113 ± 5 | 76 ± 3 | 64 ± 5 | 74 ± 2 | 92 ± 4 | 64 ± 2 | 54 ± 5 | 60 ± 3 | 83 ± 4 | 48 ± 2 | 47 ± 5 | 46 ± 3 |
| Glass | 43 ± 4 | 74 ± 4 | 71 ± 2 | 74 ± 2 | 24 ± 9 | 62 ± 3 | 52 ± 4 | 56 ± 2 | 48 ± 1 | 49 ± 3 | 45 ± 3 | 46 ± 2 |
| Ti6Al4V | 74 ± 5 | 76 ± 4 | 74 ± 2 | 74 ± 2 | 43 ± 1 | 66 ± 2 | 61 ± 2 | 59 ± 3 | 54 ± 3 | 44 ± 1 | 47 ± 3 | 47 ± 3 |
| Silicone | 113 ± 2 | 106 ± 2 | 111 ± 2 | 108 ± 2 | 100 ± 2 | 88 ± 3 | 95 ± 4 | 93 ± 7 | 82 ± 6 | 53 ± 4 | 53 ± 6 | 55 ± 5 |
| Film Thickness (µm) | θW ± sW [°] |
|---|---|
| 26 | 104 ± 4 |
| 27 | 106 ± 2 |
| 58 | 106 ± 2 |
| 273 | 104 ± 3 |
| 279 | 101 ± 2 |
| 294 | 96 ± 4 |
| 302 | 107 ± 4 |
| 317 | 94 ± 5 |
| 335 | 102 ± 5 |
| 402 | 103 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luque-Agudo, V.; Gallardo-Moreno, A.M.; González-Martín, M.L. Influence of Solvent and Substrate on Hydrophobicity of PLA Films. Polymers 2021, 13, 4289. https://doi.org/10.3390/polym13244289
Luque-Agudo V, Gallardo-Moreno AM, González-Martín ML. Influence of Solvent and Substrate on Hydrophobicity of PLA Films. Polymers. 2021; 13(24):4289. https://doi.org/10.3390/polym13244289
Chicago/Turabian StyleLuque-Agudo, Verónica, Amparo M. Gallardo-Moreno, and María Luisa González-Martín. 2021. "Influence of Solvent and Substrate on Hydrophobicity of PLA Films" Polymers 13, no. 24: 4289. https://doi.org/10.3390/polym13244289
APA StyleLuque-Agudo, V., Gallardo-Moreno, A. M., & González-Martín, M. L. (2021). Influence of Solvent and Substrate on Hydrophobicity of PLA Films. Polymers, 13(24), 4289. https://doi.org/10.3390/polym13244289






