Microstructure and Biological Properties of Electrospun In Situ Polymerization of Polycaprolactone-Graft-Polyacrylic Acid Nanofibers and Its Composite Nanofiber Dressings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
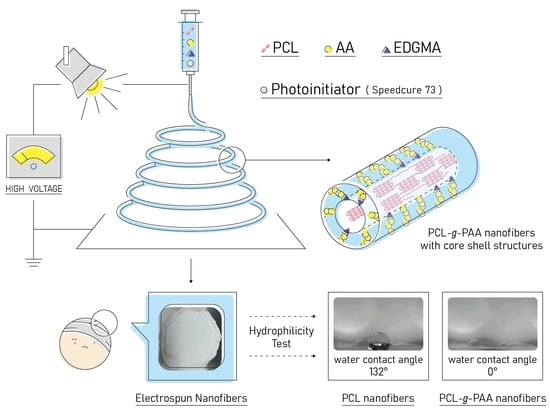
2.2. Preparation of the Electrospinning Solution
2.3. Electrospinning Process
2.4. Determination of the Degree of Grafting
2.5. Contact Angle Measurement
2.6. Morphology and Characterization of Nanofibers
2.7. Cell Viability
2.8. Antimicrobial Ability Test
2.9. Biodegradation
3. Results and Discussion
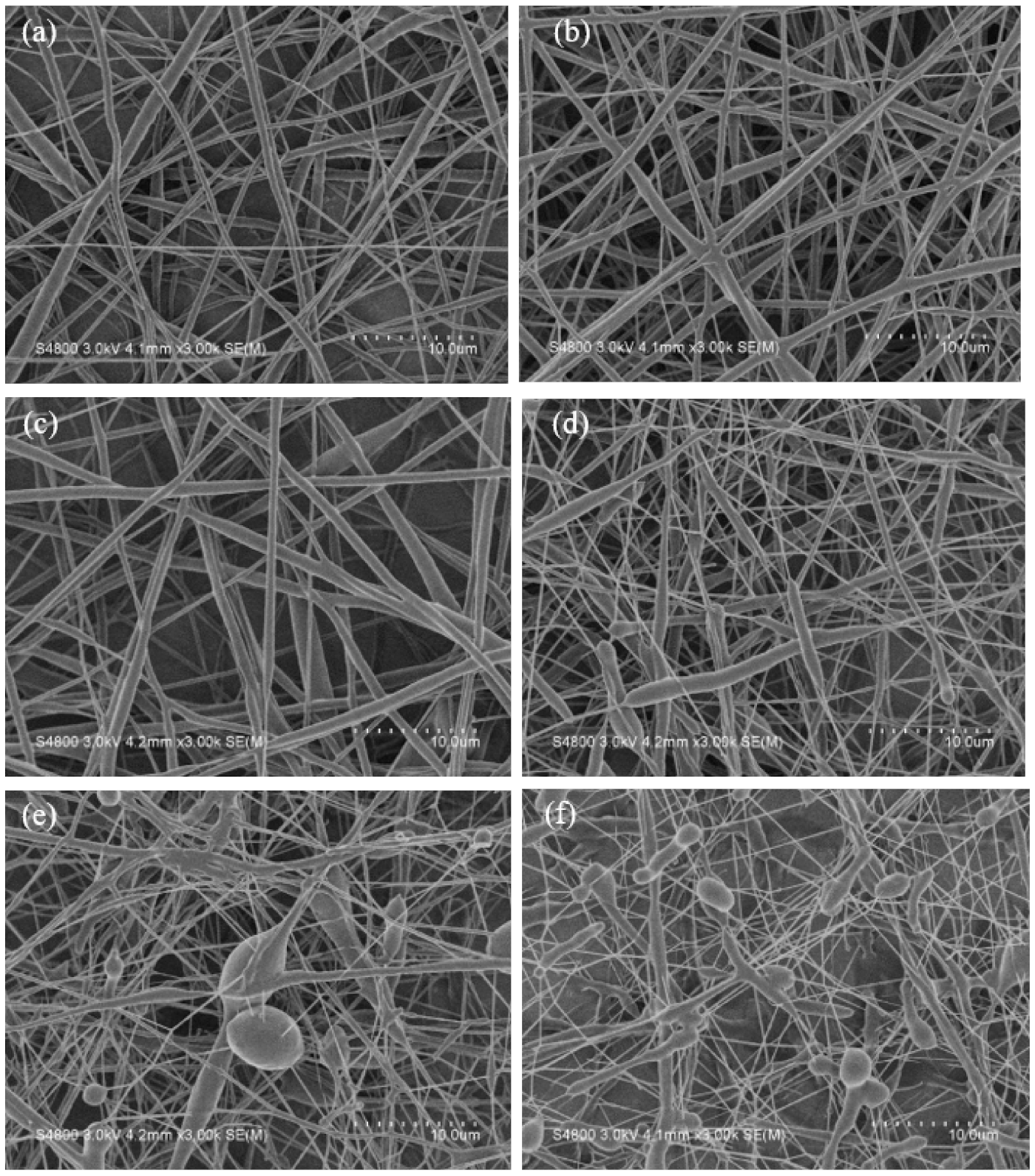
3.1. Electrospun Nanofibers with the Mixture of PCL and AA
3.2. Electrospun Nanofibers with PCL Grafted with PAA
3.3. Biodegradation of Electrospun PCL and PCL-g-PAA Nanofibers
3.4. Filled PCL and PCL-g-PAA Nanofibers with GO-g-PTA Nanosheets
3.5. Antimicrobial Ability and Cell Viability Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramos-Gallardo, G. Chronic Wounds in Burn Injury: A Case Report on Importance of Biofilms. World J. Plast. Surg. 2016, 5, 175–180. [Google Scholar] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; De Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.P.D.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-Based Strategies to Advance Therapies for Chronic Skin Wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Ge, J.; Wang, M.; Chen, M.; Niu, W.; Cheng, W.; Xue, Y.; Lin, C.; Lei, B. Bioactive Anti-inflammatory, Antibacterial, Antioxidative Silicon-Based Nanofibrous Dressing Enables Cutaneous Tumor Photothermo-Chemo Therapy and Infection-Induced Wound Healing. ACS Nano 2020, 14, 2904–2916. [Google Scholar] [CrossRef]
- Luo, M.; Wang, M.; Niu, W.; Chen, M.; Cheng, W.; Zhang, L.; Xie, C.; Wang, Y.; Guo, Y.; Leng, T.; et al. Injectable self-healing anti-inflammatory europium oxide-based dressing with high angiogenesis for improving wound healing and skin regeneration. Chem. Eng. J. 2021, 412, 128471. [Google Scholar] [CrossRef]
- Ousey, K.; Cutting, K.F.; Rogers, A.A.; Rippon, M.G. The importance of hydration in wound healing: Reinvigorating the clinical perspective. J. Wound Care 2016, 25, 122–130. [Google Scholar] [CrossRef]
- Lustig, A.; Gefen, A. Three-dimensional shape-conformation performances of wound dressings tested in a robotic sacral pressure ulcer phantom. Int. Wound J. 2021, 18, 670–680. [Google Scholar] [CrossRef]
- Rezk, A.I.; Kim, K.-S.; Kim, C.S. Poly(ε-Caprolactone)/Poly(Glycerol Sebacate) Composite Nanofibers Incorporating Hydroxyapatite Nanoparticles and Simvastatin for Bone Tissue Regeneration and Drug Delivery Applications. Polymers 2020, 12, 2667. [Google Scholar] [CrossRef]
- Bose, S.; Sarkar, N.; Banerjee, D. Effects of PCL, PEG and PLGA polymers on curcumin release from calcium phosphate matrix for in vitro and in vivo bone regeneration. Mater. Today Chem. 2018, 8, 110–120. [Google Scholar] [CrossRef]
- Hassan, A.A.; Radwan, H.A.; Abdelaal, S.A.; Al-Radadi, N.S.; Ahmed, M.K.; Shoueir, K.R.; Hady, M.A. Polycaprolactone based electrospun matrices loaded with Ag/hydroxyapatite as wound dressings: Morphology, cell adhesion, and antibacterial activity. Int. J. Pharm. 2021, 593, 120143. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Agheb, M.; Rafienia, M.; Amini Baghbadorani, M.; Navid, S.; Ebrahimpour, K.; Khodabakhshi, D.; Ghahremani, F. A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold. Sci. Rep. 2020, 10, 3063. [Google Scholar] [CrossRef] [Green Version]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and Properties of Electrospun PCL and Its Composites for Medical Applications: A Mini Review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef] [Green Version]
- Ghaee, A.; Bagheri-Khoulenjani, S.; Amir Afshar, H.; Bogheiri, H. Biomimetic nanocomposite scaffolds based on surface modified PCL-nanofibers containing curcumin embedded in chitosan/gelatin for skin regeneration. Compos. B Eng. 2019, 177, 107339. [Google Scholar] [CrossRef]
- Hosseini, Y.; Emadi, R.; Kharaziha, M. Surface modification of PCL-diopside fibrous membrane via gelatin immobilization for bone tissue engineering. Mater. Chem. Phys. 2017, 194, 356–366. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Massah, F. Improvement of hydrophilicity and cell attachment of polycaprolactone scaffolds using green synthesized carbon dots. Mater. Today Sustain. 2021, 13, 100075. [Google Scholar] [CrossRef]
- Razmshoar, P.; Bahrami, S.H.; Akbari, S. Functional hydrophilic highly biodegradable PCL nanofibers through direct aminolysis of PAMAM dendrimer. Int. J. Polym. Mater. 2020, 69, 1069–1080. [Google Scholar] [CrossRef]
- Ekram, B.; Abd El-Hady, B.M.; El-Kady, A.M.; Amr, S.M.; Gabr, H.; Waly, A.I.; Guirguis, O.W. Enhancing the Stability, Hydrophilicity, Mechanical and Biological Properties of Electrospun Polycaprolactone in Formic Acid/Acetic Acid Solvent System. Fibers Polym. 2019, 20, 715–724. [Google Scholar] [CrossRef]
- Park, K.; Ju, Y.M.; Son, J.S.; Ahn, K.-D.; Han, D.K. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. J. Biomater. Sci. Polym. Ed. 2007, 18, 369–382. [Google Scholar] [CrossRef]
- Oner, A.; Tufek, E.; Yezer, I.; Birol, A.; Demir, M.; Er, S.; Demirkol, D.O. High generation dendrimer decorated poly-Ɛ-caprolactone/polyacrylic acid electrospun nanofibers for the design of a bioelectrochemical sensing surface. React. Funct. Polym. 2021, 161, 104853. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; da Silva, L.P.; Pirraco, R.P.; Ma, M.; Yang, L.; Reis, R.L.; Chen, J. A thermo-/pH-responsive hydrogel (PNIPAM-PDMA-PAA) with diverse nanostructures and gel behaviors as a general drug carrier for drug release. Polym. Chem. 2018, 9, 4063–4072. [Google Scholar] [CrossRef]
- Gao, X.; He, C.; Xiao, C.; Zhuang, X.; Chen, X. Biodegradable pH-responsive polyacrylic acid derivative hydrogels with tunable swelling behavior for oral delivery of insulin. Polymer 2013, 54, 1786–1793. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Tai, Z.; Yang, J.; Qi, Y.; Yan, X.; Xue, Q. Synthesis of a graphene oxide–polyacrylic acid nanocomposite hydrogel and its swelling and electroresponsive properties. RSC Adv. 2013, 3, 12751–12757. [Google Scholar] [CrossRef]
- Zahid, M.; Lodhi, M.; Afzal, A.; Rehan, Z.A.; Mehmood, M.; Javed, T.; Shabbir, R.; Siuta, D.; Althobaiti, F.; Dessok, E.S. Development of Hydrogels with the Incorporation of Raphanus sativus L. Seed Extract in Sodium Alginate for Wound-Healing Application. Gels 2021, 7, 107. [Google Scholar] [CrossRef]
- Chuah, C.; Wang, J.; Tavakoli, J.; Tang, Y. Novel Bacterial Cellulose-Poly (Acrylic Acid) Hybrid Hydrogels with Controllable Antimicrobial Ability as Dressings for Chronic Wounds. Polymers 2018, 10, 1323. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Qiu, J.; Lu, C.; Jin, S.; Zhang, G.; Sakai, E. Multi-Sacrificial Bonds Enhanced Double Network Hydrogel with High Toughness, Resilience, Damping, and Notch-Insensitivity. Polymers 2020, 12, 2263. [Google Scholar] [CrossRef]
- Qian, W.; Song, T.; Ye, M.; Xu, P.; Lu, G.; Huang, X. PAA-g-PLA amphiphilic graft copolymer: Synthesis, self-assembly, and drug loading ability. Polym. Chem. 2017, 8, 4098–4107. [Google Scholar] [CrossRef]
- Schmolke, H.; Hartwig, S.; Klages, C.-P. Poly(acrylic acid)-graft-poly(ethylene glycol) preparation and adsorption on polyelectrolyte multilayers (PEMs) for custom-made antiadhesive surfaces. Phys. Status Solidi A 2011, 208, 1290–1300. [Google Scholar] [CrossRef]
- Guo, X.; Weiss, A.; Ballauff, M. Synthesis of Spherical Polyelectrolyte Brushes by Photoemulsion Polymerization. Macromolecules 1999, 32, 6043–6046. [Google Scholar] [CrossRef]
- Kim, C.H.; Cho, K.; Park, J.-K. Effect of poly(acrylic acid)-g-PCL microstructure on the mechanical properties of starch/PCL blend compatibilized with poly(acrylic acid)-g-PCL. Poly. Eng. Sci. 2001, 41, 542–553. [Google Scholar] [CrossRef]
- Song, X.; Yao, W.; Lu, G.; Li, Y.; Huang, X. TBHBMA: A novel trifunctional acrylic monomer for the convenient synthesis of PAA-g-PCL well-defined amphiphilic graft copolymer. Polym. Chem. 2013, 4, 2864–2875. [Google Scholar] [CrossRef]
- Ata, S.; Basak, S.; Mal, D.; Singha, N.K. Synthesis and self-assembly behavior of POSS tethered amphiphilic polymer based on poly(caprolactone) (PCL) grafted with poly(acrylic acid) (PAA) via ROP, ATRP, and CuAAC reaction. J. Polym. Res. 2017, 24, 1–13. [Google Scholar] [CrossRef]
- Filippi, S.; Yordanov, H.; Minkova, L.; Polacco, G.; Talarico, M. Reactive Compatibilizer Precursors for LDPE/PA6 Blends, 4. Macromol. Mater. Eng. 2004, 289, 512–523. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Mudassir, J.; Majeed, S. Synthesis and characterization of polycaprolactone/acrylic acid (PCL/AA) hydrogel for controlled drug delivery. Bull. Mater. Sci. 2011, 34, 1537–1547. [Google Scholar] [CrossRef]
- Memic, A.; Abudula, T.; Mohammed, H.S.; Joshi Navare, K.; Colombani, T.; Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl. Bio Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.-L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for Controlled and Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Li, T.; Zhao, H.; Li, X.; Gao, C.; Zhang, S.; Xie, E. Graphene-based composite materials beneficial to wound healing. Nanoscale 2012, 4, 2978–2982. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Fan, Z.; Po, K.H.L.; Wong, K.K.; Chen, S.; Lau, S.P. Polyethylenimine-Modified Graphene Oxide as a Novel Antibacterial Agent and Its Synergistic Effect with Daptomycin for Methicillin-Resistant Staphylococcus aureus. ACS Appl. Nano Mater. 2018, 1, 1811–1818. [Google Scholar] [CrossRef]
- Huang, C.-L.; Lee, K.-M.; Liu, Z.-X.; Lai, R.-Y.; Chen, C.-K.; Chen, W.-C.; Hsu, J.-F. Antimicrobial Activity of Electrospun Polyvinyl Alcohol Nanofibers Filled with Poly[2-(tert-butylaminoethyl) Methacrylate]-Grafted Graphene Oxide Nanosheets. Polymers 2020, 12, 1449. [Google Scholar] [CrossRef] [PubMed]
- Pal, J.; Skrifvars, M.; Nandan, B.; Srivastava, R.K. Electrospun composite matrices from tenside-free poly(ε-caprolactone)-grafted acrylic acid/hydroxyapatite oil-in-water emulsions. J. Mater. Sci. 2017, 52, 2254–2262. [Google Scholar] [CrossRef]
- Sasmal, A.; Sahoo, D.; Nanda, R.; Nayak, P.; Nayak, P.; Mishra, J.; Chang, Y.; Yoon, J.-Y. Biodegradable Nanocomposites from Maleated Polycaprolactone/Soy Protein Isolate Blend With Organoclay: Preparation, Characterization, and Properties. Polym. Compos. 2009, 30, 708–714. [Google Scholar] [CrossRef]
- Gupta, B.; Krishnanand, K.; Deopura, B.L. Oxygen plasma-induced graft polymerization of acrylic acid on polycaprolactone monofilament. Eur. Polym. J. 2012, 48, 1940–1948. [Google Scholar] [CrossRef]
- Chen, W.-C.; Ko, C.-L.; Yang, J.-K.; Wu, H.-Y.; Lin, J.-H. Comparison and preparation of multilayered polylactic acid fabric strengthen calcium phosphate-based bone substitutes for orthopedic applications. J. Artif. Organs 2016, 19, 70–79. [Google Scholar] [CrossRef]
- Chen, C.-K.; Lee, M.-C.; Lin, Z.-I.; Lee, C.-A.; Tung, Y.-C.; Lou, C.-W.; Law, W.-C.; Chen, N.-T.; Lin, K.-Y.A.; Lin, J.-H. Intensifying the Antimicrobial Activity of Poly[2-(tert-butylamino)ethyl Methacrylate]/Polylactide Composites by Tailoring Their Chemical and Physical Structures. Mol. Pharm. 2019, 16, 709–723. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Bhattarai, N.; Yi, H.K.; Hwang, P.H.; Cha, D.I.; Kim, H.Y. Novel biodegradable electrospun membrane: Scaffold for tissue engineering. Biomaterials 2004, 25, 2595–2602. [Google Scholar] [CrossRef]
- Wang, C.; Hsu, C.-H.; Lin, J.-H. Scaling Laws in Electrospinning of Polystyrene Solutions. Macromolecules 2006, 39, 7662–7672. [Google Scholar] [CrossRef]
- De Freitas, A.G.O.; Muraro, P.I.R.; Bortolotto, T.; Trindade, S.G.; Schmidt, V.; Lopes, L.Q.S.; Ninago, M.; Satti, A.; Ciolino, A.; Villar, M.; et al. Facile one-pot synthesis and solution behavior of poly(acrylic acid)-block-polycaprolactone copolymers. J. Mol. Liq. 2019, 273, 99–106. [Google Scholar] [CrossRef]
- Sisson, A.L.; Ekinci, D.; Lendlein, A. The contemporary role of ε-caprolactone chemistry to create advanced polymer architectures. Polymer 2013, 54, 4333–4350. [Google Scholar] [CrossRef] [Green Version]
- Crescenzi, V.; Manzini, G.; Calzolari, G.; Borri, C. Thermodynamics of fusion of poly-β-propiolactone and poly-ϵ-caprolactone. comparative analysis of the melting of aliphatic polylactone and polyester chains. Eur. Polym. J. 1972, 8, 449–463. [Google Scholar] [CrossRef]
- Xie, W.; Xu, P.; Wang, W.; Liu, Q. Preparation and antibacterial activity of a water-soluble chitosan derivative. Carbohydr. Polym. 2002, 50, 35–40. [Google Scholar] [CrossRef]










| Sample Code | PCL (wt%) | AA (wt%) | Photoinitiator (wt%) | EDGMA (wt%) | df (nm) | Water Contact Angle (°) |
|---|---|---|---|---|---|---|
| PCL/AA 100/0 | 100 | 0 | - | - | 780 ± 200 | 132 |
| PCL/AA 90/10 | 90 | 10 | - | - | 566 ± 261 | 0 |
| PCL/AA 80/20 | 80 | 20 | - | - | 557 ± 271 | 0 |
| PCL/AA 70/30 | 70 | 30 | - | - | 497 ± 361 | 0 |
| PCL/AA 60/40 | 60 | 40 | - | - | 363 ± 256 | 0 |
| PCL/AA 50/50 | 50 | 50 | - | - | 330 ± 321 | 0 |
| PCL-g-PAA-i1 | 80 | 20 | 1 | - | 660 ± 301 | 0 |
| PCL-g-PAA-i5 | 80 | 20 | 5 | - | 602 ± 273 | 0 |
| PCL-g-PAA-i10 | 80 | 20 | 10 | - | 591 ± 304 | 0 |
| PCL-g-PAA-i1c1 | 80 | 20 | 1 | 1 | 618 ± 212 | 0 |
| PCL-g-PAA-i1c10 | 80 | 20 | 1 | 10 | 513 ± 203 | 0 |
| Sample Code | PCL (wt%) | PCL-g-PAA-i1c1 (wt%) | GO-g-PTA (wt%) | df (nm) | Water Contact Angle (°) |
|---|---|---|---|---|---|
| PCL/GO-g-PTA 99/1 | 99 | - | 1 | 443 ± 130 | 134 |
| PCL/GO-g-PTA 97/3 | 97 | - | 3 | 414 ± 122 | 131 |
| PCL/GO-g-PTA 95/5 | 95 | - | 5 | 422 ± 102 | 127 |
| PCL/GO-g-PTA 93/7 | 93 | - | 7 | 481 ± 128 | 128 |
| PCL/GO-g-PTA 90/10 | 90 | - | 10 | 438 ± 122 | 126 |
| PCL-g-PAA-i1c1/GO-g-PTA 99/1 | - | 99 | 1 | 499 ± 186 | 0 |
| PCL-g-PAA-i1c1/GO-g-PTA 97/3 | - | 97 | 3 | 353 ± 94 | 0 |
| PCL-g-PAA-i1c1/GO-g-PTA 95/5 | - | 95 | 5 | 520 ± 204 | 0 |
| PCL-g-PAA-i1c1/GO-g-PTA 93/7 | - | 93 | 7 | 490 ± 202 | 0 |
| PCL-g-PAA-i1c1/GO-g-PTA 90/10 | - | 90 | 10 | 431 ± 138 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-J.; Huang, C.-L.; Lai, R.-Y.; Zhuang, C.-H.; Chiu, W.-H.; Lee, K.-M. Microstructure and Biological Properties of Electrospun In Situ Polymerization of Polycaprolactone-Graft-Polyacrylic Acid Nanofibers and Its Composite Nanofiber Dressings. Polymers 2021, 13, 4246. https://doi.org/10.3390/polym13234246
Huang Y-J, Huang C-L, Lai R-Y, Zhuang C-H, Chiu W-H, Lee K-M. Microstructure and Biological Properties of Electrospun In Situ Polymerization of Polycaprolactone-Graft-Polyacrylic Acid Nanofibers and Its Composite Nanofiber Dressings. Polymers. 2021; 13(23):4246. https://doi.org/10.3390/polym13234246
Chicago/Turabian StyleHuang, Yi-Jen, Chien-Lin Huang, Ruo-Yu Lai, Cheng-Han Zhuang, Wei-Hao Chiu, and Kun-Mu Lee. 2021. "Microstructure and Biological Properties of Electrospun In Situ Polymerization of Polycaprolactone-Graft-Polyacrylic Acid Nanofibers and Its Composite Nanofiber Dressings" Polymers 13, no. 23: 4246. https://doi.org/10.3390/polym13234246
APA StyleHuang, Y.-J., Huang, C.-L., Lai, R.-Y., Zhuang, C.-H., Chiu, W.-H., & Lee, K.-M. (2021). Microstructure and Biological Properties of Electrospun In Situ Polymerization of Polycaprolactone-Graft-Polyacrylic Acid Nanofibers and Its Composite Nanofiber Dressings. Polymers, 13(23), 4246. https://doi.org/10.3390/polym13234246