Advanced Hydrogels for Cartilage Tissue Engineering: Recent Progress and Future Directions
Abstract
:1. Introduction
2. Cartilage Structure
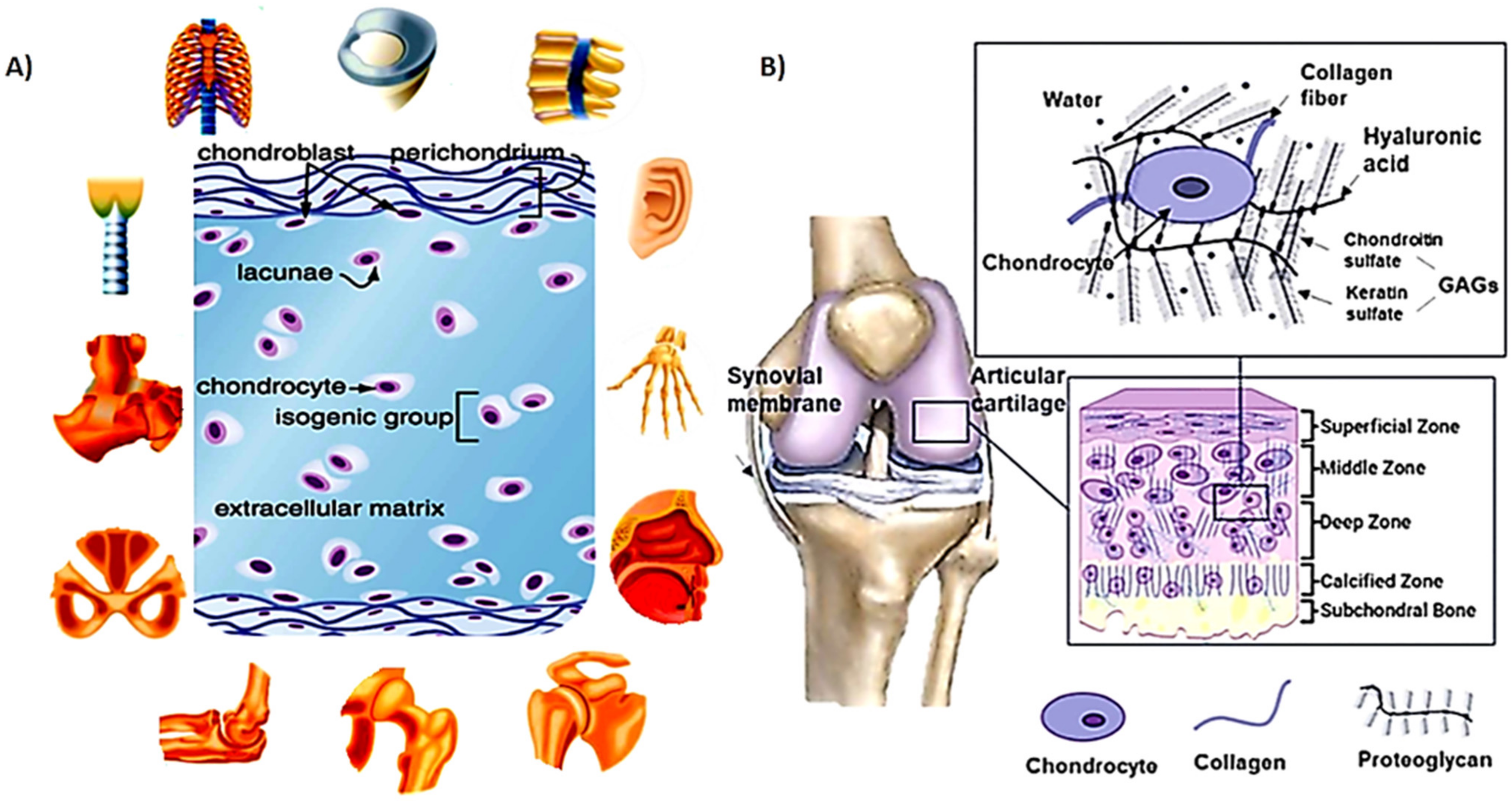
2.1. Mechanical Properties of Cartilage
2.2. Damage and Treatment of Cartilage
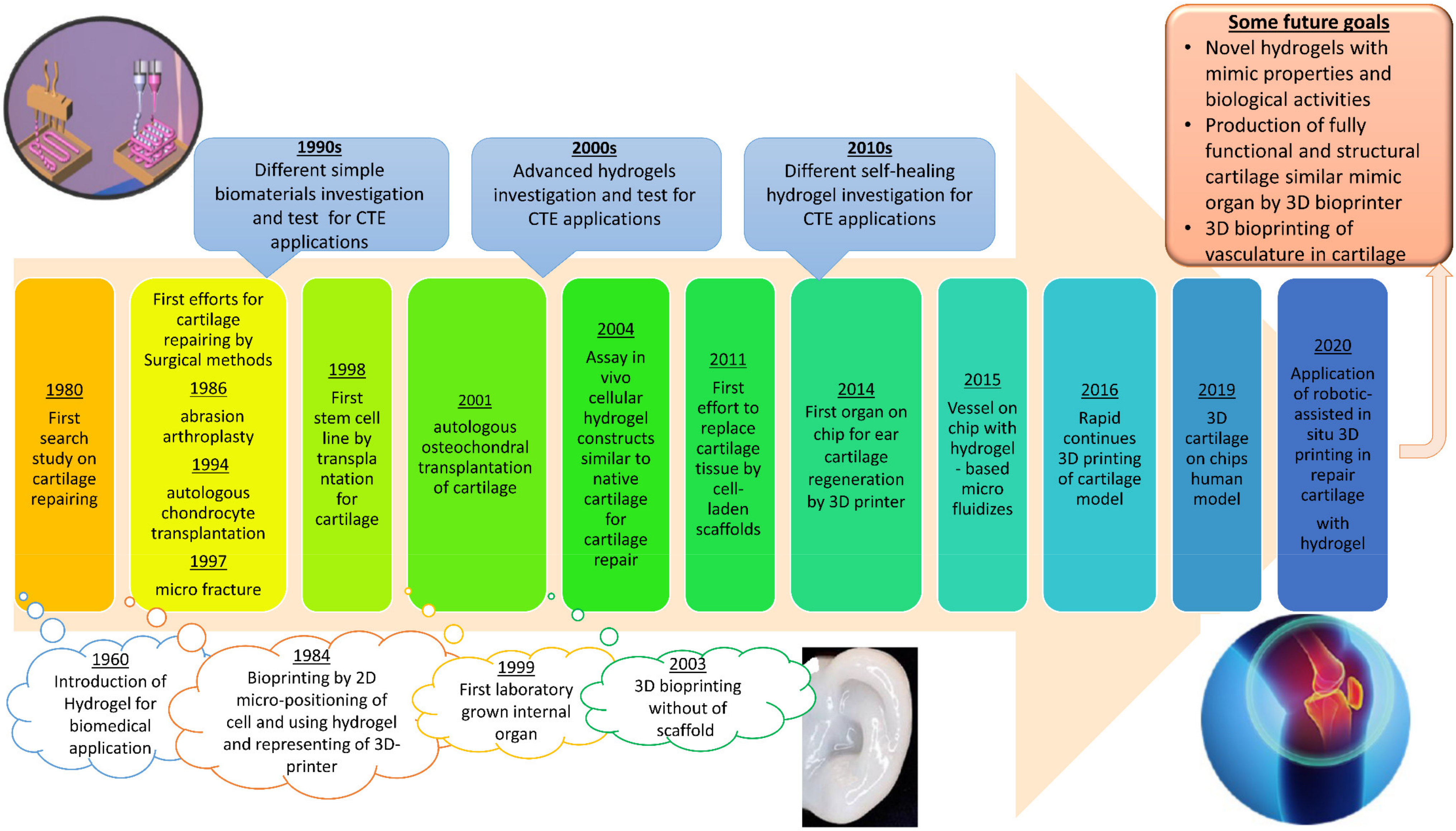
3. Cartilage Tissue Engineering
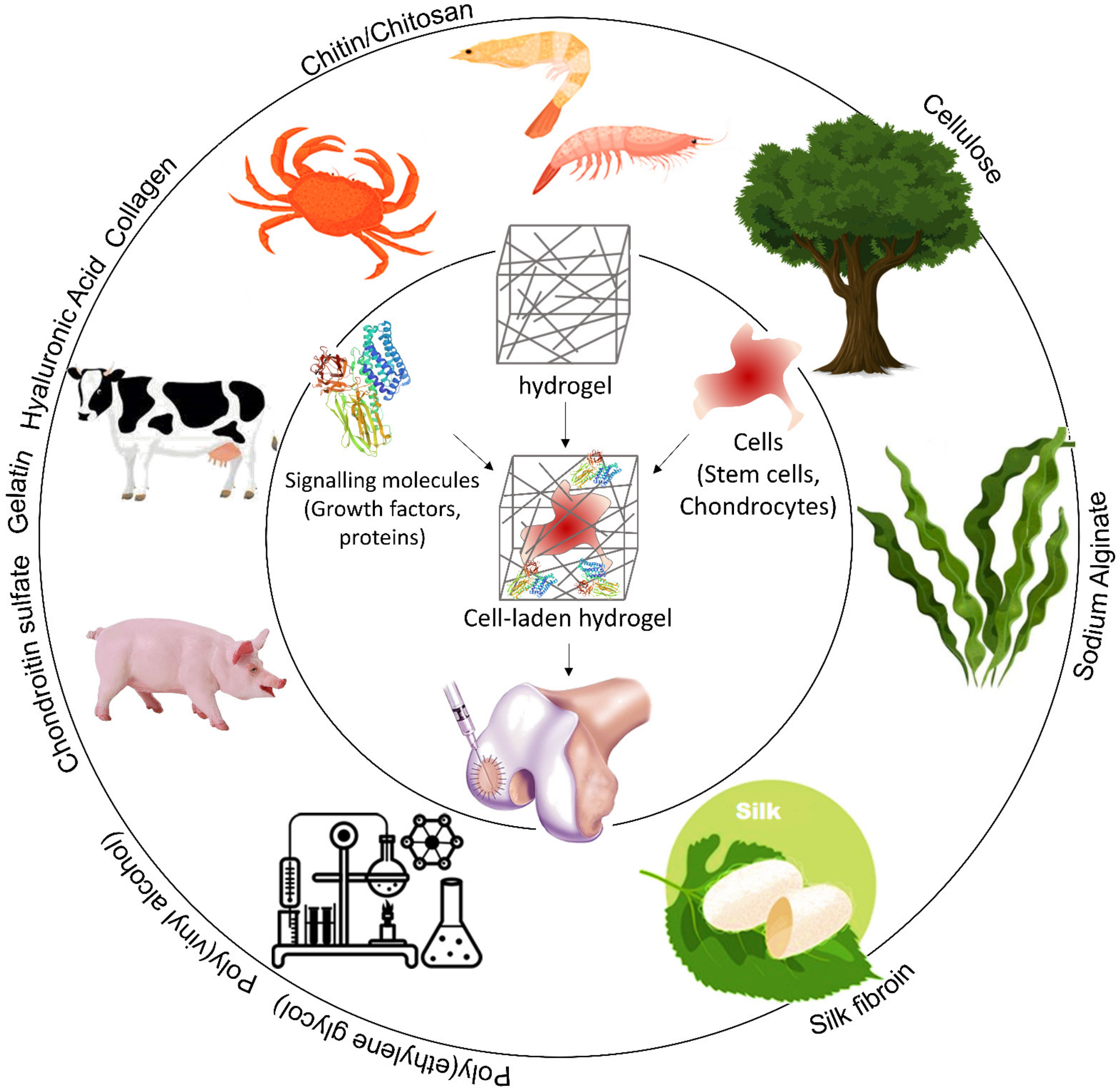
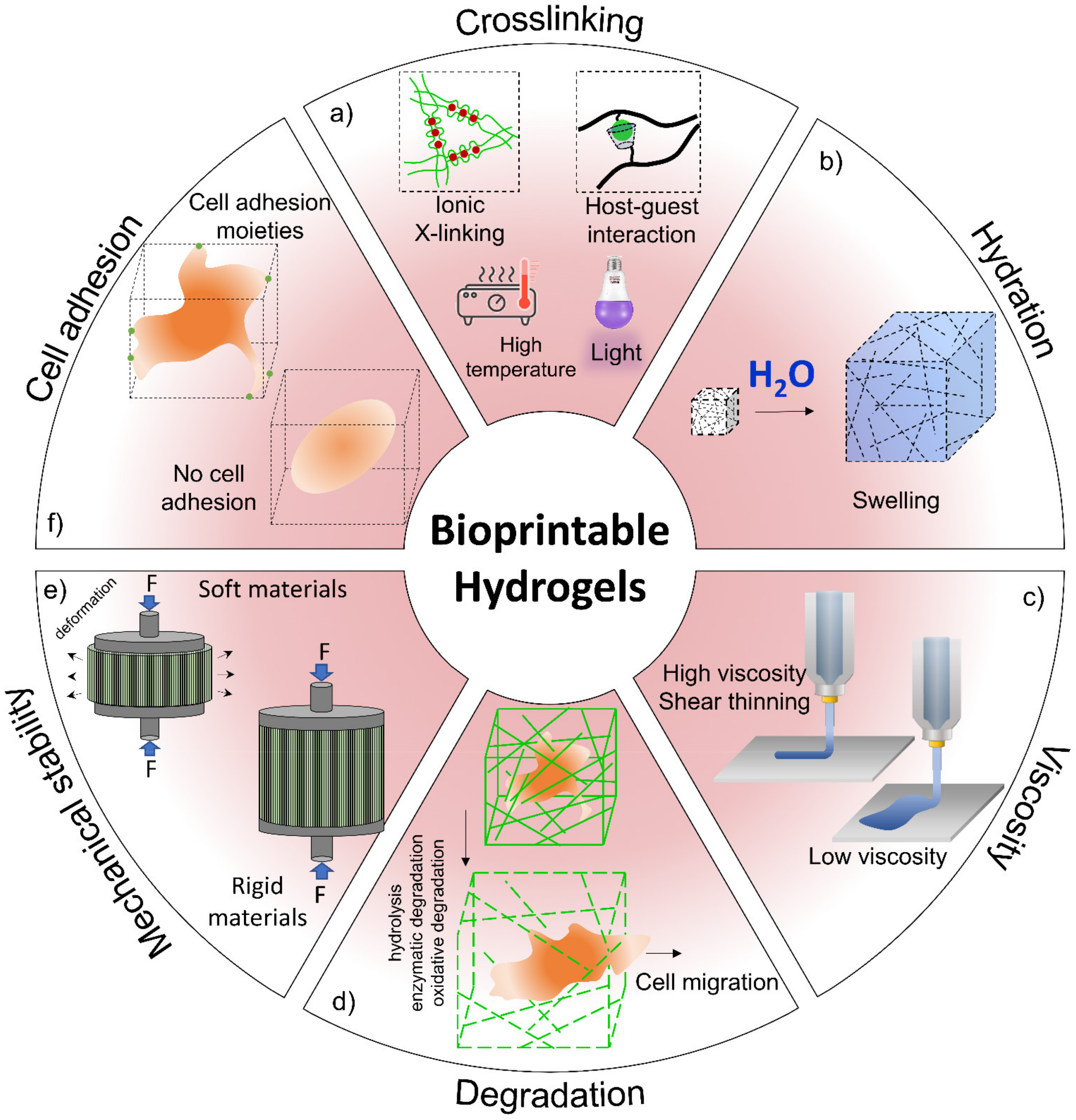
3.1. Hydrogels for Cartilage Healing
3.2. Advanced Hydrogel for Cartilage Tissue Engineering
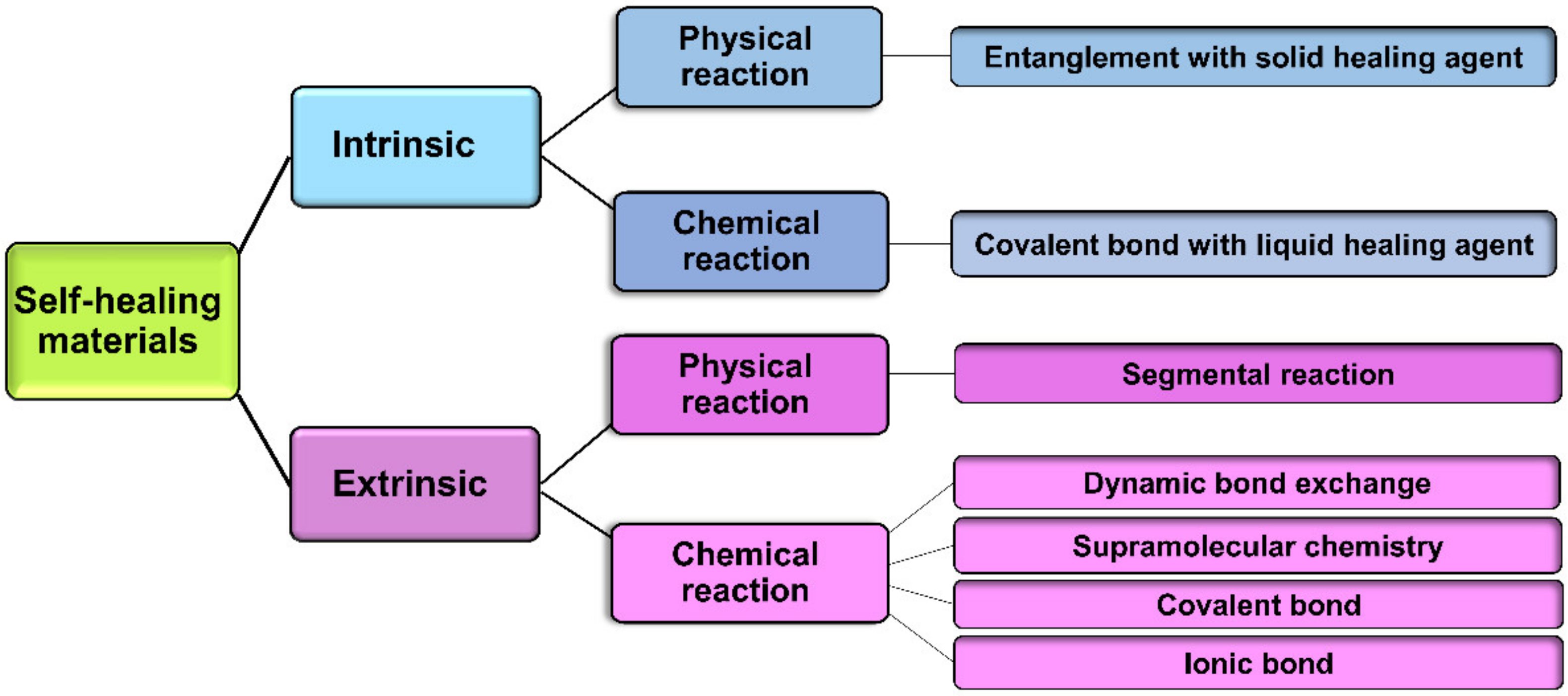
4. Self-Healing Hydrogel in Cartilage Tissue Engineering
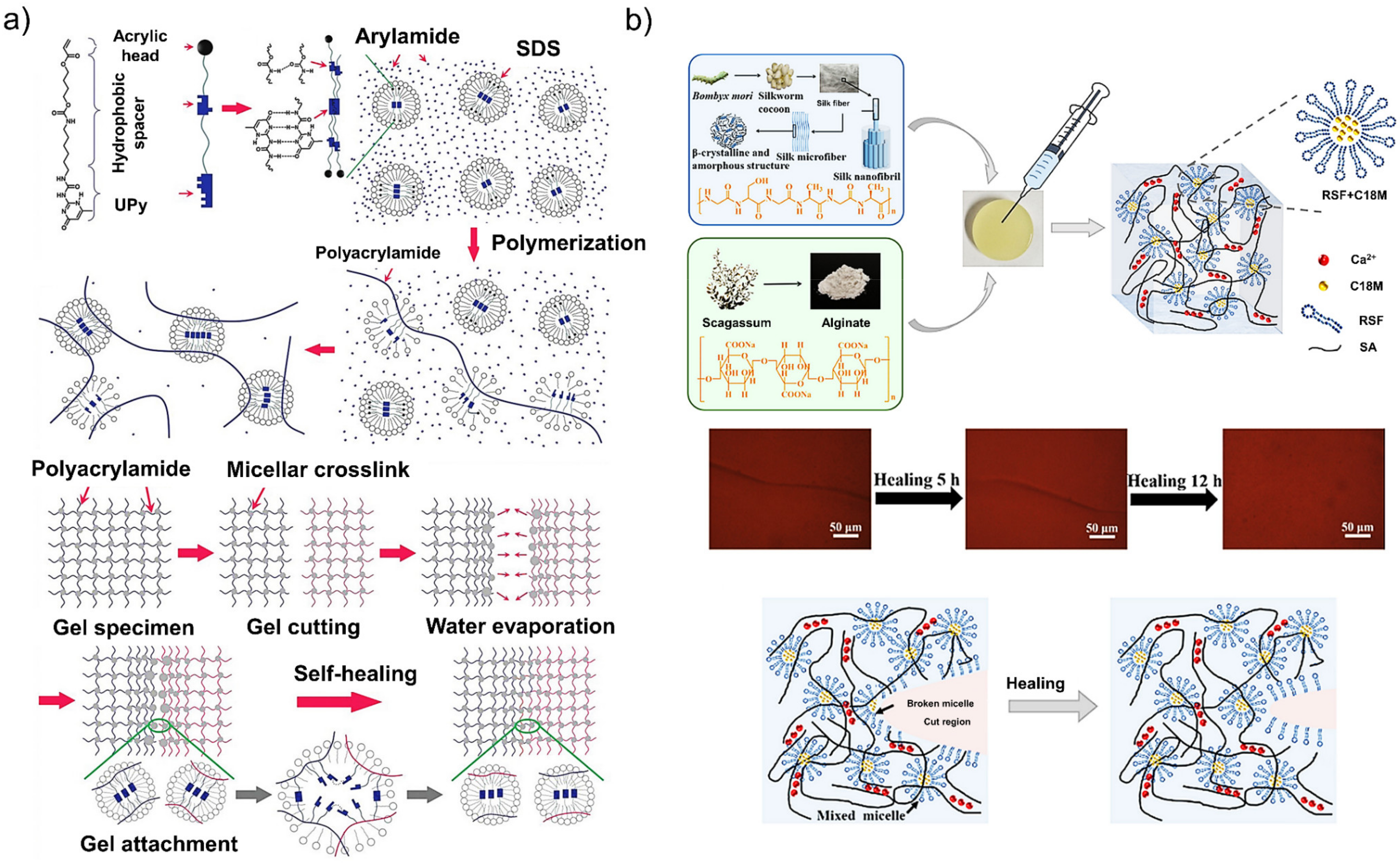
4.1. Materials
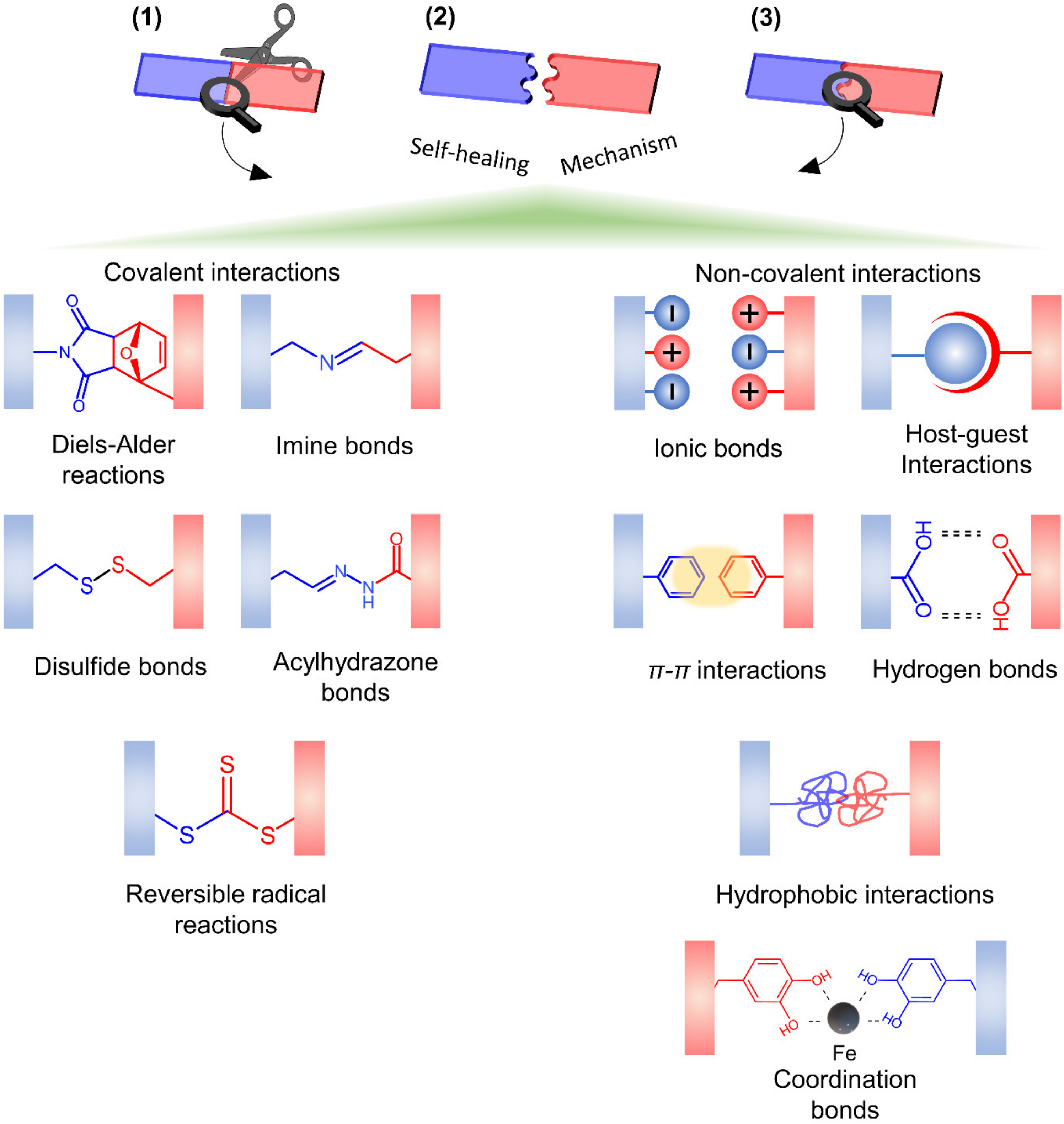
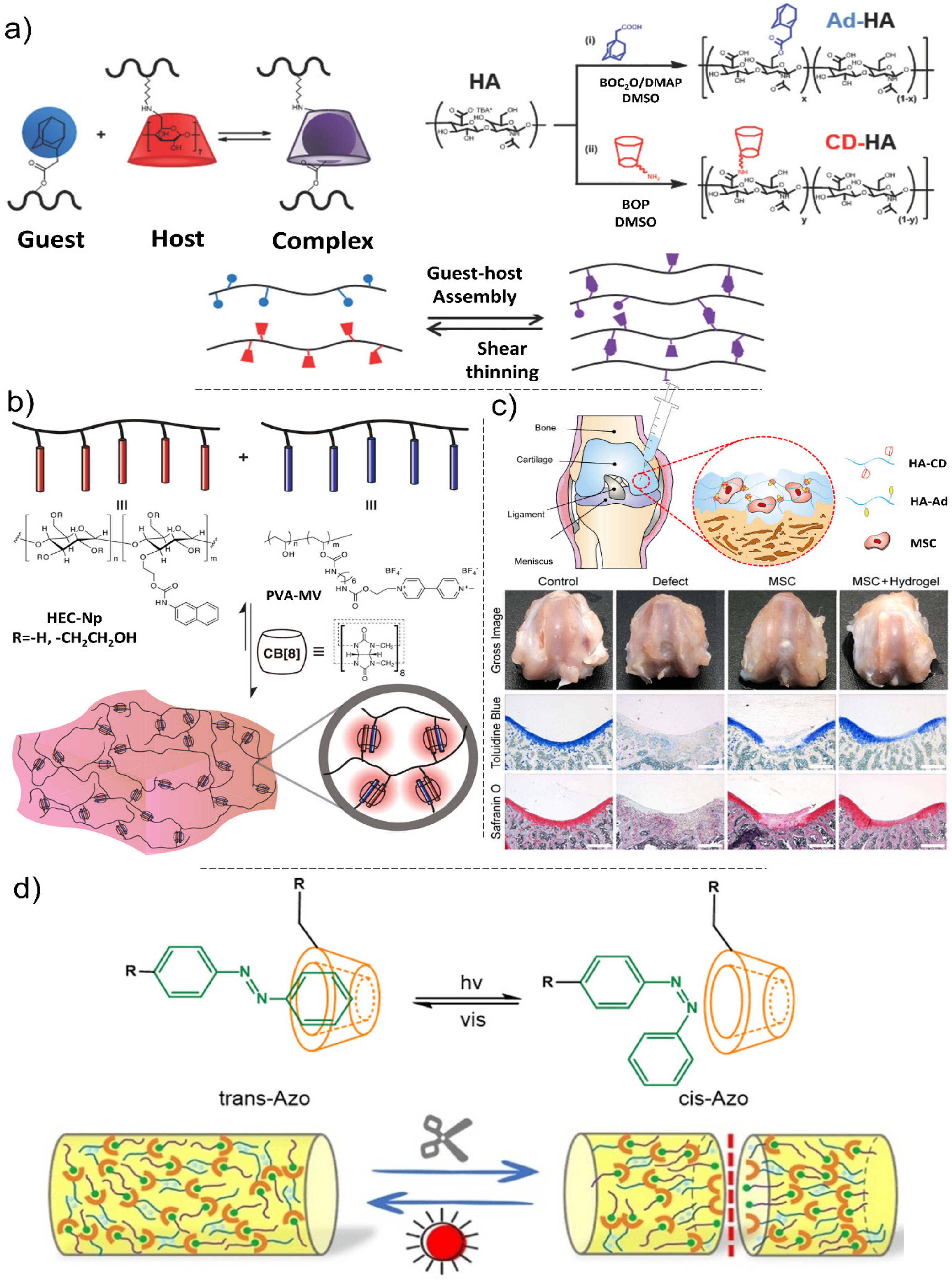
4.2. Mechanisms of Self-Healing
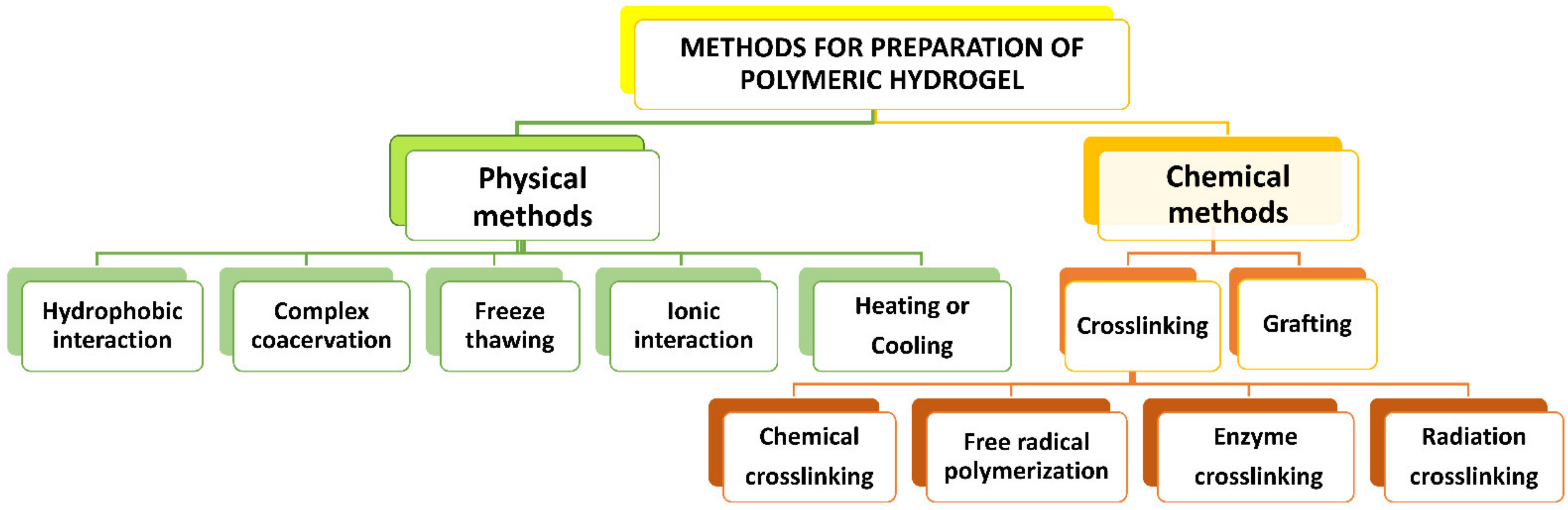
5. Fabrication Methods
6. Conclusions and Perspective Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71, 51–69. [Google Scholar] [CrossRef]
- Killen, M.-C.; Charalambous, C.P. Advances in cartilage restoration techniques. In Advances in Medical and Surgical Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 71–83. [Google Scholar]
- Ngadimin, K.D.; Stokes, A.; Gentile, P.; Ferreira, A.M. Biomimetic hydrogels designed for cartilage tissue engineering. Biomater. Sci. 2021, 9, 4246–4259. [Google Scholar] [CrossRef]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact. Mater. 2021, 6, 998–1011. [Google Scholar] [CrossRef]
- Lin, H.; Yin, C.; Mo, A.; Hong, G. Applications of Hydrogel with Special Physical Properties in Bone and Cartilage Regeneration. Materials 2021, 14, 235. [Google Scholar] [CrossRef]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G. Self-healing hydrogels: The next paradigm shift in tissue engineering. Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [Green Version]
- Ghomi, E.R.; Neisiany, R.E.; Khorasani, S.N.; Dinari, M.; Ataei, S.; Koochaki, M.S.; Ramakrishna, S. Development of an epoxy self-healing coating through the incorporation of acrylic acid-co-acrylamide copolymeric gel. Prog. Org. Coat. 2020, 149, 105948. [Google Scholar] [CrossRef]
- Panahi, P.; Khorasani, S.N.; Koochaki, M.S.; Dinari, M.; Das, O.; Neisiany, R.E. Synthesis of Cloisite 30B-acrylamide/acrylic acid nanogel composite for self-healing purposes. Appl. Clay Sci. 2021, 210, 106174. [Google Scholar] [CrossRef]
- Wang, Y.; Adokoh, C.K.; Narain, R. Recent development and biomedical applications of self-healing hydrogels. Expert Opin. Drug Deliv. 2018, 15, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Esmaeely Neisiany, R.; Enayati, M.S.; Sajkiewicz, P.; Pahlevanneshan, Z.; Ramakrishna, S. Insight Into the Current Directions in Functionalized Nanocomposite Hydrogels. Front. Mater. 2020, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Wang, X.; Park, S.; Jin, X.; Ma, P.X. Rapid self-integrating, injectable hydrogel for tissue complex regeneration. Adv. Healthc. Mater. 2015, 4, 1491–1495. [Google Scholar] [CrossRef]
- Taylor, D.L.; in het Panhuis, M. Self-healing hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Yang, M. Application of hydrogels in cartilage tissue engineering. Curr. Stem Cell Res. Ther. 2018, 13, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cells Mater. 2017, 33, 59. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A.; de la Guardia, M. Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Biotechnol. J. 2020, 15, 2000095. [Google Scholar] [CrossRef]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F. Agarose-based biomaterials: Opportunities and challenges in cartilage tissue engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef]
- Little, C.J.; Bawolin, N.K.; Chen, X. Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng. Part B: Rev. 2011, 17, 213–227. [Google Scholar] [CrossRef]
- Lin, W.; Klein, J. Recent progress in cartilage lubrication. Adv. Mater. 2021, 33, 2005513. [Google Scholar] [CrossRef]
- Jahn, S.; Seror, J.; Klein, J. Lubrication of articular cartilage. Annu. Rev. Biomed. Eng. 2016, 18, 235–258. [Google Scholar] [CrossRef]
- Kuiper, N.; Sharma, A. A detailed quantitative outcome measure of glycosaminoglycans in human articular cartilage for cell therapy and tissue engineering strategies. Osteoarthr. Cartil. 2015, 23, 2233–2241. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yu, J.; Ren, K.; Zuo, J.; Ding, J.; Chen, X. Thermosensitive hydrogels as scaffolds for cartilage tissue engineering. Biomacromolecules 2019, 20, 1478–1492. [Google Scholar] [CrossRef]
- Cutcliffe, H.C.; DeFrate, L.E. Comparison of cartilage mechanical properties measured during creep and recovery. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oinas, J.; Ronkainen, A.; Rieppo, L.; Finnilä, M.; Iivarinen, J.; van Weeren, P.; Helminen, H.; Brama, P.; Korhonen, R.; Saarakkala, S. Composition, structure and tensile biomechanical properties of equine articular cartilage during growth and maturation. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Peters, A.E.; Akhtar, R.; Comerford, E.J.; Bates, K.T. The effect of ageing and osteoarthritis on the mechanical properties of cartilage and bone in the human knee joint. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Bos, E.J.; Pluemeekers, M.; Helder, M.; Kuzmin, N.; van der Laan, K.; Groot, M.-L.; van Osch, G.; van Zuijlen, P. Structural and mechanical comparison of human ear, alar, and septal cartilage. Plast. Reconstr. Surg. Glob. Open 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Giretová, M.; Medvecký, Ľ.; Petrovová, E.; Čížková, D.; Mudroňová, D.; Danko, J. Effects of cell seeding methods on chondrogenic differentiation of rat mesenchymal stem cells in polyhydroxybutyrate/chitosan scaffolds. FOLIA 2019, 63, 6–16. [Google Scholar]
- Frisch, J.; Venkatesan, J.K.; Rey-Rico, A.; Madry, H.; Cucchiarini, M. Current progress in stem cell-based gene therapy for articular cartilage repair. Curr. Stem Cell Res. Ther. 2015, 10, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Link, J.M.; Salinas, E.Y.; Hu, J.C.; Athanasiou, K.A. The tribology of cartilage: Mechanisms, experimental techniques, and relevance to translational tissue engineering. Clin. Biomech. 2020, 79, 104880. [Google Scholar] [CrossRef]
- Liao, J.; Shi, K.; Ding, Q.; Qu, Y.; Luo, F.; Qian, Z. Recent developments in scaffold-guided cartilage tissue regeneration. J. Biomed. Nanotechnol. 2014, 10, 3085–3104. [Google Scholar] [CrossRef]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular cartilage regeneration in osteoarthritis. Cells 2019, 8, 1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeznach, O.; Kołbuk, D.; Sajkiewicz, P. Injectable hydrogels and nanocomposite hydrogels for cartilage regeneration. J. Biomed. Mater. Res. Part A 2018, 106, 2762–2776. [Google Scholar] [CrossRef]
- Ondresik, M.; Azevedo Maia, F.R.; da Silva Morais, A.; Gertrudes, A.C.; Dias Bacelar, A.H.; Correia, C.; Goncalves, C.; Radhouani, H.; Amandi Sousa, R.; Oliveira, J.M. Management of knee osteoarthritis. Current status and future trends. Biotechnol. Bioeng. 2017, 114, 717–739. [Google Scholar] [CrossRef]
- Kwon, J.S.; Yoon, S.M.; Kwon, D.Y.; Tai, G.Z.; Jin, L.M.; Song, B.; Lee, B.; Kim, J.H.; Han, D.K.; Min, B.H. Injectable in situ-forming hydrogel for cartilage tissue engineering. J. Mater. Chem. B 2013, 1, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.; Yang, P.; Duan, G.; Liu, X.; Gu, Z.; Li, Y. Polyphenol scaffolds in tissue engineering. Mater. Horiz. 2021, 8, 145–167. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef]
- Xiao, Y.; Friis, E.A.; Gehrke, S.H.; Detamore, M.S. Mechanical testing of hydrogels in cartilage tissue engineering: Beyond the compressive modulus. Tissue Eng. Part B: Rev. 2013, 19, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Vyas, C.; Mishbak, H.; Cooper, G.; Peach, C.; Pereira, R.F.; Bartolo, P. Biological perspectives and current biofabrication strategies in osteochondral tissue engineering. Biomanufacturing Rev. 2020, 5, 1–24. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, 434–437. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Sivashanmugam, A.; Kumar, R.A.; Priya, M.V.; Nair, S.V.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.H.; Park, A.; Rim, M.; Youn, J.; Lee, W.; Song, J.E.; Khang, G. Advanced gellan gum-based glycol chitosan hydrogel for cartilage tissue engineering biomaterial. Int. J. Biol. Macromol. 2020, 158, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Sadeghianmaryan, A.; Naghieh, S.; Sardroud, H.A.; Yazdanpanah, Z.; Soltani, Y.A.; Sernaglia, J.; Chen, X. Extrusion-based printing of chitosan scaffolds and their in vitro characterization for cartilage tissue engineering. Int. J. Biol. Macromol. 2020, 164, 3179–3192. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, Y.; Barboiu, M.; Maumus, M.; Noël, D.; Jorgensen, C.; Li, S. Biobased pH-responsive and self-healing hydrogels prepared from O-carboxymethyl chitosan and a 3-dimensional dynamer as cartilage engineering scaffold. Carbohydr. Polym. 2020, 244, 116471. [Google Scholar] [CrossRef]
- Li, H.; Chen, R.; Jia, Z.; Wang, C.; Xu, Y.; Li, C.; Xia, H.; Meng, D. Porous fish collagen for cartilage tissue engineering. Am. J. Transl. Res. 2020, 12, 6107. [Google Scholar]
- Mirtaghavi, A.; Baldwin, A.; Tanideh, N.; Zarei, M.; Muthuraj, R.; Cao, Y.; Zhao, G.; Geng, J.; Jin, H.; Luo, J. Crosslinked porous three-dimensional cellulose nanofibers-gelatine biocomposite scaffolds for tissue regeneration. Int. J. Biol. Macromol. 2020, 164, 1949–1959. [Google Scholar] [CrossRef]
- Nawaz, H.A.; Schröck, K.; Schmid, M.; Krieghoff, J.; Maqsood, I.; Kascholke, C.; Kohn-Polster, C.; Schulz-Siegmund, M.; Hacker, M.C. Injectable oligomer-cross-linked gelatine hydrogels via anhydride–amine-conjugation. J. Mater. Chem. B 2021, 9, 2295–2307. [Google Scholar] [CrossRef]
- Park, H.; Lee, H.J.; An, H.; Lee, K.Y. Alginate hydrogels modified with low molecular weight hyaluronate for cartilage regeneration. Carbohydr. Polym. 2017, 162, 100–107. [Google Scholar] [CrossRef]
- Fenn, S.L.; Oldinski, R.A. Visible light crosslinking of methacrylated hyaluronan hydrogels for injectable tissue repair. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2016, 104, 1229–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef]
- He, C.; Ji, H.; Qian, Y.; Wang, Q.; Liu, X.; Zhao, W.; Zhao, C. Heparin-based and heparin-inspired hydrogels: Size-effect, gelation and biomedical applications. J. Mater. Chem. B 2019, 7, 1186–1208. [Google Scholar] [CrossRef]
- Sim, H.J.; Thambi, T.; Lee, D.S. Heparin-based temperature-sensitive injectable hydrogels for protein delivery. J. Mater. Chem. B 2015, 3, 8892–8901. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Bryant, S.J. The role of chondroitin sulfate in regulating hypertrophy during MSC chondrogenesis in a cartilage mimetic hydrogel under dynamic loading. Biomaterials 2019, 190, 51–62. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Tsang, W.P.; Wan, C.; Wu, C. Fabrication of injectable high strength hydrogel based on 4-arm star PEG for cartilage tissue engineering. Biomaterials 2017, 120, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Hara, M. Clusters of neural stem/progenitor cells cultured on a soft poly (vinyl alcohol) hydrogel crosslinked by gamma irradiation. J. Biosci. Bioeng. 2016, 121, 584–590. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yu, F.; Zheng, L.; Wang, R.; Yan, W.; Wang, Z.; Xu, J.; Wu, J.; Shi, D.; Zhu, L. Natural hydrogels for cartilage regeneration: Modification, preparation and application. J. Orthop. Transl. 2019, 17, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Dehghan-Baniani, D.; Chen, Y.; Wang, D.; Bagheri, R.; Solouk, A.; Wu, H. Injectable in situ forming kartogenin-loaded chitosan hydrogel with tunable rheological properties for cartilage tissue engineering. Colloids Surf. B: Biointerfaces 2020, 192, 111059. [Google Scholar] [CrossRef]
- Kolanthai, E.; Sindu, P.A.; Khajuria, D.K.; Veerla, S.C.; Kuppuswamy, D.; Catalani, L.H.; Mahapatra, D.R. Graphene oxide—a tool for the preparation of chemically crosslinking free alginate–chitosan–collagen scaffolds for bone tissue engineering. ACS Appl. Mater. Interfaces 2018, 10, 12441–12452. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choi, B.; Hu, J.; Lee, M. Injectable chitosan hyaluronic acid hydrogels for cartilage tissue engineering. Acta Biomater. 2013, 9, 4779–4786. [Google Scholar] [CrossRef]
- Kashi, M.; Baghbani, F.; Moztarzadeh, F.; Mobasheri, H.; Kowsari, E. Green synthesis of degradable conductive thermosensitive oligopyrrole/chitosan hydrogel intended for cartilage tissue engineering. Int. J. Biol. Macromol. 2018, 107, 1567–1575. [Google Scholar] [CrossRef]
- Piątkowski, M.; Kitala, D.; Radwan-Pragłowska, J.; Janus, Ł.; Klama-Baryła, A.; Łabuś, W.; Tomanek, E.; Glik, J.; Matýsek, D.; Kawecki, M. Chitosan/aminoacid hydrogels with antimicrobial and bioactive properties as new scaffolds for human mesenchymal stem cells culture applicable in wound healing. Express Polym. Lett. 2018, 12, 100–112. [Google Scholar] [CrossRef]
- Bang, S.; Jung, U.-W.; Noh, I. Synthesis and biocompatibility characterizations of in situ chondroitin sulfate–gelatin hydrogel for tissue engineering. Tissue Eng. Regen. Med. 2018, 15, 25–35. [Google Scholar] [CrossRef]
- Dai, M.; Sui, B.; Hua, Y.; Zhang, Y.; Bao, B.; Lin, Q.; Liu, X.; Zhu, L.; Sun, J. A well defect-suitable and high-strength biomimetic squid type II gelatin hydrogel promoted in situ costal cartilage regeneration via dynamic immunomodulation and direct induction manners. Biomaterials 2020, 240, 119841. [Google Scholar] [CrossRef]
- Fernandez, J.G.; Seetharam, S.; Ding, C.; Feliz, J.; Doherty, E.; Ingber, D.E. Direct bonding of chitosan biomaterials to tissues using transglutaminase for surgical repair or device implantation. Tissue Eng. Part A 2017, 23, 135–142. [Google Scholar] [CrossRef]
- Kontturi, L.-S.; Järvinen, E.; Muhonen, V.; Collin, E.C.; Pandit, A.S.; Kiviranta, I.; Yliperttula, M.; Urtti, A. An injectable, in situ forming type II collagen/hyaluronic acid hydrogel vehicle for chondrocyte delivery in cartilage tissue engineering. Drug Deliv. Transl. Res. 2014, 4, 149–158. [Google Scholar] [CrossRef]
- Zeng, L.; Yao, Y.; Wang, D.-A.; Chen, X. Effect of microcavitary alginate hydrogel with different pore sizes on chondrocyte culture for cartilage tissue engineering. Mater. Sci. Eng. C 2014, 34, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, Q.; Liu, J.; Pei, Y.; Tang, K. A unique high mechanical strength dialdehyde microfibrillated cellulose/gelatin composite hydrogel with a giant network structure. RSC Adv. 2016, 6, 71999–72007. [Google Scholar] [CrossRef]
- Heo, J.; Koh, R.H.; Shim, W.; Kim, H.D.; Yim, H.-G.; Hwang, N.S. Riboflavin-induced photo-crosslinking of collagen hydrogel and its application in meniscus tissue engineering. Drug Deliv. Transl. Res. 2016, 6, 148–158. [Google Scholar] [CrossRef]
- Oh, B.H.; Bismarck, A.; Chan-Park, M.B. Injectable, Interconnected, High-Porosity Macroporous Biocompatible Gelatin Scaffolds Made by Surfactant-Free Emulsion Templating. Macromol. Rapid Commun. 2015, 36, 364–372. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Jayakrishnan, A.; Banerjee, R. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014, 10, 3650–3663. [Google Scholar] [CrossRef] [PubMed]
- Antich, C.; de Vicente, J.; Jiménez, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez-Martín, P.; Marchal, J.A. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Chen, F.; Ni, Y.; Liu, B.; Zhou, T.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y. Self-crosslinking and injectable hyaluronic acid/RGD-functionalized pectin hydrogel for cartilage tissue engineering. Carbohydr. Polym. 2017, 166, 31–44. [Google Scholar] [CrossRef]
- Domingues, R.M.; Silva, M.; Gershovich, P.; Betta, S.; Babo, P.; Caridade, S.G.; Mano, J.O.F.; Motta, A.; Reis, R.L.; Gomes, M.E. Development of injectable hyaluronic acid/cellulose nanocrystals bionanocomposite hydrogels for tissue engineering applications. Bioconjugate Chem. 2015, 26, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Lin, S.; Zhang, K.; Dong, C.; Wu, T.; Huang, H.; Yan, X.; Zhang, L.; Li, G.; Bian, L. Sulfated hyaluronic acid hydrogels with retarded degradation and enhanced growth factor retention promote hMSC chondrogenesis and articular cartilage integrity with reduced hypertrophy. Acta Biomater. 2017, 53, 329–342. [Google Scholar] [CrossRef] [PubMed]
- La Gatta, A.; Ricci, G.; Stellavato, A.; Cammarota, M.; Filosa, R.; Papa, A.; D’Agostino, A.; Portaccio, M.; Delfino, I.; De Rosa, M. Hyaluronan hydrogels with a low degree of modification as scaffolds for cartilage engineering. Int. J. Biol. Macromol. 2017, 103, 978–989. [Google Scholar] [CrossRef]
- Mikael, P.E.; Kim, H.S.; Nukavarapu, S.P. Hybrid extracellular matrix design for cartilage-mediated bone regeneration. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2018, 106, 300–309. [Google Scholar] [CrossRef]
- Palumbo, F.S.; Fiorica, C.; Di Stefano, M.; Pitarresi, G.; Gulino, A.; Agnello, S.; Giammona, G. In situ forming hydrogels of hyaluronic acid and inulin derivatives for cartilage regeneration. Carbohydr. Polym. 2015, 122, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Levinson, C.; Hertl, M.; Broguiere, N.; Brück, O.; Mustjoki, S.; Gerstenberg, A.; Weber, D.; Salzmann, G.; Steinwachs, M. Characterization of polydactyly chondrocytes and their use in cartilage engineering. Sci. Rep. 2019, 9, 1–15. [Google Scholar]
- Broguiere, N.; Cavalli, E.; Salzmann, G.M.; Applegate, L.A.; Zenobi-Wong, M. Factor XIII cross-linked hyaluronan hydrogels for cartilage tissue engineering. ACS Biomater. Sci. Eng. 2016, 2, 2176–2184. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Cao, X.; Li, Y.; Zeng, L.; Zhu, J.; Wang, G.; Chen, X. Diels–Alder crosslinked HA/PEG hydrogels with high elasticity and fatigue resistance for cell encapsulation and articular cartilage tissue repair. Polym. Chem. 2014, 5, 5116–5123. [Google Scholar] [CrossRef]
- Almeida, H.; Eswaramoorthy, R.; Cunniffe, G.; Buckley, C.; O’Brien, F.; Kelly, D. Fibrin hydrogels functionalized with cartilage extracellular matrix and incorporating freshly isolated stromal cells as an injectable for cartilage regeneration. Acta Biomater. 2016, 36, 55–62. [Google Scholar] [CrossRef]
- Benavides, O.M.; Quinn, J.P.; Pok, S.; Petsche Connell, J.; Ruano, R.; Jacot, J.G. Capillary-like network formation by human amniotic fluid-derived stem cells within fibrin/poly (ethylene glycol) hydrogels. Tissue Eng. Part A 2015, 21, 1185–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rial, R.; Liu, Z.; Ruso, J.M. Soft actuated hybrid hydrogel with bioinspired complexity to control mechanical flexure behavior for tissue engineering. Nanomaterials 2020, 10, 1302. [Google Scholar] [CrossRef]
- You, F.; Chen, X.; Cooper, D.; Chang, T.; Eames, B.F. Homogeneous hydroxyapatite/alginate composite hydrogel promotes calcified cartilage matrix deposition with potential for three-dimensional bioprinting. Biofabrication 2018, 11, 015015. [Google Scholar] [CrossRef]
- Critchley, S.; Cunniffe, G.; O’Reilly, A.; Diaz-Payno, P.; Schipani, R.; McAlinden, A.; Withers, D.; Shin, J.; Alsberg, E.; Kelly, D.J. Regeneration of osteochondral defects using developmentally inspired cartilaginous templates. Tissue Eng. Part A 2019, 25, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Wang, H.; Trinh, P.; Heilshorn, S.C.; Yang, F. Elastin-like protein-hyaluronic acid (ELP-HA) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials 2017, 127, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Khorshidi, S.; Karkhaneh, A. A self-crosslinking tri-component hydrogel based on functionalized polysaccharides and gelatin for tissue engineering applications. Mater. Lett. 2016, 164, 468–471. [Google Scholar] [CrossRef]
- Jaikumar, D.; Sajesh, K.; Soumya, S.; Nimal, T.; Chennazhi, K.; Nair, S.V.; Jayakumar, R. Injectable alginate-O-carboxymethyl chitosan/nano fibrin composite hydrogels for adipose tissue engineering. Int. J. Biol. Macromol. 2015, 74, 318–326. [Google Scholar] [CrossRef]
- Fathi, A.; Mithieux, S.M.; Wei, H.; Chrzanowski, W.; Valtchev, P.; Weiss, A.S.; Dehghani, F. Elastin based cell-laden injectable hydrogels with tunable gelation, mechanical and biodegradation properties. Biomaterials 2014, 35, 5425–5435. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, F.; Krüger, M.; De Torre, I.G.; Sierra, L.Q.; Rodrigo, M.A.; Kock, L.; Rodriguez-Cabello, J.C. Cartilage regeneration in preannealed silk elastin-like co-recombinamers injectable hydrogel embedded with mature chondrocytes in an ex vivo culture platform. Biomacromolecules 2018, 19, 4333–4347. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yu, S.; Liu, B.; Ni, Y.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y.; Yan, D. An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci. Rep. 2016, 6, 1–12. [Google Scholar]
- Wiltsey, C.; Kubinski, P.; Christiani, T.; Toomer, K.; Sheehan, J.; Branda, A.; Kadlowec, J.; Iftode, C.; Vernengo, J. Characterization of injectable hydrogels based on poly (N-isopropylacrylamide)-g-chondroitin sulfate with adhesive properties for nucleus pulposus tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 837–847. [Google Scholar] [CrossRef]
- Paul, A.; Manoharan, V.; Krafft, D.; Assmann, A.; Uquillas, J.A.; Shin, S.R.; Hasan, A.; Hussain, M.A.; Memic, A.; Gaharwar, A.K. Nanoengineered biomimetic hydrogels for guiding human stem cell osteogenesis in three dimensional microenvironments. J. Mater. Chem. B 2016, 4, 3544–3554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leppiniemi, J.; Lahtinen, P.; Paajanen, A.; Mahlberg, R.; Metsä-Kortelainen, S.; Pinomaa, T.; Pajari, H.; Vikholm-Lundin, I.; Pursula, P.; Hytönen, V.P. 3D-printable bioactivated nanocellulose–alginate hydrogels. ACS Appl. Mater. Interfaces 2017, 9, 21959–21970. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wang, L.; Yu, X.; Wang, C.; Wang, Z. Synthesis and characterization of a novel double cross-linked hydrogel based on Diels-Alder click reaction and coordination bonding. Mater. Sci. Eng. C 2018, 82, 299–309. [Google Scholar] [CrossRef]
- Mehrali, M.; Thakur, A.; Pennisi, C.P.; Talebian, S.; Arpanaei, A.; Nikkhah, M.; Dolatshahi-Pirouz, A. Nanoreinforced hydrogels for tissue engineering: Biomaterials that are compatible with load-bearing and electroactive tissues. Adv. Mater. 2017, 29, 1603612. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Peters, B.; Burger, T.J.; Boomstra, J.; Dhert, W.J.; Melchels, F.P.; Malda, J. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 2013, 5, 035007. [Google Scholar] [CrossRef]
- Rodell, C.B.; Mealy, J.E.; Burdick, J.A. Supramolecular guest–host interactions for the preparation of biomedical materials. Bioconjugate Chem. 2015, 26, 2279–2289. [Google Scholar] [CrossRef]
- Yang, L.; Tan, X.; Wang, Z.; Zhang, X. Supramolecular polymers: Historical development, preparation, characterization, and functions. Chem. Rev. 2015, 115, 7196–7239. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Zhang, D.; Xu, Z.; Liu, W. A highly tough and stiff supramolecular polymer double network hydrogel. Polymer 2018, 153, 193–200. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Mihajlovic, M.; Dankers, P.Y.; Masereeuw, R.; Sijbesma, R.P. Carbon nanotube reinforced supramolecular hydrogels for bioapplications. Macromol. Biosci. 2019, 19, 1800173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Yang, P.; Zhang, Z.; Wang, L.; Liu, L.; Wang, Y. Self-healing polyurethane nanocomposite films with recoverable surface hydrophobicity. J. Appl. Polym. Sci. 2018, 135, 46421. [Google Scholar] [CrossRef]
- Teng, L.; Chen, Y.; Jia, Y.-G.; Ren, L. Supramolecular and dynamic covalent hydrogel scaffolds: From gelation chemistry to enhanced cell retention and cartilage regeneration. J. Mater. Chem. B 2019, 7, 6705–6736. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, M.; Kim, T.Y.; Kim, H.; Ju, J.H.; Hahn, S.K. Supramolecular Injectable Hyaluronate Hydrogels for Cartilage Tissue Regeneration. ACS Appl. Bio Mater. 2020, 3, 5040–5047. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Xavier, J.R.; Carrow, J.K.; Desai, P.; Alge, D.; Gaharwar, A.K. Mechanically stiff nanocomposite hydrogels at ultralow nanoparticle content. ACS Nano 2016, 10, 246–256. [Google Scholar] [CrossRef]
- Asadi, N.; Alizadeh, E.; Salehi, R.; Khalandi, B.; Davaran, S.; Akbarzadeh, A. Nanocomposite hydrogels for cartilage tissue engineering: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Liu, M.; Zhang, Y.; Yin, J.; Pei, R. Nanocomposite hydrogels for tissue engineering applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef]
- Piluso, S.; Labet, M.; Zhou, C.; Seo, J.W.; Thielemans, W.; Patterson, J. Engineered three-dimensional microenvironments with starch nanocrystals as cell-instructive materials. Biomacromolecules 2019, 20, 3819–3830. [Google Scholar] [CrossRef]
- Asadi, N.; Alizadeh, E.; Rahmani Del Bakhshayesh, A.; Mostafavi, E.; Akbarzadeh, A.; Davaran, S. Fabrication and in vitro evaluation of Nanocomposite hydrogel scaffolds based on gelatin/PCL–PEG–PCL for cartilage tissue engineering. ACS Omega 2019, 4, 449–457. [Google Scholar] [CrossRef]
- Bonhome-Espinosa, A.B.; Campos, F.; Durand-Herrera, D.; Sánchez-López, J.D.; Schaub, S.; Durán, J.D.; Lopez-Lopez, M.T.; Carriel, V. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103619. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liang, Y.; Jia, Z.; Chen, J.; Duan, L.; Liu, W.; Zhu, F.; Liang, Q.; Zhu, W.; You, W. Development of magnetic nanocomposite hydrogel with potential cartilage tissue engineering. ACS Omega 2018, 3, 6182–6189. [Google Scholar] [CrossRef]
- Nejadnik, M.R.; Yang, X.; Bongio, M.; Alghamdi, H.S.; Van den Beucken, J.J.; Huysmans, M.C.; Jansen, J.A.; Hilborn, J.; Ossipov, D.; Leeuwenburgh, S.C. Self-healing hybrid nanocomposites consisting of bisphosphonated hyaluronan and calcium phosphate nanoparticles. Biomaterials 2014, 35, 6918–6929. [Google Scholar] [CrossRef]
- Schlichting, K.E.; Copeland-Johnson, T.M.; Goodman, M.; Lipert, R.J.; Prozorov, T.; Liu, X.; McKinley, T.O.; Lin, Z.; Martin, J.A.; Mallapragada, S.K. Synthesis of a novel photopolymerized nanocomposite hydrogel for treatment of acute mechanical damage to cartilage. Acta Biomater. 2011, 7, 3094–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yu, H.; Yang, H.; Hao, X.; Tang, Q.; Zhang, X. An Injectable Interpenetrating Polymer Network Hydrogel with Tunable Mechanical Properties and Self-Healing Abilities. Macromol. Chem. Phys. 2017, 218, 1700348. [Google Scholar] [CrossRef]
- Hu, M.; Yang, J.; Xu, J. Structural and biological investigation of chitosan/hyaluronic acid with silanized-hydroxypropyl methylcellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Drug Deliv. 2021, 28, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Schipani, R.; Scheurer, S.; Florentin, R.; Critchley, S.E.; Kelly, D.J. Reinforcing interpenetrating network hydrogels with 3D printed polymer networks to engineer cartilage mimetic composites. Biofabrication 2020, 12, 035011. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.R.; Zimmermann, J.; Bader, R.; van Rienen, U. Numerical simulation of electroactive hydrogels for cartilage–tissue engineering. Materials 2019, 12, 2913. [Google Scholar] [CrossRef] [Green Version]
- Davoodi, P.; Lee, L.Y.; Xu, Q.; Sunil, V.; Sun, Y.; Soh, S.; Wang, C.-H. Drug delivery systems for programmed and on-demand release. Adv. Drug Deliv. Rev. 2018, 132, 104–138. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Fallahi, A.; El-Sokkary, A.M.; Salehi, S.; Akl, M.A.; Jafari, A.; Tamayol, A.; Fenniri, H.; Khademhosseini, A.; Andreadis, S.T. Stimuli-responsive hydrogels for manipulation of cell microenvironment: From chemistry to biofabrication technology. Prog. Polym. Sci. 2019, 98, 101147. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Brudno, Y.; Mooney, D.J. On-demand drug delivery from local depots. J. Control. Release 2015, 219, 8–17. [Google Scholar] [CrossRef]
- Gandavarapu, N.R.; Azagarsamy, M.A.; Anseth, K.S. Photo-click living strategy for controlled, reversible exchange of biochemical ligands. Adv. Mater. 2014, 26, 2521–2526. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, J.; Carling, C.-J.; Almutairi, A. Photocontrolled release using one-photon absorption of visible or NIR light. J. Control. Release 2015, 219, 18–30. [Google Scholar] [CrossRef]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef]
- Salehi, M.; Naseri-Nosar, M.; Azami, M.; Nodooshan, S.J.; Arish, J. Comparative study of poly (L-lactic acid) scaffolds coated with chitosan nanoparticles prepared via ultrasonication and ionic gelation techniques. Tissue Eng. Regen. Med. 2016, 13, 498–506. [Google Scholar] [CrossRef]
- Karimi, F.; Collins, J.; Heath, D.E.; Connal, L.A. Dynamic covalent hydrogels for triggered cell capture and release. Bioconjugate Chem. 2017, 28, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bellinger, A.M.; Glettig, D.L.; Barman, R.; Lee, Y.-A.L.; Zhu, J.; Cleveland, C.; Montgomery, V.A.; Gu, L.; Nash, L.D. A pH-responsive supramolecular polymer gel as an enteric elastomer for use in gastric devices. Nat. Mater. 2015, 14, 1065–1071. [Google Scholar] [CrossRef]
- Yang, J.-A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Davoodi, P.; Srinivasan, M.P.; Wang, C.-H. Synthesis of intracellular reduction-sensitive amphiphilic polyethyleneimine and poly (ε-caprolactone) graft copolymer for on-demand release of doxorubicin and p53 plasmid DNA. Acta Biomater. 2016, 39, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Skaalure, S.C.; Chu, S.; Bryant, S.J. An enzyme-sensitive PEG hydrogel based on aggrecan catabolism for cartilage tissue engineering. Adv. Healthc. Mater. 2015, 4, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.J.; Park, M.H.; Joo, M.K.; Jeong, B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem. Soc. Rev. 2012, 41, 4860–4883. [Google Scholar] [CrossRef]
- Ruel-Gariepy, E.; Leroux, J.-C. In situ-forming hydrogels—review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- Sá-Lima, H.; Tuzlakoglu, K.; Mano, J.F.; Reis, R.L. Thermoresponsive poly (N-isopropylacrylamide)-g-methylcellulose hydrogel as a three-dimensional extracellular matrix for cartilage-engineered applications. J. Biomed. Mater. Res. Part A 2011, 98, 596–603. [Google Scholar] [CrossRef]
- Park, K.M.; Lee, S.Y.; Joung, Y.K.; Na, J.S.; Lee, M.C.; Park, K.D. Thermosensitive chitosan–Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009, 5, 1956–1965. [Google Scholar] [CrossRef]
- Abbadessa, A.; Mouser, V.H.; Blokzijl, M.M.; Gawlitta, D.; Dhert, W.J.; Hennink, W.E.; Malda, J.; Vermonden, T. A synthetic thermosensitive hydrogel for cartilage bioprinting and its biofunctionalization with polysaccharides. Biomacromolecules 2016, 17, 2137–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strehin, I.; Nahas, Z.; Arora, K.; Nguyen, T.; Elisseeff, J. A versatile pH sensitive chondroitin sulfate–PEG tissue adhesive and hydrogel. Biomaterials 2010, 31, 2788–2797. [Google Scholar] [CrossRef] [Green Version]
- Halacheva, S.S.; Freemont, T.J.; Saunders, B.R. pH-responsive physical gels from poly (meth) acrylic acid-containing crosslinked particles: The relationship between structure and mechanical properties. J. Mater. Chem. B 2013, 1, 4065–4078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sá-Lima, H.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Stimuli-responsive chitosan-starch injectable hydrogels combined with encapsulated adipose-derived stromal cells for articular cartilage regeneration. Soft Matter 2010, 6, 5184–5195. [Google Scholar] [CrossRef] [Green Version]
- Levett, P.A.; Melchels, F.P.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Giammanco, G.E.; Carrion, B.; Coleman, R.M.; Ostrowski, A.D. Photoresponsive polysaccharide-based hydrogels with tunable mechanical properties for cartilage tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 14423–14429. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.M.; Dijkstra, P.J.; Karperien, M.; Van Blitterswijk, C.; Zhong, Z.; Feijen, J. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogushi, Y.; Sakai, S.; Kawakami, K. Synthesis of enzymatically-gellable carboxymethylcellulose for biomedical applications. J. Biosci. Bioeng. 2007, 104, 30–33. [Google Scholar] [CrossRef]
- Ruiz-Cantu, L.; Gleadall, A.; Faris, C.; Segal, J.; Shakesheff, K.; Yang, J. Multi-material 3D bioprinting of porous constructs for cartilage regeneration. Mater. Sci. Eng. C 2020, 109, 110578. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Pourbashir, S.; Shahrousvand, M.; Ghaffari, M. Preparation and characterization of semi-IPNs of polycaprolactone/poly (acrylic acid)/cellulosic nanowhisker as artificial articular cartilage. Int. J. Biol. Macromol. 2020, 142, 298–310. [Google Scholar] [CrossRef]
- Dang-i, A.Y.; Kousar, A.; Liu, J.; Mukwaya, V.; Zhao, C.; Wang, F.; Hou, L.; Feng, C.-L. Mechanically stable C2-phenylalanine hybrid hydrogels for manipulating cell adhesion. ACS Appl. Mater. Interfaces 2019, 11, 28657–28664. [Google Scholar] [CrossRef]
- Luo, X.B.; Ma, M.F.; Zhou, X.J. Hydroxyapatite-poly (vinyl alcohol)-sodium Alginate Porous Hydrogels with Poly (vinyl alcohol) Surface Layer Used for Articular Cartilage Repair. Proc. Mater. Sci. Forum 2016, 852, 1155–1161. [Google Scholar] [CrossRef]
- Chen, J.X.; Cao, L.J.; Shi, Y.; Wang, P.; Chen, J.H. In situ supramolecular hydrogel based on hyaluronic acid and dextran derivatives as cell scaffold. J. Biomed. Mater. Res. Part A 2016, 104, 2263–2270. [Google Scholar] [CrossRef]
- Dinescu, S.; Galateanu, B.; Radu, E.; Hermenean, A.; Lungu, A.; Stancu, I.C.; Jianu, D.; Tumbar, T.; Costache, M. A 3D porous gelatin-alginate-based-IPN acts as an efficient promoter of chondrogenesis from human adipose-derived stem cells. Stem Cells Int. 2015, 2015, 252909. [Google Scholar] [CrossRef] [PubMed]
- Pirinen, S.; Karvinen, J.; Tiitu, V.; Suvanto, M.; Pakkanen, T.T. Control of swelling properties of polyvinyl alcohol/hyaluronic acid hydrogels for the encapsulation of chondrocyte cells. J. Appl. Polym. Sci. 2015, 132, 42272. [Google Scholar] [CrossRef]
- Skaalure, S.C.; Dimson, S.O.; Pennington, A.M.; Bryant, S.J. Semi-interpenetrating networks of hyaluronic acid in degradable PEG hydrogels for cartilage tissue engineering. Acta Biomater. 2014, 10, 3409–3420. [Google Scholar] [CrossRef]
- Snyder, T.N.; Madhavan, K.; Intrator, M.; Dregalla, R.C.; Park, D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J. Biol. Eng. 2014, 8, 1–11. [Google Scholar]
- Ingavle, G.C.; Frei, A.W.; Gehrke, S.H.; Detamore, M.S. Incorporation of aggrecan in interpenetrating network hydrogels to improve cellular performance for cartilage tissue engineering. Tissue Eng. Part A 2013, 19, 1349–1359. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.; Zhu, M.; Sun, Y.; Xu, J.; Feng, Q.; Lin, S.; Wu, T.; Xu, J.; Tian, F.; Xia, J. Robust biopolymeric supramolecular “Host− Guest Macromer” hydrogels reinforced by in situ formed multivalent nanoclusters for cartilage regeneration. Macromolecules 2016, 49, 866–875. [Google Scholar] [CrossRef]
- Jung, H.; Park, J.S.; Yeom, J.; Selvapalam, N.; Park, K.M.; Oh, K.; Yang, J.-A.; Park, K.H.; Hahn, S.K.; Kim, K. 3D tissue engineered supramolecular hydrogels for controlled chondrogenesis of human mesenchymal stem cells. Biomacromolecules 2014, 15, 707–714. [Google Scholar] [CrossRef]
- Saygili, E.; Kaya, E.; Ilhan-Ayisigi, E.; Saglam-Metiner, P.; Alarcin, E.; Kazan, A.; Girgic, E.; Kim, Y.-W.; Gunes, K.; Eren-Ozcan, G.G. An alginate-poly (acrylamide) hydrogel with TGF-β3 loaded nanoparticles for cartilage repair: Biodegradability, biocompatibility and protein adsorption. Int. J. Biol. Macromol. 2021, 172, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhao, X.; Ye, L. Construction of Dual Orientation Crystalline Structure in Poly (vinyl alcohol)/Graphene Oxide Nano-Composite Hydrogels and Reinforcing Mechanism. Ind. Eng. Chem. Res. 2019, 58, 10908–10921. [Google Scholar] [CrossRef]
- Su, W.; Hu, Y.; Zeng, M.; Li, M.; Lin, S.; Zhou, Y.; Xie, J. Design and evaluation of nano-hydroxyapatite/poly (vinyl alcohol) hydrogels coated with poly (lactic-co-glycolic acid)/nano-hydroxyapatite/poly (vinyl alcohol) scaffolds for cartilage repair. J. Orthop. Surg. Res. 2019, 14, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, C.; Figueiredo, L.; Pace, R.; Lesoeur, J.; Rouillon, T.; Le Visage, C.; Tassin, J.-F.; Weiss, P.; Guicheux, J.; Rethore, G. Laponite nanoparticle-associated silated hydroxypropylmethyl cellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Acta Biomater. 2018, 65, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Nojoomi, A.; Tamjid, E.; Simchi, A.; Bonakdar, S.; Stroeve, P. Injectable polyethylene glycol-laponite composite hydrogels as articular cartilage scaffolds with superior mechanical and rheological properties. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Mirahmadi, F.; Tafazzoli-Shadpour, M.; Shokrgozar, M.A.; Bonakdar, S. Enhanced mechanical properties of thermosensitive chitosan hydrogel by silk fibers for cartilage tissue engineering. Mater. Sci. Eng. C 2013, 33, 4786–4794. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, C.; Wen, J.; Wu, Y.; Shan, Y.; Liao, J. The design, mechanism and biomedical application of self-healing hydrogels. Chin. Chem. Lett. 2017, 28, 1857–1874. [Google Scholar] [CrossRef]
- Khan, M.; Koivisto, J.T.; Hukka, T.I.; Hokka, M.; Kellomäki, M. Composite hydrogels using bioinspired approach with in situ fast gelation and self-healing ability as future injectable biomaterial. ACS Appl. Mater. Interfaces 2018, 10, 11950–11960. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Tu, Y.; Chen, N.; Li, C.; Liu, H.; Zhu, R.; Chen, S.; Xiao, Q.; Liu, J.; Ramakrishna, S.; He, L. Advances in injectable self-healing biomedical hydrogels. Acta Biomater. 2019, 90, 1–20. [Google Scholar] [CrossRef]
- Binder, W.H. Self-Healing Polymers: From Principles to Applications; Wiley Online Library: Weinheim, Germany, 2013. [Google Scholar]
- Maiz-Fernández, S.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Polysaccharide-based in situ self-healing hydrogels for tissue engineering applications. Polymers 2020, 12, 2261. [Google Scholar] [CrossRef]
- Leach, J.B.; Bivens, K.A.; Collins, C.N.; Schmidt, C.E. Development of photocrosslinkable hyaluronic acid-polyethylene glycol-peptide composite hydrogels for soft tissue engineering. J. Biomed. Mater. Res. Part A: Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 70, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, M.; Guadagno, L. Healing efficiency of epoxy-based materials for structural applications. Polym. Compos. 2013, 34, 1525–1532. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef]
- Ding, N.; Cai, X.; Zhang, P.; Dong, S.; Du, B.; Nie, J.; Yu, P. Mimicking the Mechanical Properties of Cartilage Using Ionic-and Hydrogen-Bond Cross-Linked Hydrogels with a High Equilibrium Water Content above 70%. ACS Appl. Polym. Mater. 2021, 3, 2709–2721. [Google Scholar] [CrossRef]
- Jiang, H.; Duan, L.; Ren, X.; Gao, G. Hydrophobic association hydrogels with excellent mechanical and self-healing properties. Eur. Polym. J. 2019, 112, 660–669. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Banerjee, R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef]
- Roh, H.-H.; Kim, H.-S.; Kim, C.; Lee, K.-Y. 3D Printing of Polysaccharide-Based Self-Healing Hydrogel Reinforced with Alginate for Secondary Cross-Linking. Biomedicines 2021, 9, 1224. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, W.; Wang, Q.; Xu, C.; Tang, Q.; Yang, H. An injectable, dual responsive, and self-healing hydrogel based on oxidized sodium alginate and hydrazide-modified poly (ethyleneglycol). Molecules 2018, 23, 546. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Cao, X.; Du, J.; Wang, G.; Chen, X. Multifunctional hydrogel with good structure integrity, self-healing, and tissue-adhesive property formed by combining Diels–Alder click reaction and acylhydrazone bond. ACS Appl. Mater. Interfaces 2015, 7, 24023–24031. [Google Scholar] [CrossRef]
- Wypych, G. Self-Healing Materials; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Döhler, D.; Michael, P.; Binder, W. Principles of Self-Healing Polymers. In Self-Healing Polymers; Wiley Online Library: Hoboken, NJ, USA, 2013; pp. 5–60. [Google Scholar]
- Ferreira, N.; Ferreira, L.; Cardoso, V.; Boni, F.; Souza, A.; Gremião, M. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Urban, M.W. Self-healing of polymers via supramolecular chemistry. Adv. Mater. Interfaces 2018, 5, 1800384. [Google Scholar] [CrossRef]
- Meng, L.; Shao, C.; Cui, C.; Xu, F.; Lei, J.; Yang, J. Autonomous self-healing silk fibroin injectable hydrogels formed via surfactant-free hydrophobic association. ACS Appl. Mater. Interfaces 2019, 12, 1628–1639. [Google Scholar] [CrossRef]
- Liu, J.; Tan, C.S.Y.; Yu, Z.; Li, N.; Abell, C.; Scherman, O.A. Tough supramolecular polymer networks with extreme stretchability and fast room-temperature self-healing. Adv. Mater. 2017, 29, 1605325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Heilshorn, S.C. Adaptable hydrogel networks with reversible linkages for tissue engineering. Adv. Mater. 2015, 27, 3717–3736. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.J.; Appel, E.A.; Meijer, E.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2016, 15, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 48668. [Google Scholar] [CrossRef] [Green Version]
- Appel, E.; Loh, X.; Jones, S.; Biedermann, F.; Dreiss, C.; Scherman, O. High-water-content hydrogels from renewable resources through host–guest interactions. J. Am. Chem. Soc. 2012, 134, 11767–11773. [Google Scholar] [CrossRef]
- Appel, E.A.; Loh, X.J.; Jones, S.T.; Biedermann, F.; Dreiss, C.A.; Scherman, O.A. Ultrahigh-water-content supramolecular hydrogels exhibiting multistimuli responsiveness. J. Am. Chem. Soc. 2012, 134, 11767–11773. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Lee, Y.; Son, J.; Bae, J.; Park, K. In situ. Supramolecular Assembly and Modular Modification of Hyaluronic Acid Hydrogels for 3D Cellular Engineering. ACS Nano 2012, 6, 2960. [Google Scholar]
- Rodell, C.B.; MacArthur, J.W., Jr.; Dorsey, S.M.; Wade, R.J.; Wang, L.L.; Woo, Y.J.; Burdick, J.A. Shear-thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv. Funct. Mater. 2015, 25, 636–644. [Google Scholar] [CrossRef]
- Appel, E.A.; del Barrio, J.; Loh, X.J.; Scherman, O.A. Supramolecular polymeric hydrogels. Chem. Soc. Rev. 2012, 41, 6195–6214. [Google Scholar] [CrossRef]
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter 2012, 8, 260–272. [Google Scholar] [CrossRef]
- He, F.; Wang, L.; Yang, S.; Qin, W.; Feng, Y.; Liu, Y.; Zhou, Y.; Yu, G.; Li, J. Highly stretchable and tough alginate-based cyclodextrin/Azo-polyacrylamide interpenetrating network hydrogel with self-healing properties. Carbohydr. Polym. 2021, 256, 117595. [Google Scholar] [CrossRef]
- Liu, F.; Li, F.; Deng, G.; Chen, Y.; Zhang, B.; Zhang, J.; Liu, C.-Y. Rheological images of dynamic covalent polymer networks and mechanisms behind mechanical and self-healing properties. Macromolecules 2012, 45, 1636–1645. [Google Scholar] [CrossRef]
- Liu, Q.; Ji, N.; Xiong, L.; Sun, Q. Rapid gelling, self-healing, and fluorescence-responsive chitosan hydrogels formed by dynamic covalent crosslinking. Carbohydr. Polym. 2020, 246, 116586. [Google Scholar] [CrossRef]
- Li, S.; Pei, M.; Wan, T.; Yang, H.; Gu, S.; Tao, Y.; Liu, X.; Zhou, Y.; Xu, W.; Xiao, P. Self-healing hyaluronic acid hydrogels based on dynamic Schiff base linkages as biomaterials. Carbohydr. Polym. 2020, 250, 116922. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, D.; Lin, H.; Xiao, Y.; Zhang, X. Cellulose Nanocrystal Reinforced Collagen-Based Nanocomposite Hydrogel with Self-Healing and Stress-Relaxation Properties for Cell Delivery. Biomacromolecules 2020, 21, 2400–2408. [Google Scholar] [CrossRef]
- Furlani, F.; Sacco, P.; Cok, M.; de Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Biomimetic, Multiresponsive, and Self-Healing Lactose-Modified Chitosan (CTL)-Based Gels Formed via Competitor-Assisted Mechanism. ACS Biomater. Sci. Eng. 2019, 5, 5539–5547. [Google Scholar] [CrossRef]
- Barcan, G.A.; Zhang, X.; Waymouth, R.M. Structurally dynamic hydrogels derived from 1, 2-dithiolanes. J. Am. Chem. Soc. 2015, 137, 5650–5653. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Li, X.; Wang, S.; Yuan, L.; Ge, L.; Li, D.; Mu, C. Facile fabrication of biocompatible gelatin-based self-healing hydrogels. ACS Appl. Polym. Mater. 2019, 1, 1350–1358. [Google Scholar] [CrossRef]
- Hafeez, S.; Ooi, H.W.; Morgan, F.L.; Mota, C.; Dettin, M.; Van Blitterswijk, C.; Moroni, L.; Baker, M.B. Viscoelastic oxidized alginates with reversible imine type crosslinks: Self-healing, injectable, and bioprintable hydrogels. Gels 2018, 4, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, F.; Wu, S.; Wang, S.; Xiong, Y.; Li, Y.; Li, B.; Deng, H.; Du, Y.; Xiao, L.; Shi, X. A dynamic and self-crosslinked polysaccharide hydrogel with autonomous self-healing ability. Soft Matter 2015, 11, 3971–3976. [Google Scholar] [CrossRef] [PubMed]
- Lü, S.; Gao, C.; Xu, X.; Bai, X.; Duan, H.; Gao, N.; Feng, C.; Xiong, Y.; Liu, M. Injectable and self-healing carbohydrate-based hydrogel for cell encapsulation. ACS Appl. Mater. Interfaces 2015, 7, 13029–13037. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Qi, T.; Li, G.L. Towards dynamic but supertough healable polymers through biomimetic hierarchical hydrogen-bonding interactions. Angew. Chem. Int. Ed. 2018, 57, 13838–13842. [Google Scholar] [CrossRef]
- Guo, M.; Pitet, L.M.; Wyss, H.M.; Vos, M.; Dankers, P.Y.; Meijer, E. Tough stimuli-responsive supramolecular hydrogels with hydrogen-bonding network junctions. J. Am. Chem. Soc. 2014, 136, 6969–6977. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Zhang, X. Ultrarobust, tough and highly stretchable self-healing materials based on cartilage-inspired noncovalent assembly nanostructure. Nat. Commun. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, X.; Gan, S.; Zhao, J.; Rong, J. Dual ionically cross-linked hydrogels with ultra-tough, stable, and self-healing properties. J. Mater. Sci. 2019, 54, 14218–14232. [Google Scholar] [CrossRef]
- Izzo, D.; Palazzo, B.; Scalera, F.; Gullotta, F.; Lapesa, V.; Scialla, S.; Sannino, A.; Gervaso, F. Chitosan scaffolds for cartilage regeneration: Influence of different ionic crosslinkers on biomaterial properties. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 936–945. [Google Scholar] [CrossRef]
- Bai, T.; Liu, S.; Sun, F.; Sinclair, A.; Zhang, L.; Shao, Q.; Jiang, S. Zwitterionic fusion in hydrogels and spontaneous and time-independent self-healing under physiological conditions. Biomaterials 2014, 35, 3926–3933. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Yang, Y.; He, J.; Li, M.; Guo, B. Novel supramolecular self-healing silk fibroin-based hydrogel via host–guest interaction as wound dressing to enhance wound healing. Chem. Eng. J. 2021, 417, 128278. [Google Scholar] [CrossRef]
- Loebel, C.; Rodell, C.B.; Chen, M.H.; Burdick, J.A. Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat. Protoc. 2017, 12, 1521. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ma, X.; Wu, S.; Tian, H. A rapidly self-healing supramolecular polymer hydrogel with photostimulated room-temperature phosphorescence responsiveness. Angew. Chem. 2014, 126, 14373–14376. [Google Scholar] [CrossRef]
- Jia, Y.-G.; Jin, J.; Liu, S.; Ren, L.; Luo, J.; Zhu, X. Self-healing hydrogels of low molecular weight poly (vinyl alcohol) assembled by host–guest recognition. Biomacromolecules 2018, 19, 626–632. [Google Scholar] [CrossRef]
- Duan, J.; Jiang, J.; Li, J.; Liu, L.; Li, Y.; Guan, C. The preparation of a highly stretchable cellulose nanowhisker nanocomposite hydrogel. J. Nanomater. 2015, 16, 75. [Google Scholar] [CrossRef]
- Qin, B.; Zhang, S.; Sun, P.; Tang, B.; Yin, Z.; Cao, X.; Chen, Q.; Xu, J.F.; Zhang, X. Tough and Multi-Recyclable Cross-Linked Supramolecular Polyureas via Incorporating Noncovalent Bonds into Main-Chains. Adv. Mater. 2020, 32, 2000096. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, Y.; Nan, Y.; Okuro, K.; Aida, T. Mechanically robust, readily repairable polymers via tailored noncovalent cross-linking. Science 2018, 359, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Guo, G.; Lu, X.; Ji, S.; Tan, G.; Gao, L. Facile soaking strategy toward simultaneously enhanced conductivity and toughness of self-healing composite hydrogels through constructing multiple noncovalent interactions. ACS Appl. Mater. Interfaces 2018, 10, 19133–19142. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Joshi, N.; Thorat, K.; Kaur, S.; Chandan, R.; Banerjee, R. A tumor responsive self healing prodrug hydrogel enables synergistic action of doxorubicin and miltefosine for focal combination chemotherapy. J. Mater. Chem. B 2019, 7, 2920–2925. [Google Scholar] [CrossRef]
- Long, T.; Li, Y.; Fang, X.; Sun, J. Salt-Mediated Polyampholyte Hydrogels with High Mechanical Strength, Excellent Self-Healing Property, and Satisfactory Electrical Conductivity. Adv. Funct. Mater. 2018, 28, 1804416. [Google Scholar] [CrossRef]
- Li, S.; Chen, C.; Zhang, Z.; Wang, D.; Lv, S. Illustration and application of enhancing effect of arginine on interactions between nano-clays: Self-healing hydrogels. Soft Matter 2019, 15, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Cui, X.; Liu, X.; Qu, X.; Sun, J. Highly tough, stretchable, self-healing, and recyclable hydrogels reinforced by in situ-formed polyelectrolyte complex nanoparticles. Macromolecules 2019, 52, 3141–3149. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Liow, S.S.; Xue, K.; Zhang, X.; Li, Z.; Loh, X.J. Autonomous chitosan-based self-healing hydrogel formed through noncovalent interactions. ACS Appl. Polym. Mater. 2019, 1, 1769–1777. [Google Scholar] [CrossRef]
- Liao, M.; Wan, P.; Wen, J.; Gong, M.; Wu, X.; Wang, Y.; Shi, R.; Zhang, L. Wearable, healable, and adhesive epidermal sensors assembled from mussel-inspired conductive hybrid hydrogel framework. Adv. Funct. Mater. 2017, 27, 1703852. [Google Scholar] [CrossRef]
- Gavel, P.K.; Dev, D.; Parmar, H.S.; Bhasin, S.; Das, A.K. Investigations of peptide-based biocompatible injectable shape-memory hydrogels: Differential biological effects on bacterial and human blood cells. ACS Appl. Mater. Interfaces 2018, 10, 10729–10740. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small 2019, 15, 1900046. [Google Scholar] [CrossRef]
- Xu, J.; Wang, G.; Wu, Y.; Ren, X.; Gao, G. Ultrastretchable wearable strain and pressure sensors based on adhesive, tough, and self-healing hydrogels for human motion monitoring. ACS Appl. Mater. Interfaces 2019, 11, 25613–25623. [Google Scholar] [CrossRef]
- Chen, J.; An, R.; Han, L.; Wang, X.; Zhang, Y.; Shi, L.; Ran, R. Tough hydrophobic association hydrogels with self-healing and reforming capabilities achieved by polymeric core-shell nanoparticles. Mater. Sci. Eng. C 2019, 99, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kang, M.; Li, K.; Yao, F.; Oderinde, O.; Fu, G.; Xu, L. Polysaccharide-templated preparation of mechanically-tough, conductive and self-healing hydrogels. Chem. Eng. J. 2018, 334, 2222–2230. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Huang, C.-F.; Wei, Y.; Hsu, S.-H. Novel chitosan–cellulose nanofiber self-healing hydrogels to correlate self-healing properties of hydrogels with neural regeneration effects. NPG Asia Mater. 2019, 11, 1–17. [Google Scholar] [CrossRef]
- Tang, L.; Liao, S.; Qu, J. Self-healing and multistimuli-responsive hydrogels formed via a cooperation strategy and their application in detecting biogenic amines. ACS Appl. Mater. Interfaces 2018, 10, 27365–27373. [Google Scholar] [CrossRef]
- Rodell, C.B.; Dusaj, N.N.; Highley, C.B.; Burdick, J.A. Injectable and cytocompatible tough double-network hydrogels through tandem supramolecular and covalent crosslinking. Adv. Mater. 2016, 28, 8419–8424. [Google Scholar] [CrossRef]
- Agarwal, T.; Chiesa, I.; Presutti, D.; Irawan, V.; Vajanthri, K.Y.; Costantini, M.; Nakagawa, Y.; Tan, S.-A.; Makvandi, P.; Zare, E.N. Recent advances in bioprinting technologies for engineering different cartilage-based tissues. Mater. Sci. Eng. C 2021, 112005. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Eng. Part B: Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Nematollahi, Z.; Tafazzoli-Shadpour, M.; Zamanian, A.; Seyedsalehi, A.; Mohammad-Behgam, S.; Ghorbani, F.; Mirahmadi, F. Fabrication of chitosan silk-based tracheal scaffold using freeze-casting method. Iran. Biomed. J. 2017, 21, 228. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, N.; Farzin, A.; Bayat, F.; Goodarzi, A.; Salehi, M.; Karimi, R.; Mohamadnia, A.; Parhiz, A.; Ai, J. Optimization of 3D alginate scaffold properties with interconnected porosity using freeze-drying method for cartilage tissue engineering application. Arch. Neurosci. 2019, 6, e85122. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.; Sankar, M.R.; Katiyar, V. State of art on solvent casting particulate leaching method for orthopedic scaffoldsfabrication. Mater. Today: Proc. 2017, 4, 898–907. [Google Scholar] [CrossRef]
- Jia, Z.; Liu, Y.; Wang, Y.; Peng, S.; Jia, P.; Zhang, W.; Tan, X. Gas-foaming three-dimensional electrospun nanofiber scaffold improved three-dimensional cartilage regeneration. Mater. Res. Express 2021, 8, 085403. [Google Scholar] [CrossRef]
- Munir, N.; Callanan, A. Novel phase separated polycaprolactone/collagen scaffolds for cartilage tissue engineering. Biomed. Mater. 2018, 13, 051001. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chyu, J.; Zumwalt, M. Recent progress of fabrication of cell scaffold by electrospinning technique for articular cartilage tissue engineering. Int. J. Biomater. 2018, 2018, 1953636. [Google Scholar] [CrossRef] [Green Version]
- Nikbakht, M.; Karbasi, S.; Rezayat, S.M.; Tavakol, S.; Sharifi, E. Evaluation of the effects of hyaluronic acid on poly (3-hydroxybutyrate)/chitosan/carbon nanotubes electrospun scaffold: Structure and mechanical properties. Polym. -Plast. Technol. Mater. 2019, 58, 2031–2040. [Google Scholar] [CrossRef]
- Li, C.; Wang, K.; Zhou, X.; Li, T.; Xu, Y.; Qiang, L.; Peng, M.; Xu, Y.; Xie, L.; He, C. Controllable fabrication of hydroxybutyl chitosan/oxidized chondroitin sulfate hydrogels by 3D bioprinting technique for cartilage tissue engineering. Biomed. Mater. 2019, 14, 025006. [Google Scholar] [CrossRef]
- Kundu, J.; Shim, J.H.; Jang, J.; Kim, S.W.; Cho, D.W. An additive manufacturing-based PCL–alginate–chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef]
- Semba, J.A.; Mieloch, A.A.; Rybka, J.D. Introduction to the state-of-the-art 3D bioprinting methods, design, and applications in orthopedics. Bioprinting 2020, 18, e00070. [Google Scholar] [CrossRef]
- Zhu, W.; Qu, X.; Zhu, J.; Ma, X.; Patel, S.; Liu, J.; Wang, P.; Lai, C.S.E.; Gou, M.; Xu, Y. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture. Biomaterials 2017, 124, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Roseti, L.; Cavallo, C.; Desando, G.; Parisi, V.; Petretta, M.; Bartolotti, I.; Grigolo, B. Three-dimensional bioprinting of cartilage by the use of stem cells: A strategy to improve regeneration. Materials 2018, 11, 1749. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Gu, H.; Mi, H.; Rao, C.; Fu, J.; Turng, L.-s. Fabrication of scaffolds in tissue engineering: A review. Front. Mech. Eng. 2018, 13, 107–119. [Google Scholar] [CrossRef]
- Facklam, A.L.; Volpatti, L.R.; Anderson, D.G. Biomaterials for personalized cell therapy. Adv. Mater. 2020, 32, 1902005. [Google Scholar] [CrossRef]
- Fox, I.J.; Daley, G.Q.; Goldman, S.A.; Huard, J.; Kamp, T.J.; Trucco, M. Use of differentiated pluripotent stem cells in replacement therapy for treating disease. Science 2014, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbabi, V. Multi-Physics Computational Models of Articular Cartilage for Estimation of Its Mechanical and Physical Properties. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2016. [Google Scholar]
- Pearce, D.; Fischer, S.; Huda, F.; Vahdati, A. Applications of computer modeling and simulation in cartilage tissue engineering. Tissue Eng. Regen. Med. 2020, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
| Mechanical Property | Value | Test Method | Ref. |
|---|---|---|---|
| Aggregate modulus (MPa) | 0.10–2.1 | Confined compression | [22] |
| Hydraulic permeability (m2/Pa.s) | 10−16–10−15 | Unconfined compression, indentation | [22] |
| Compressive Young’s modulus (MPa) | 0.23–0.85 | Unconfined compression | [22] |
| Poisson’s ratio | 0.05–0.30 | Unconfined compression | [22] |
| Tensile equilibrium modulus (MPa) | 5.0–12.0 | Tensile stress relaxation | [22] |
| Tensile Young’s modulus (MPa) | 5.0–25.0 | Tensile constant strain rate | [22] |
| Tensile strength (MPa) | 0.7–25.0 | Equilibrium shear | [22] |
| Equilibrium shear modulus (MPa) | 0.05–0.40 | Equilibrium shear | [22] |
| Complex shear modulus (MPa) | 0.2–2.5 | Dynamic shear | [22] |
| Shear loss angle (°) | 10–15 | Dynamic shear | [22] |
| Biological property | Value | Ref. | |
| Initial cell seeding | ≥63 million cells/mL | [28] | |
| Osmolality | Physiological osmolality | [29] | |
| Extracellular pH | 7–8 | [29] | |
| Pore size | 2.5–6.5 nm | [29] | |
| Growth factors | PDGF, TGF-β, FGF, BMP, IGF | [29] | |
| Mechanical loading (dynamic compression) | 2–10% strain or 0.5–1.0 MPa at physiological frequency 0.01 to 1.0 Hz | [29] | |
| Main Base | Main Materials | Advantages | Highlighted Achievements | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Chitosan | Chitosan, kartogenin | Increased mechanical properties, excellent biocompatibility, biodegradability, and cell adhesion | Significant statistical models to predict the properties | Immunogenic | [58] |
| Gellan gum (GG)/nanoparticles/graphene oxide/hydroxyethyl cellulose/dialdehyde starch/ poly (vinyl alcohol)/gelatin/ hyaluronic acid | Controllable properties, degradation rate, and pore size | [43,59,60] | |||
| Chitosan, Pyrrole | Good thermo-sensitive gelation | High gelation time, swelling, and degradation time | [61] | ||
| Chitosan, PLA, calcium phosphate, hydroxyapatite | - | Bioinert | [62] | ||
| Collagen/gelatin | hyaluronic acid/dialdehyde micro fibrillated cellulose (DAMFC)/transglutaminase enzyme | Biosafe, excellent mechanical and biochemical properties, biocompatibility, and cell viability, low cost, biodegradable, ECM production of cartilage | - | Immunogenic | [63,64,65,66,67,68] |
| Riboflavin, collagen, hyaluronic acid | - | Delayed enzyme-triggered degradation time | [69] | ||
| Gelatin, graft-poly(N-isopropyl acrylamide) | Low water/oil interfacial tensions, thermo-responsive | [70] | |||
| Alginate, borax | Reduced inflammatory effect | [71] | |||
| Hyaluronic acid | Alginate/cellulose nanocrystals, adipic acid dihydrazide/fibrin/ lysine methyl ester/divinyl sulfone, functionalized inulin | Bioprintable, biocompatible, good proliferation, stable, enhanced cell adhesion, proliferation, and differentiation | - | Weak mechanical integrity, fast degradation in vivo | [72,73,74,75,76,77,78] |
| Polydactyly chondrocytes, heparin/fibrin | Cartilage-like matrix | [79] | |||
| Trans glutaminase crosslinked hyaluronan | Excellent mitogen chondrification, superior adhesion to native cartilage | [80] | |||
| PEG, chondrocytes | Superior mechanical properties, improved metabolic viability | Fast degradation | [81] | ||
| Fibrin | ECM microparticle, alginate microbeads/PEG, human amniotic fluid-derived stem cells | Stable, biocompatible, injectable | - | [82,83] | |
| Alginate | Gelatin, Hydroxyapatite, protein (BSA),Alginate, Fibrinogen | Tunable mechanical properties similar to native cartilage, excellent osteochondral regeneration and proliferation, 3D printable, excellent cell adhesion and biocompatibility | Interconnected mesh structure, great flexibility and degradation | Slow and unpredictable degradation in vivo | [84] |
| Hydroxyapatite (HAP) complex | - | [85] | |||
| Bone marrow-derived mesenchymal stem cells/polymethacrylate hybrid, collagen type I/hyaluronic acid, elastin-like protein (ELP) | - | [86,87] | |||
| - | Excellent viscoelasticity | [88] | |||
| Gelatin | High degradation | [89] | |||
| Elastin | Poly(N-isopropylacrylamide- co-polylactide-2 hydroxyethyl methacrylate-co-oligo (ethylene glycol) monomethyl ether methacrylate (PNPHO) | Biocompatible, proper mechanical properties, good structural stable, cell proliferation, injectable | - | Difficult to integrate with surrounding tissue, Immunogenic | [90] |
| Silk | Cell interactions | [91] | |||
| Chondroitin sulfate | Pullulan/poly (N-isopropylacrylamide) (NIPAAm) | Biocompatible, cytocompatible, increased cell proliferation, mechanically stable, improved cartilaginous ECM deposition, good mechanical properties, injectable | Self-healing | Immunogenic | [92] |
| No cytotoxicity | [93] |
| Advanced Hydrogel Type | Main Materials | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Multi materials | Chondrocyte-laden GelMA, PCL | Porous structure, cell proliferation, excellent mechanical and thermo-reversible properties, printable | Long-time UV exposure and low cell viability | [146] |
| PCL, Pluronic F-127 | Biocompatible, biodegradable, finite antigenicity | Immune response and therapeutic efficacy have not determined | [147] | |
| Poly(vinyl alcohol), poly(ε-caprolactone), Gelatin methacrylamide/Gellan gum, Alginate | Great differentiation, ability to produce complex structure and support components | Low shape fidelity | [98] | |
| IPN | Polycaprolactone, Poly (acrylic acid), Cellulosic nano-whisker, Acrylic-urethane cross-linker | Improved the mechanical properties, water absorption of about 30%, excellent hydrophilic property | Need to optimization of physicochemical surface conditions for cell adhesion and proliferation | [148] |
| Carboxymethyl dextran, Amino dextran | Excellent mechanical stable, adhesion, and spreading behavior of fibroblast cells, biodegradable and biocompatible | Immune responses have not been determined | [149] | |
| Hydroxyapatite particles, Alginate | Proper osteochondral healing, suitable compressive modulus and swelling property, high porosity, uniform pores | Using of poor supramolecular gelation agent | [150] | |
| Conjugated dextran with 2-naphthylacetic, HA, β-cyclodextrin | Excellent resilience, good biocompatibility | N/A | [151] | |
| Gelatin, Alginate polyacrylamide | Enhanced mechanical properties, excellent cell proliferation, finite cytotoxicity, chondrogenic gene expression, and structural stability, great porosity in long-term | Uncontrollable porosity, Formation of a thin superficial layer that does not allow cell penetration | [152] | |
| Ethylene diamine-functionalized HA, Divinyl sulfone-inulin | Biodegradable, FDA-approved, good mechanical properties | Low cell viability | [78] | |
| Low-molecular-weight PVA, High molecular weight HA | Biocompatible, excellent swelling properties and cell viability | Fast gelation in room temperature | [153] | |
| Poly(ethylene glycol), Low-molecular weight HA | Excellent solubility in GAG deposition during structure maturation, support of collagen biosynthesis | Low enzyme degradation | [154] | |
| Methacrylated HA, Fibrin | Biocompatible, Support of differentiation | Unstable and unsuitable mechanical properties in low concentration | [155] | |
| Methacrylated chondroitin sulfate, Agarose-poly(ethylene glycol) diacrylate | Enhanced collagen biosynthesis and GAGs in the cell-matrix, low cost | Low cell viability | [156] | |
| Supramolecular | Adamantane-functionalized HA, monoacrylated β-cyclodextrin | Great drying and re-swelling without changes in water content or shape, excellent collagen deposition, suitable biophysical properties, rapid stress relaxation, self-healing | N/A | [157] |
| Cucurbituril, diaminohexane | Controlled dexamethasone release, enhanced cell proliferation, GAG synthesis, and chondrogenic gene expression, in vivo neocartilage production | N/A | [158] | |
| Nanomaterials | Alginate, Poly(acrylamide) hydrogel, poly(lactide-co-glycolide) (PLGA) nanoparticles | Great viscoelasticity, biodegradable, biocompatible and protein absorber, excellent cell proliferation and mechanical strength, stable | N/A | [159] |
| Poly(vinyl alcohol), Graphene oxide | Great bio-mechanical and bio-friction properties, excellent shear-thinning, printability, and printing accuracy, proper compressive and tribological properties | Unsuitable pore size | [160] | |
| Nano hydroxyapatite, Poly(vinyl alcohol), Poly(lactic-co-glycolic acid) | Biocompatible, practicable, excellent mechanical properties, sensitive to compressive stress, suitable chondrocyte adhesion and proliferation | N/A | [161] | |
| Poly(vinyl alcohol), Nano-hydroxyapatite, magnetic Nanoparticles (Fe2O3) | Proper mechanical properties, great mesenchymal stem cells growth | Variable crystallinity | [112] | |
| Hydroxypropyl methylcellulose, Laponites | Excellent mechanical properties, oxygen diffusion, and cell expression | Some toxicity, decreased cell density | [162] | |
| PEG, Laponite particles | Good elastic modulus, biocompatible, excellent mechanical properties | Low cell viability | [163] | |
| Silk fibers, Chitosan/Glycerophosphate | Excellent mechanical properties, GAG, and collagen type II expression | Unsuitable biological properties, toxic gelation agent | [164] |
| Mechanism Type | Materials | Self- Healing Conditions | Time of Healing | Properties | Main Reactions | Healing Efficiency | Ref. |
|---|---|---|---|---|---|---|---|
| Dynamic Covalent interaction | Poly(ethylene oxide) | Room temperature (RT), acidic pH | 48 h | Biocompatible, cell viability, good viscoelasticity, improved mechanical stability | Acylhydrazone exchange reactions, disulfide exchange reactions | N/A | [197] |
| Chitosan, Dialdehyde debranched starch (DADBS) | 25 °C | <30 min | Fast crosslinking time under 30 s, tunable self-healing, excellent viscoelasticity, and mechanical properties, excellent 3D printability, obvious responsiveness to fluorescence light | Crosslinking by Schiff-base reactions between the aldehyde groups in DADBS and the amino groups in chitosan | 100% | [198] | |
| O-carboxymethyl chitosan | RT | - | Electrostatic attraction, porous and interconnected morphology, storage modulus, excellent pH sensitive swelling properties | Schiff base reaction between the amino groups on the chitosan and aldehyde groups of crosslink agent, host-guest reaction of poly(β-cyclodextrin) with diamantine | ≥97% | [45] | |
| Dialdehyde—modified hyaluronic acid (AHA), Cystamine dihydrochloride (Cys) | Ambient temperature | 10 min | Fast crosslinking, improved mechanical properties, bioprintable, biocompatible | Schiff base reaction between the di-aldehyde groups on AHA and amino groups on Cys | ~100% | [199] | |
| Aldehyde—functionalized surface-modified cellulose nanocrystals (a-CNCs) | RT | - | Biocompatible, injectable in situ, rapid shear thinning, cell viability, good viscoelasticity, improved mechanical stability | Schiff-base reaction between the aldehyde groups on a-CNCs and amine groups on collagen | ~100% | [200] | |
| Lactose-modified chitosan (CTL), Boric acid, Mannitol | RT | 5 min | Biocompatible, excellent viscoelasticity | Schiff base reactions between the bronic groups in boric acid and the amino groups in CTL | 100% | [201] | |
| Triblock(ABA) copolymers with a central poly(ethylene oxide) block and terminal dithiolane blocks | 25 °C | 24 h, | Biocompatible, excellent Stiffness and viscoelasticity, photosensitive, mucoadhesive | The reversible ring-opening of disulfide exchange, the intracellular redox potential | N/A | [202] | |
| Gelatin, Dialdehyde carboxymethyl cellulose | 37 °C | 1 h | Excellent biocompatibility, biodegradability and non-immunogenicity, good fatigue resistance | Schiff base reaction between amino-gelatin and dialdehyde carboxymethyl cellulose | 90% | [203] | |
| Oxidized alginate (OA), Semicarbazone (or hydrazine) | RT | 10 min (or 30 min) | Biocompatibility, excellent stiffness, viscoelasticity, spreading of fibroblasts and cell adhesion, printability, non-cytotoxic | The Divalent bond between amino bonds of OA and Ca+2 of semi-carbazone | 70% (40%) | [204] | |
| Acrylamide-modified chitin, Oxidized alginate | Basic pH, 25 °C | 2 h | Good biocompatibility and biodegradability, excellent viability | Schiff base reactions between imine linkages amine groups of acrylamide-modified chitin and dialdehyde groups on oxidized alginate | N/A | [205] | |
| Chondroitin sulfate multiple aldehydes (CSMA), N-succinyl chitosan (SC) | 20 °C, high moisture | 2 h | Excellent viability, good biocompatibility, and biodegradability, finite inflammatory, injectable | Schiff base reactions between aldehyde groups on CSMA and amino groups on SC | N/A | [206] | |
| Hydrogen interaction | Urethane, Urea, 2-ureido-4[1H]-pyrimidinone | RT | 48 h | Excellent toughness, tensile strength, and mechanical properties | Hierarchical hydrogen bonding of urethane and supramolecular interaction | 90% | [207] |
| Ureido- pyrimidinone (UPy), Functionalized dextran | 20 °C | 10 min | Biocompatible, good mechanical properties | Ureido-pyrimidinone (UPy)- functionalized dextran | 100% | [11] | |
| 2-ureido-4[1H]-pyrimidinone (UPy), Poly(ethylene glycol) (PEG) | RT | N/A | Tunable mechanical properties, shape memory behavior. Tough | Hydrogen-bonding between UPy and PEG | N/A | [208] | |
| Polyurethane (PU), Tannin, Acid- modified nano tungsten disulfide | RT | 12 h | Excellent mechanical strength and tensile | Noncovalent bonding connection of nano filer, interfacial hydrogen bonds between TA-WS2 and PU | 100% | [209] | |
| Cucurbit[8]uril (CB[8]), Acrylamide, N,N′-bismethylene bisacrylamide | RT | Very fast | Good mechanical properties | Hydrogen bond and Supramolecular interaction between CB[8] and acrylamide, covalent | N/A | [185] | |
| Ionic interaction | 2-hydroxypropyltrimethyl ammonium chloride chitosan (HACC), Poly(acrylic acid) (PAAc)-Fe3+ | 70 °C | 48 h | Excellent mechanical properties, tough and transparent | Both macromolecular positively charged HACC and Fe3+ metal ions acted as cross-linkers to form ionic bonds with negatively charged PAAc | 74% | [210] |
| Chitosan, Arginine (Arg), Tripolyphosphate (TPP) | RT | 48 h | Tunable structural and chemical physical properties | Reaction of Polyanions of TPP and cations of amino acid arginin | N/A | [211] | |
| Ammonium persulfate (APS), N,N,N′,N′- tetramethylethylenedi amine (TEMED) | RT, pH ≤ 3 | N/A | Anti-fatigue, good mechanical properties, time-independent healing | Positively and negatively charged groups of APS and TEMED | 66–73% | [212] | |
| Supramolecular Interaction | β-cyclodextrin modified alginate (Alg-CD), Adamantine modified graphene oxide, | RT | 12 h | Injectable, good cell adhesion and differentiation, excellent mechanical properties | Guest–host interactions | 100% | [213] |
| Adamantane functionalized hyaluronic acid, β-Cyclodextrin | RT | 12 h | Photo-cross-linkable compressible | Guest–host interactions | N/A | [157] | |
| β-cyclodextrin, adamantine bound by peptide tether to Hyaluronic acid | 37 °C | Fast | Injectable, good cell adhesion and differentiation, excellent mechanical stability | Guest–host interactions | 100% | [214] | |
| β-cyclodextrin-, α-bromonaphthalene functionalized acrylamide | 20 °C | 1 min–1 h | Injectable, excellent mechanical properties | Guest–host interactions | N/A | [215] | |
| β Cholic-acid, β-cyclodextrin-functionalized N,N′-dimethylacrylamide | 20 °C | <1 min | Injectable, degradable | Guest–host interaction | 97% | [216] | |
| Hydrophobic interaction | Acrylamide, Octyl phenol polyethoxy ether acrylate copolymer | RT | 6 days | Excellent mechanical properties, flexible | Micelles between the hydrophobic acrylates and sodium dodecyl sulfate | 70% | [175] |
| Cellulose nanowhiskers (CNW), Acrylamide (AM), Stearyl methacrylate, Sodium dodecylsulfat (SDS) | RT | 60 min | Excellent mechanical properties, stretchable | Hydrophobic interaction of CNW and AM | 100% | [217] |
| Hydrogels (Materials) | Bonding Mechanisms | Properties | Ref. |
|---|---|---|---|
| Polyvinyl alcohol/poly(3,4-ethylenedioxythiophene)/sulfosuccinic acid | H-bonding | High water content (75 wt %) | [220] |
| Crystallization | High tensile stress (~2.5 MPa) | ||
| Electrostatic interactions | Large elongation (>600%) | ||
| Conductivity (~25 mS/cm) | |||
| Carboxymethyl cellulose/borate/gelatin | Schiff-base reaction | pH and glucose responsive | [221] |
| Boronate-diol complexation | |||
| P(urea-IL1-SPMA1)-3d IL: imidazolium-based ionic liquid SPMA: 3-sulfopropyl methacrylate potassium salt | H-bonding | Tensile strength of ~1.3 MPa | [222] |
| Ionic interaction | Strain at break of ~720% | ||
| Toughness of ~6.7 MJ/m3 | |||
| Laponite® nano-clay, hydroxyapatite, poly-L-arginine, sodium polyacrylate | H-bonding | - | [223] |
| Electrostatic interactions | |||
| Poly(diallyldimethylammonium chloride)/branched poly(ethylenimine)/poly(sodium 4-styrenesulfonate)/poly(acrylic acid) | H-bonding | Tensile strength: 1.26 MPa | [224] |
| Electrostatic interactions | Strain at break: 2434.2% | ||
| Toughness: 19.53 MJ/m3 | |||
| Free radical polymerization of acrylic acid/acrylamide in the presence of chitosan | H-bonding | High water content (<90%) | [225] |
| Electrostatic interactions | Strain at break <625%) | ||
| High self-healing efficiency (<88%) | |||
| Functionalized single-wall carbon nanotube/polyvinyl alcohol/polydopamine | H-bonding | Fast self-healing ability (~2 s) | [226] |
| π-π interactions | High self-healing efficiency (99%) | ||
| Robust adhesiveness | |||
| Amoc (9-anthracenemethoxycarbonyl)-capped dipeptides | H-bonding | Antibacterial efficacy | [227] |
| π-π interactions | |||
| Hyaluronic acid-graft-dopamine and reduced graphene oxide/using a H2O2/HPR (horseradish peroxidase) | H-bonding | Antioxidant activity | [228] |
| Photothermal effect | |||
| π-π interactions | Adhesive hydrogel | ||
| Hemostatic hydrogel | |||
| Conductive hydrogel | |||
| Casein sodium salt from bovine milk/polydopamine/polyacrylamide | H-bonding | Super-stretchability | [229] |
| π-π interactions | Excellent fatigue resistance | ||
| Rapid self-healing | |||
| Poly (styrene-acrylic acid) core-shell nanoparticles/free radical copolymerization of acrylamide and stearyl methylacrylate | H-bonding | Excellent self-healing | [230] |
| Hydrophobic interactions | Good mechanical properties | ||
| Alginate aldehyde/poly (acrylamide) | Schiff-base reaction | Excellent self-healing and mechanical properties | [231] |
| H-bonding | |||
| Glycol chitosan/cellulose nanofiber/telechelic difunctional polyethylene glycol | Schiff-base reaction | Injectability (neural stem cells delivery) | [232] |
| H-bonding | |||
| Salicylaldehyde benzoyl hydrazone-terminal poly(ethylene glycol)/Ni2+ | Metal–ligand coordination | Rapid self-healing Reversible pH-responsiveness | [233] |
| Hydrophobic interactions | |||
| Adamantane and β-cyclodextrin modified hyaluronic acid/methacrylated hyaluronic acid | Michael addition crosslinking (covalent reaction) | Injectability | [234] |
| Rapid self-healing | |||
| Host-guest interactions | Cytocompatibility | ||
| Mechanical toughness |
| Method | Main Characteristic | Resulted Porosity | Cell Viability | Ref. |
|---|---|---|---|---|
| Freeze casting | Ceramic slurries are used in this method; then, water is evaporated. It produces pores due to formation of ice crystals. | <85% | <90% | [237] |
| Freeze-drying | It is an easy procedure that can be applied with natural materials such as collagen and fibers. The porosity can be improved by freezing temperature alterations and changing of the concentration of materials. | 30%–80% | <90% | [238] |
| Solvent casting and Particle leaching | It uses casting molds to produce 3D scaffolds by polymer solution. Then, it requires leaching by using organic solvents to simplify the addition of drugs or growth factors to scaffolds. | 50%–90% | 75%–88% | [239] |
| Gas foaming | Using high-pressure carbon dioxide for expanding the polymer matrix without applying high temperature or toxic solvents. Changing pressure can also create scaled porous scaffolds. | <90% | N/A | [240] |
| Phase separation | Changing temperature for polymer and solvent separation results in a solid polymer due to phase separation. Finally, a desirable, homogenous, and interconnected porous scaffold is produced depending on cooling rates. | 60%–98% | <98% | [241] |
| Electrospinning | Nanoscale or microscale fibers are produced by tuning process parameters and chemicals in this method. | 80%–95% | <80% | [242,243] |
| Sol–gel | Colloidal metal oxides are applied traditionally to create tunable porous scaffolds in the sol–gel method with desirable chemistry. Double phasic chitosan scaffolds with a conjunction peptide have demonstrated the capability to recruit stem cells for cartilage repair. | N/A | N/A | [244] |
| Additive manufacturing | Extrusion methods in biomedical applications are often polymer-based and provide benefits in cost, size, and flexibility against old manufacturing methods. Both polymers and metals can be used in solid free-form sintering, while laser melting is limited to metals. | 80%–90% | 60%–95% | [245] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafezi, M.; Nouri Khorasani, S.; Zare, M.; Esmaeely Neisiany, R.; Davoodi, P. Advanced Hydrogels for Cartilage Tissue Engineering: Recent Progress and Future Directions. Polymers 2021, 13, 4199. https://doi.org/10.3390/polym13234199
Hafezi M, Nouri Khorasani S, Zare M, Esmaeely Neisiany R, Davoodi P. Advanced Hydrogels for Cartilage Tissue Engineering: Recent Progress and Future Directions. Polymers. 2021; 13(23):4199. https://doi.org/10.3390/polym13234199
Chicago/Turabian StyleHafezi, Mahshid, Saied Nouri Khorasani, Mohadeseh Zare, Rasoul Esmaeely Neisiany, and Pooya Davoodi. 2021. "Advanced Hydrogels for Cartilage Tissue Engineering: Recent Progress and Future Directions" Polymers 13, no. 23: 4199. https://doi.org/10.3390/polym13234199
APA StyleHafezi, M., Nouri Khorasani, S., Zare, M., Esmaeely Neisiany, R., & Davoodi, P. (2021). Advanced Hydrogels for Cartilage Tissue Engineering: Recent Progress and Future Directions. Polymers, 13(23), 4199. https://doi.org/10.3390/polym13234199







